Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Journal of the Southern African Institute of Mining and Metallurgy
versão On-line ISSN 2411-9717
versão impressa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.121 no.7 Johannesburg Jul. 2021
http://dx.doi.org/10.17159/2411-9717/1442/2020
STUDENT PAPERS
Beneficiation of recycled process water at DRDGOLD's ERGO plant, and its effect on gold recovery
A. NarainI, *; J. H. PotgieterI, II; G. E. RenckenI; J. SmithI
ISchool of Chemical and Metallurgical Engineering, University of the Witwatersrand, South Africa
IIDepartment of Natural Science, Manchester Metropolitan University, UK
SYNOPSIS
To conserve fresh water resources and comply with environmental regulations, DRDGOLD, a South African gold producer re-treating surface tailings, has transitioned to a fully closed water circulation system. Consequently, the accumulation of contaminants, as well as addition of reagents, has led to changes in water composition that have compromised leach performance and overall gold recovery. A two-sample t-test confirmed a significant difference in gold recoveries between the use of Rand Water, which was used as a benchmark, and untreated process water. Atomic absorption analysis of ERGO's process water, confirmed the presence of iron, nickel, zinc, and manganese. A study of the effect of the identified contaminants on gold recovery showed that iron, nickel, and zinc have the largest negative effect on gold recovery, with iron and nickel being more detrimental than zinc. Sulphates were shown to have a possible passivation effect, which also influenced gold recoveries, although to a lesser extent than the heavy metals. Calcium, when present in excess, had a positive influence on gold recovery indicating the possible formation of a calcium aurocyanide complex. Lime softening successfully reduced the heavy metal and sulphate concentrations, and the gold recoveries obtained with the treated process water were similar to those as achieved with Rand Water.
Keywords: gold tailings, re-processing, water quality, gold recovery.
Introduction
DRDGOLD is a South African gold producer and a world leader in the recovery of gold from the re-treatment of surface tailings. DRDGOLD ERGO Mining (Pty) Ltd (ERGO) specialises in the re-treatment of gold mine tailings, with numerous operations and tailings reclamation sites spanning 163 km from the western to the central and eastern regions of the Witwatersrand. The current study focuses on the ERGO plant operations in Brakpan, which predominantly reclaims tailings dams in the eastern regions of the Witwatersrand.
Approximately 64 000 t of tailings material are treated daily, requiring an estimated 60 ML/d of water. Since water is a strategic resource in water-scarce South Africa , several mining operations are at risk due to a limited supply of water. This has resulted in an increased utilization of recycled water, with the focus on recycling process water. ERGO's metallurgical research department has, however, demonstrated improved gold recoveries when utilizing clean water compared with recycled process water in laboratory test work. This has led to a growing interest in research to determine the effect/s of recycled process water on gold recovery.
While there is extensive literature available on the effect and interactions of metal-cyanide complexes with the aurocyanide complex, little is known about the effects of water quality on cyanide gold leaching (Rees, 2000). Cyanide has been used globally as a lixiviant for gold leaching since the process was first patented over 100 years ago. However, the processing and extraction of gold has become more complex as the simple, free-milling oxide ores have become depleted over the past decade (Rees, 2000). Due to the presence of various metal species in the ore, as well as in the process water, cyanide complexes of antimony (Sb), arsenic (As), cobalt (Co), copper (Cu), iron (Fe), nickel (Ni), thallium (Tl), and zinc (Zn) may form in the leach solution that is contacted with activated carbon (Sheya and Palmer, 1989). These complexes could result in lower gold recoveries.
Rees (2000) showed that copper is the most problematic metal in gold leaching due to the rapid formation of copper cyanide complexes, which can consume a great deal of the available cyanide.
Boehme and Potter (1983) proved that silver has an adverse effect on the loading rate of gold, with ratios of silver to gold of 1:1 and 2:1. They also demonstrated that as the copper concentration in a slurry increased, the rate of gold loading onto activated carbon decreased.
Hedley and Tabachnick (1968) showed that the zinc cyanide complex formed was deleterious to gold dissolution and subsequent gold recovery. Fleming and Nicol (1984) demonstrated that activated carbon adsorbs organic solvents, which affect the rate of gold loading. Activated carbon also adsorbs small quantities of cyanide, metal hydroxides, calcium (Ca), and iron sulphide, which contaminate the carbon, resulting in a decrease loading capacity. Fleming and Nicol (1984) concluded that activated carbon may have a stronger affinity for both Cu and Ni than for gold.
