Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Journal of the Southern African Institute of Mining and Metallurgy
versión On-line ISSN 2411-9717
versión impresa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.119 no.6 Johannesburg jun. 2019
http://dx.doi.org/10.17159/2411-9717/602/2019
PAPERS OF GENERAL INTEREST
Kell hydrometallurgical extraction of precious and base metals from flotation concentrates - Piloting, engineering, and implementation advances
K.S. LiddellI; M.D. AdamsI; L.A. SmithI; B. MullerII
IOezone Ltd & KellTech Ltd, Mauritius
IIThe Simulus Group, Australia
SYNOPSIS
Kell is a hydrometallurgical treatment option for the recovery of platinum group metals (PGMs), gold, silver, and base metals from flotation concentrates to refined products on site. The process is cyanide-free, while eliminating emissions of sulphur dioxide, arsenic trioxide, and other toxic species often emitted by smelters and roasters. Kell has been tested on a range of concentrates, including PGMs (UG2, Merensky, Platreef, Great Dyke, Great Lakes, Lake Superior and others), refractory gold-silver, copper-gold, and polymetallic concentrates. High extraction efficiencies (>95%) are achieved for value metals (Pt, Pd, Rh, Au, Ag, Ni, Cu, Co) and secondary metals (Sb, Zn, Pb, and others). Studies comparing Kell with smelter-refining show economic benefits: capex 18-33% of smelting; opex 51-66%; electricity consumption 13-46%; and environmental benefits: greenhouse gas emissions 57-61% of smelting, acidification 5-27%, human toxicity 37-62%, and freshwater ecotoxicity 43-55% of smelting. Synergies for Kell plants alongside smelter-refineries include utilization of recovered acid from sulphur abatement systems and excess capacity in precious metal refineries. Kell is unconstrained by concentrate grade and impurities, allowing the co-location of Kell plants for treatment of non-smeltable concentrates alongside existing smelters.
Kell has been demonstrated in a nine-week integrated pilot campaign at 1:1000 scale, supporting a bankable feasibility study for a 110 000 t/a plant treating a UG2-Merensky concentrate at Pilanesberg Platinum Mine (PPM), owned by Sedibelo Platinum Ltd, which has along with South African Industrial Development Corporation (IDC), invested in Kell. Zimbabwean Mining Development Corporation (ZMDC) has signed a Memorandum of Agreement (MOA) with KellTech for a centralized PGM concentrate processing plant.
Keywords: Kell, platinum group metals, base metals, hydrometallurgy, recovery, refining, environment.
Introduction
Metalliferous concentrates from the mining of ore deposits have for millennia been treated by pyrometallurgical processing methods such as smelting and roasting. In the last 125 years, hydrometallurgical processes have played an increasingly important role, with the now widespread use of pre-oxidation techniques and cyanide leaching in gold ore and concentrate processing