Fink and Putnam (1950) found that the addition of trace amounts of sodium sulphide to the cyanide solution dramatically hindered gold leaching, and postulated that the sulphide ions passivate the surface of the gold by forming a layer of Au2S. Jeffrey and Breuer (2000) obtained similar results using an ore containing 5% silver. Most naturally occurring gold sulphide ores contain trace amounts of silver, which has been shown to increase the cyanide leaching reaction rate (Jeffrey and Breuer, 2000).
As previous work focused mostly on the effects of heavy metals on gold loading on activated carbon, this investigation aims to extend current knowledge by considering the impact of typical water contaminants like Ca, magnesium (Mg), and sulphate on gold recovery. McDougall, et al. (1980) postulated that the mechanism of adsorption of aurocyanide onto activated carbon involves adsorption of aurocyanide as the less soluble Mn+[(Au(CN)2]n complex (M = K+, Ca+, Mg+) as the initial adsorption stage.
Therefore, in this work we examined the quality of the recycled process water streams on a typical sulphide ore gold processing plant. Laboratory-scale gold flotation and cyanide leach test work were carried out to establish the effects of contaminants such as metal cyanide complexes, sulphates, Ca, and Mg, on gold recovery. An investigation of treatment options for process water at various temperatures, with the respective effect on gold recovery, was also undertaken.
Material and methods
Sample preparation
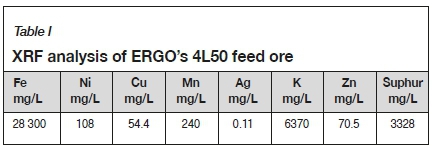
A low-grade, refractory sulphide gold tailings material containing approximately 0.20 g/t Au was used as the sample. The material, which had previously undergone milling, was sourced from the 4L50 reclamation site. The elemental composition was determined by X-ray fluorescence (XFR) analysis of a composite sample of the 4L50 feed. The contaminants present in the highest concentrations are summarized in Table I.
Reagents
In addition to using Rand Water and ERGO's recycled process water for the experimental work, a number of reagents were required for leaching, flotation, and water treatment. These included calcium oxide (lime), sodium carbonate (soda ash), sodium cyanide, commercial flotation reagents (sodium normal-propyl xanthate (SNPX), 1,1,3 triethyloxybutane (Senfroth), alkyl dithiophosphate (Senkol)), nickel sulphate, iron sulphate, manganese sulphate, magnesium sulphate, and zinc sulphate. All chemicals used were analytical grade, and used as received, without further purification.
Reagent dosage for chemical precipitation
The Minnesota Rural Water Association (2009) states that chemical precipitation is an effective and common method utilized for water purification. The addtion of chemicals such as lime (calcium hydroxide, Ca(OH)2) and soda ash (sodium carbonate, Na2CO3) increases the water's pH and results in precipitation of the ions that cause hardness. If the total hardness of water is less than or equal to the total alkalinity, then hardness is attributed to carbonate hardness only, with no non-carbonate hardness. If the total hardness is greater than the total alkalinity, the carbonate hardness is equal to the total alkalinity concentration, and the non-carbonate hardness is calculated from the difference between total hardness and total alkalinity concentration. (Minnesota Rural Water Association, 2009). If total hardness is equal to or less than total alkalinity, then the lime dosage is calculated as shown in Equation [1] (Minnesota Rural Water Association, 2009):
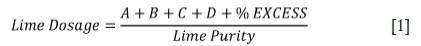
where
A = The carbon dioxide concentration in source water
B = Bicarbonate alkalinity in source water
C = Hydroxide alkalinity in source water
D = Magnesium concentration in source water
% excess = Amount of lime fed in excess to ensure pH > 10.5.
When treating water that contains non-carbonate hardness, soda ash is required. The amount of soda ash is estimated using Equation [2]:
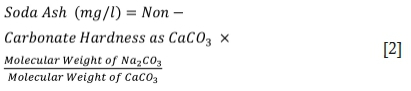
Analytical techniques
Atomic absorption calibration
Atomic absorption spectroscopy (AAS) was used for the quantitative determination of elements in solution. Standards of the various metallic ions were made up from pure chemicals and calibration curves constructed by preparing dilutions from the different stock solutions.
Fire assay
The gold content of the solid samples was determined by fire assay at the metallurgical laboratory of Mechanical Analysis and Engineering Design (MAED) in Sandton.
X-ray diffraction
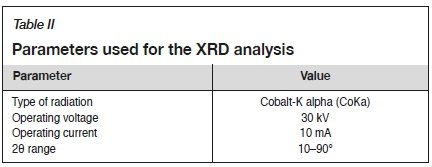
X-ray diffraction (XRD) analysis was used to characterize the crystalline materials formed during the chemical precipitation, using a Philips PW 1800 X-ray diffractometer. The XRD analysis was performed as a qualitative analysis to determine the mineral phases that had formed. The parameters used for the analysis are described in Table II.
Preparation of contaminant standards
One of the aims of this study was to establish which ions, or combination of ions, present in the process water were responsible for the reduced gold recoveries compared to when Rand Water was used. To achieve this, Rand Water was spiked with individual chemical species to determine the effect on gold recovery. In this way it was possible to identify the individual ion or combination of ions that had the greatest effect on gold recovery. The contaminants present in the highest concentrations were identified from an initial ICP-MS analysis undertaken on a composite sample of the ERGO process water (refer to Table IX for the ICP-MS analysis). Standard solutions of the identified contaminants were prepared by dissolution of a known amount of the analyte in cyanide solution. Stock solutions of 1 g/L of Ni, Fe, Zn, Ca, and Mg were individually prepared. Fe, Zn, Ni and Mg were added as sulphate salts and Ni(CN)42-, Zn(CN)2, Fe(CN)64-, and Mg(CN)2 complexes were formed in solution by the addition of the respective molar requirement of sodium cyanide. The reagents used to prepare standard solutions and their respective stoichiometric dissociation reactions with sodium cyanide are summarized in Table III.
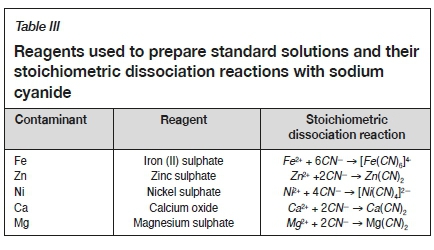
Sulphate addition was controlled and varied by the addition of stock solutions of sulphuric acid. The stoichiometric dissociation reaction to produce the anionic sulphate is: provided in Table IV.

Experimental procedures
Leach tests
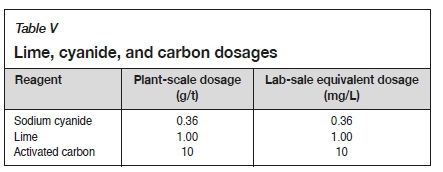
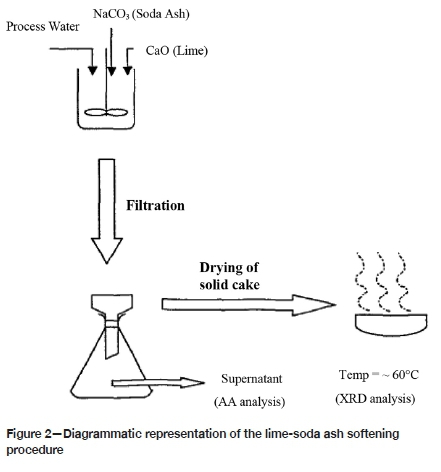
A dry composite sample was collected from the 4L50 reclamation site. The solid sample was slurried with each of the respective water sources to a density of 1.45 kg/L. A 1-litre head sample was collected, filtered, dried, and assayed for gold content. Three 1-litre samples were placed in separate 5-litre bottles. The lime, cyanide, and carbon dosages shown in Table V were added and a bottle roll leach was undertaken for 7 hours, corresponding to the leach circuit residence time at ERGO. After leaching, the carbon was screened and washed. The slurry was then filtered and the filtrate analysed for gold content using AAS. The filter cake was re-pulped and filtered twice before being dried in an oven at 110°C. The dried residue and carbon were analysed for gold by fire assay. Figure 1 illustrates a block flow diagram of the leach test work.
Lime and soda ash softening
A composite sample of process water was analysed to determine the initial calcium, sulphate, and heavy metal concentrations. The process water samples were treated with unslaked lime, as well as with a combination of unslaked lime and soda ash, at the required dosages. A one-hour waiting period was allowed for complete precipitation of contaminants. The resulting solution was filtered through a vacuum filter and the precipitate collected, dried at 90°C and weighed. A 250 mL volume of filtrate was used for the analysis of Ca, sulphate, and heavy metal concentration, and the remainder of the filtrate was used for the flotation and leach test work to observe the effect on gold recovery. The above procedure was repeated after heating the process water to temperatures of 20°C, 60°C, and 90°C. A diagrammatic representation of the lime-soda ash softening procedure is presented in Figure 2.
Flotation tests
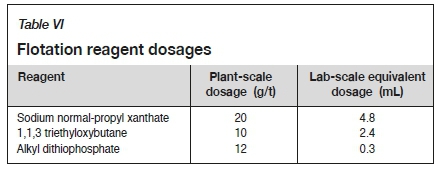
A composite 4L50 solid sample was slurried with each of the water sources to a density of 1.32 kg/L. A 5-litre portion of the slurry was transferred into a Denver laboratory-scale flotation cell. The initial pH of the slurry was measured and pH values outside of the optimal range of between pH 6-8 were adjusted using lime for pH values below 6, or sulphuric acid for pH values above 8. The flotation reagents and the required dosages are shown in Table VI. The concentrate was collected by scraping the froth at 10-second intervals after conditioning for 5 minutes. The concentrate was transferred into a labelled sample dish and dried in an oven at 110°C. The dried concentrate was analysed for gold content. The tails were transferred into a bucket and allowed to settle before being prepared for the bottle roll leach test by decanting the water and thickening the sample to a density of 1.45 kg/L. Figure 3 illustrates a block flow diagram summarizing the flotation and tails leach test work.
Results and discussion
Statistical analysis
Ten leach tests were performed to determine if there were significant differences in gold recovery between using Rand Water and recycled, process water. The margins of experimental error and repeatability of the experimental results were calculated, and the averaged statistical analysis results are presented in Table VII.
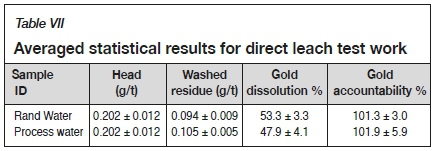
A two-sample t-test was undertaken using the experimentally determined washed residue values to determine if the differences in gold recoveries between using Rand Water and process water were significant. A two-sample t-test is an inferential test that determines if there is a significant difference between the means of two data-sets and whether the two data-sets are from the same or different populations (Central Virginia Governor's School for Science and Technology, 2003). A summary of the t-test results is presented in Table VIII.
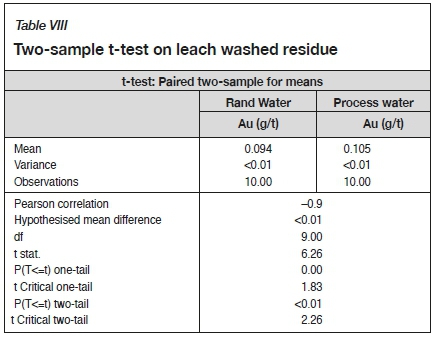
The null hypothesis for the t-test states that the means of the two washed residues are the same (Central Virginia Governor's School for Science and Technology, 2003). The calculated t-statistic value of 6.26 was greater than the t-critical value of 2.26, and the p-critical value obtained was less than 0.05; thus the null hypothesis was rejected, indicating that a significant difference exists between the gold recoveries when using Rand Water and process water.
Effect of individual contaminants on gold recovery
The contaminants present in the recycled process water were identified from an initial ICP-MS analysis on a composite sample of the ERGO process water. The contaminants present in the highest concentrations are summarized in Table IX, as well as the corresponding typical Rand Water concentrations (Mohotsi, 2020).
Rand Water was spiked with increasing amounts of each individual contaminant species to determine the effect on gold recovery. The main aim of the test work was to identify an individual ion or group of ions that had the greatest effect of gold recovery. This would facilitate the consideration of more comprehensive beneficiation options rather than following a 'blanket' overall treatment approach.
Effect of sulphur/sulphates on gold recovery
The tailings processed at ERGO are sulphidic in nature. Jeffrey and Breuer (2000) showed that many sulphide minerals are to some extent soluble in cyanide solution; and thus some sulphur species were expected to be present in the leach solution. Elemental sulphur is one of the by-products of the breakdown of pyrite, and agglomerations of elemental sulphur were observed in filtered solutions prior to ICP analysis. This sulphur is thermodynamically stable as a sulphate ion under the cyanidation conditions (Rees, 2000). This study was aimed at establishing the effects of sulphates on the leaching efficiency of gold from a sulphide ore. The leach test results using Rand Water spiked with stock solutions of sulphuric acid to vary the sulphate concentration is presented in Figure 4. An approximate 10% reduction in gold recovery was observed after the addition of 50 mg/L of sulphate, and gold recoveries decreased further as the sulphate concentrations were increased. To prevent volatilization of toxic HCN gas, lime (CaO) was added to increase the leach pH to greater than 10.5. At this high pH, the calcium ions could have reacted with the sulphate ions to produce gypsum (CaSO4. 2H2O), which could cause fouling of the sites on the activated carbon and thus reduce gold recovery.
The presence of hydrogen sulphide ions (HS-) has been found to be detrimental to the leaching of gold (Jeffrey and Breuer, 2000). This finding was pertinent in the present study because the gold would react directly with the sulphide as the pyrite was destroyed. At high pH values, there would be limited free hydrogen ions available to react with the sulphide. Furthermore, all metals are known to react readily with sulphide to form insoluble metal sulphide complexes, which may passivate the surface of the gold and thus reduce gold dissolution.
Effect of calcium on gold recovery
The Elsner equation [Equation 3] accurately describes the cyanidation of gold under controlled conditions. However, increased calcium concentrations resulting from lime addition may have a significant effect on subsequent gold dissolution and the solubility of the resulting aurocyanide complex. The effects of calcium on the leaching of gold from a sulphide ore were investigated by adding increasing concentrations of calcium (as CaO) to Rand Water and conducting laboratory-scale leach tests on ERGO's 4L50 sulphide ore. The leach results are presented in Figure 5.
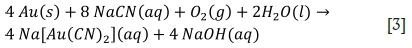
A 20% decrease in gold recovery occurred with the addition of 50 mg/L of calcium to Rand Water. This may have resulted from poor gold adsorption caused by passivation of the carbon surfaces by calcium foulants. As the initial calcium concentrations were increased above 50 mg/L, gold recoveries showed improvement with an increase in recovery of approximately 8.4% observed with a calcium addition of 500 mg/L compared to 50 mg/L. Davidson and Solet (2007) hypothesised the formation of a calcium aurocyanide complex that rapidly and strongly adsorbs onto activated carbon. These results would support the formation of a calcium aurocyanide complex as the gold recovery increased with an increased calcium concentration. Davidson and Solet (2007) further stated that the preferential adsorption of calcium aurocyanide onto activated carbon is due to the limited solubility of the calcium aurocyanide complex compared with the higher solubility of potassium and sodium aurocyanide (Davidson and Solet, 2007).
Effect of magnesium on gold recovery
The effect of magnesium addition to Rand Water on gold recovery is presented in Figure 6. The addition of 50 mg/L of magnesium resulted in a 15.8% decrease in gold recovery compared to that without magnesium. With increases in magnesium concentration above 50 mg/L, gold recoveries improved and an increase in recovery of approximately 2.5% was observed at 500 mg/L of magnesium. Although excess concentrations of Mg aided gold recovery, recoveries remain more than 10% lower than when using clean Rand Water.
Effect of heavy metals on gold recovery
Gold tailings are considered as refractory in nature as most of the free-milling gold has been leached previously, and generally contain sulphide minerals, organic carbon, and heavy metals such as iron and nickel (Rees, 2000). This leads to an increased cyanide consumption attributed to the formation of metal cyanide species that compete with the aurocyanide complex for sites on activated carbon.
The stability constants of various metal cyanide species (Rees, 2000) are given in Table X. Aurocyanide is one of the most stable metal cyano-complexes. Iron (II) and iron (III) also form strong complexes, with the iron (III) complex marginally stronger, and the iron (II) complex marginally weaker than the gold cyanide complex (Rees, 2000). Nickel forms a strong cyanide complex in the +2 oxidation state, with Zn forming a weaker cyanide complex (Rees, 2000). Due to their similar stabilities, these complexes are all present to some extent in leach solutions. Their predominance is determined by the free cyanide concentration and is strongly pH-dependent (Rees, 2000).
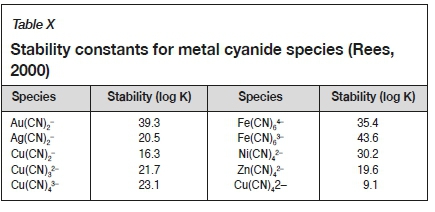
The stability constants are listed in terms of log K, where K is the equilibrium constant. The larger the equilibrium constant, the greater the stability. Ferrocyanide is shown as an example in Equation [4].

Effect of iron on gold recovery
The effect of iron additions to Rand Water on leach recoveries are presented in Figure 7. An increase in iron concentration, together with the addition of a stoichiometric amount of cyanide, resulted in a decrease in gold recovery. Since the cyanide addition was stoichiometric based on the oxidative breakdown of pyritic ore, the iron is expected to be present in the ferrous form and in the presence of cyanide would predominantly form the Fe(CN)64-complex (Rees, 2000). This is a very stable complex, and opinion in the literature is divided as to whether it would dissociate. As shown in Table X, the stability constants of aurocyanide and the Fe(CN)64- complex are similar (Kyle, 1997). The results show that as the initial iron concentration was increased from 50 mg/L to 500 mg/L, a 28% reduction of gold recovery occurred. These results are supported by Kyle (1997) who states that excess ferrous ions will readily displace gold from the aurocyanide complex.
Effect of nickel on gold recovery
The leach test results using various nickel concentrations in Rand Water are presented in Figure 8. An increase in nickel concentration, together with the addition of the stoichiometric amount of cyanide, resulted in reduced gold recovery. The stability constants in Table X show that nickel forms a medium strength cyanide complex. This complex is nine orders of magnitude less stable than the aurocyanide complex (Kyle, 1997). Additionally, the Ni(CN)42- complex contains four CN-ions compared to two in Au(CN)2- resulting in higher cyanide consumption. This allows a better dispersion in the solution without forming clusters, which enables Ni to be adsorbed onto activated carbon when present in high concentration (Sayiner and Acarkan, 2013). Similar behaviour was evident in the current study, resulting in a 23% decrease in gold recovery as the nickel concentration was increased from 50 mg/L to 500 mg/L. Addition of 100 mg/L of Ni resulted in an approximately 50% reduction in gold recovery compared to unspiked Rand Water.
Effect of zinc on gold recovery
Zinc can be a major constituent of the process water due to the cyanide leaching of zinc minerals and the zinc cementation applied to precipitate gold and silver (Merrill-Crowe process), which is the process implemented at ERGO Brakpan. The leach results using various concentrations of zinc in Rand Water are presented in Figure 9. An increase in zinc concentration, together with the addition of the stoichiometric amount of cyanide, resulted in a depressed gold recovery. Kyle (1997) explains that zinc predominantly forms a tetracyano complex at a pH of 10. Due to the lower stability of the Zn(CN)42- complex compared to the Ni(CN)42- and Fe(CN)64- complexes, it is evident that the zinc cyanide complex adversely affects gold recovery but to a lesser degree than the nickel and iron cyanide complexes. It is postulated that the bulk of the zinc introduced would form zinc hydroxide, which rapidly precipitated out of solution due to the high leach pH (Kyle, 1997).
Lime and soda ash softening - chemical precipitation
The leach test results showed that heavy metals such as Fe, Ni, and Zn have the largest negative effect on gold recovery. Sulphates also showed possible passivation effects, influencing gold recovery.
Since lime addition is the general practice for increasing the leach pH at most gold leach plants, and given its easy availability, lime softening was considered a viable and economical beneficiation option. Furthermore, the solubilities of Ca, Mg, and silica (Si) are significantly reduced by increased temperature and they are therefore, more effectively removed by warm/hot softening than by cold/ambient softening (Suez, n.d.).
Figure 10 shows two process water samples that were treated with unslaked lime and soda ash. The test was repeated at temperatures of 20°C, 60°C, and 90°C.

The lime softening and soda ash softening test work parameters, final pH values, and resultant precipitate masses are summarized in Tables XI and XII.
A one-hour waiting period was allowed for complete precipitation of contaminants. Figure 11 illustrates the settling of precipitants formed during the lime and soda ash treatment.

The filtered precipitate from the lime plus soda ash softening test at ambient conditions is illustrated in Figure 12:

Figure 13 shows a comparison between ERGO's untreated recycled process water and samples that underwent lime and soda ash treatment/softening. The beaker on the far left contains untreated process water. The middle sample was treated with unslaked lime only, and that on the far right was treated with both unslaked lime and soda ash.

X-ray diffraction analysis of precipitates
The precipitates formed from each water treatment procedure were subjected to X-ray diffraction (XRD) analysis. The main constituents were identified as quartz, magnesium silicate hydrate, calcium silicate, cordierite (identified as anthophyllite), bassanite, and gypsum. The formation of gypsum (CaSO4-2H2O) and bassanite (CaSO4-0.5H2O) would consume free sulphates in solution, thus reducing the concentration of sulphates in the process water.
XRD scans of the precipitates formed when the process water was treated with a combination of lime followed by soda ash indicated that lime treatment alone was more efficient. At ambient conditions, it was evident that only calcium-containing compounds (gypsum and calcite) , and quartz had precipitated. With the addition of soda ash, it was expected that magnesium-containing compounds would also be present in the precipitate. The addition of soda ash produced precipitates with fewer identifiable calcium- and magnesium-containing compounds than the tests where only lime treatment was used. This behaviour was observed at all temperatures investigated.
Atomic absorption analysis
Tables XIII and XIV summarize the atomic absorption results from the various treatment techniques undertaken on the ERGO process water, at the respective treatment temperatures.
The data shows that lime softening alone was sufficient to remove more than 99% of the heavy metals (Zn, Fe, and Ni) from ERGO process water. The apparent increase in potassium concentrations is attributed to impurities in the reagents and experimental error, since potassium concentration should not increase with lime and soda ash dosing. The increase in sodium concentration is attributed to the soda ash addition.
Treatment of process water with a combination of lime and soda ash at ambient conditions was effective for the complete removal of Mg. However, a similar reduction in Mg concentration (> 99%) can be achieved using only lime treatment at elevated temperatures of 60°C and 90°C. A reduction of 87% Mg is, from a practical perspective, equally sufficient. It was also evident that the reduction in nickel concentration was negatively affected by soda ash addition as well as an increase in treatment temperature. Slatter et al. (2009) demonstrated that while sodium ions increase silver loading, the presence of sodium has a negligible effect on gold loading.
From the XRD and atomic absorption results, it was concluded that lime softening/treatment on its own was sufficient to significantly reduce heavy metal concentrations. Furthermore, lime addition reduced the sulphate content due to the precipitation of gypsum.
Leach results for gold recovery
The lime and soda ash softened process water was utilized to perform laboratory-scale direct leach tests with the aim of determining the viability of the treatment options for gold recovery. Table XV summarizes the averaged data from triplicate leach tests undertaken using the differently treated waters at varying temperatures. Figure 14 gives a graphical representation of the results obtained.
The data in Table XV shows that there are differences in gold dissolution and thus gold recovery, when using Rand Water and ERGO process water for the direct leaching of the 4L50 reclaimed material. Rand Water produced a greater dissolution of gold from solids, which in turn resulted in a lower washed residue and a greater recovery of gold from the feed material.
Treatment of ERGO process water with lime (lime softening only) produced a much lower washed residue than treatment with a combination of lime and soda ash. Tests where only lime treatment was applied to ERGO process water produced a higher gold dissolution than the corresponding lime-soda ash treatment at all temperatures tested.
The addition of soda ash as part of the proposed treatment of ERGO process water proved to be detrimental to gold recovery. Leach tests using process water that had been treated with soda ash yielded the lowest percentage gold dissolution with the highest washed residue concentrations. Furthermore, the results of tests with soda ash treated water were poorer than those using untreated process water. This could be a result of the carbonates from the soda ash addition reacting with calcium to produce CaCO3, a known foulant of activated carbon that occupies active sites, thus resulting in a reduction in gold recovery (Davidson and Solet, 2007).
Process water treated with lime at 60°C provided the most significant improvement in gold recovery. However, heating 60 ML of process water per day from ambient (20°C) to 60°C will require approximately 2930 MWh of energy per day, and is thus not an economically viable option. At a unit cost of R1.00 per kilowatt-hour, the additional daily energy cost would be R2.93 million. Rand Water, at a unit cost of R30.92 per litre, is also not an economically viable option.
Flotation and tails leach results for gold recovery
The lime and soda ash softened process water was used to perform laboratory-scale leach and flotation tests to assess the viability of the treatment options for gold recovery. Table XVI summarizes the averaged data from triplicate flotation and leach test work using the different water sources. Figures 15 and 16 give a graphical representation of these results.
The data from Figures 15 and 16 shows that addition of soda ash to treat ERGO process water may prove detrimental to gold recovery. The 4L50 material, with a gold head grade of 0.20 g/t, was slurried with the different water sources and subjected to flotation. The concentrate was collected, dried, and analysed for gold by fire assay, and the flotation tails underwent leach tests. It is important to note that the deviation in the tails head grades presented in Table XIV is attributed to efficiency of the flotation. With effective flotation, a lower tails head grade is expected due to the higher gold grades in the flotation concentrate. Therefore, a higher gold grade of the tails prior to leaching is attributed to the corresponding lower concentrate gold grade from ineffective flotation. The tails leach tests which utilized process water treated with soda ash resulted in the lowest gold dissolutions, as well as the highest washed residue values. The results of these tests were also inferior to those with ERGO process water. .
Conclusions
➤ Experimental work and statistical analysis indicated a significant difference in gold recoveries between using Rand Water and untreated process water.
➤ Analysis of a composite sample of ERGO process water confirmed the presence of appreciable concentrations of heavy metals.
➤ Leach tests conducted using Rand Water spiked with known concentrations of the identified contaminants showed that iron, nickel, and zinc had the largest adverse effect on gold recovery, with iron and nickel having a greater effect than zinc.
➤ The presence of sulphates indicated possible passivation effects, although these were less deleterious than the effects of heavy metal contamination.
➤ The increased gold recoveries observed as calcium concentrations increased could possibly be ascribed to the formation of a calcium aurocyanide complex.
➤ Due to the negligible differences in gold recoveries when using the lime treated process water and Rand Water, the use of lime was chosen as an attractive treatment option.
➤ Lime softening proved effective for the reduction of heavy metal concentrations by metal hydroxide precipitation, as well as reducing sulphate concentration by the precipitation of gypsum.
➤ While the leach results indicated that lime-treated process water at elevated temperatures produced higher gold recoveries; the heating requirement for 60 ML of process water per day makes this option economically unviable.
Recommendations
It is not clear whether these negative effects of heavy metals on gold recovery were due to cyanide consumption by metal-cyano complexes, fouling of the activated carbon, or precipitates passivating the gold surfaces before adsorption. This should be investigated in future work to establish the mechanism of this reduced gold recovery.
Acknowledgements
DRDGold (Pty) Ltd is gratefully acknowledged for financial support of this investigation, and a bursary for the corresponding author. The authors owe a huge debt to Mr. Bruce Ebell from DRDGold for his insight and contribution to this work.
References
Boehme, W. and Potter, G. 1983. Carbon adsorption of gold. Maximum loading and ionic contaminant effect on loading rates. Gold and Silver Heap and Dump Leaching Practice. Hiskey, J. ( ed.). American Institute of Mining, Metallurgy and Petroleum Engineers, Salt Lake City, NV. pp. 129-137. [ Links ]
Central Virginia Governor's School for Science and Technology. 2003. T Test. http:// www.cvgs.k12.va.us/DIGSTATS/main/inferant/d_tdist.htm [accessed 25 May 2020]. [ Links ]
Davidson, R. and Solet, M. 2007. The major role played by calcium in gold plant circuits. Journal of the Southern African Institute of Mining and Metallurgy, vol. 107, no. 7. pp. 463-468. [ Links ]
Fink, C. and Putnam, G. 1950. The action of sulphie ion and of metal salts on the dissolution of gold in cyanide solutions. Mining Engineering, vol. 187. pp. 952-955. [ Links ]
Fleming, C. and Nicol, M. 1984. The adsorption of gold onto activated carbon III. Factors influencing the rate of loading and equilibrium capacity. Journal of the South African Institute of Mining and Metallurgy, vol. 84, no. 4. pp. 85-93. [ Links ]
Hedley, N. and Tabachnick, H. 1968. Chemistry of cyanidation. Mineral Dressing Notes no. 23. American Cyanamid Company, New Jersey. 54. pp. [ Links ]
Jeffrey, M. and Breuer, P. 2000. The cyanide leaching of gold in solutions containing sulfide. Minerals Engineering, vol. 13, no. 10. pp. 1097-1106. [ Links ]
Kyle, J.H. 1997. Stability of metal-cyanide and hydroxide complexes. Proceedings of the World Gold '97 Conference, Singapore. Australasian Institute of Mining and Metalurgy, Melbourne. pp. 163-169. [ Links ]
McDougall, G., Hancock, R., Nicol, M., Wellington, o., and Copperthwaite, R. 1980. The mechanism of the adsorption of gold cyanide on activated carbon. Journal of the South African Institute of Mining and Metallurgy, vol. 80, no. 9. pp. 344-356. [ Links ]
Minnesota Rural Water Association. 2009. Lime softening. Minnesota Water Works Operations Manual. 4th edn. Elbow Lake, MN. Chapter 16. [ Links ]
Mohotsi, M. 2020. Rand Water - Reports for Johannesburg Metro. Water Quality Information Management, Johannesburg. [ Links ]
Rees, K.L. 2000. Leaching and adsorption behaviour of gold ores, PhD thesis, Dearatment of Chemical Engineering, University of Melbourne. [ Links ]
Sayiner, B. and Acarkan, N. 2013. Effect of silver, nickel and copper cyanides on gold adsorption on activated carbon in cyanide leach solutions. Physicochemical Problems of Mineral Processing, vol. 1, no. 50. pp. 277-287. [ Links ]
Sheya, A. and Palmer, G. 1989. Effect of metal impurities on adsorption of gold by activated carbon. Cyanide Solutions. US Bureau of Mines. [ Links ]
Slatter, K.A., Plint, N.D., Cole, M., Dilsook, v., and de Vaux, D., Palm. N., and Oostendorp, B. 2009. Water management in Anglo American process operations. Proceedings of the International Mine Water Conference, Pretoria. International Mine Water Association. pp. 46-55. [ Links ]
Suez. Not dated. Suez water technologies & solutions. https://www.suezwatertechnologies.com/handbook/chapter-07-precipitation-softening [accessed 1 October 2019]. [ Links ] ♦
 Correspondence:
Correspondence:
A. Narain
Email: arshir.narain@yahoo.com
Received: 30 Nov. 2020
Revised: 13 May 2021
Accepted: 28 Jul. 2021
Published: July 2021
ORCID:
A. Narain https://orcid.org/0000-0002-3757-0571
J.H. Potgieter https://orcid.org/0000-0003-2833-7986
J. Smith https://orcid.org/0000-0002-2395-3109
* Paper written on project work carried out in partial fulfilment of M.Sc (Metallurgical Engineering) degree














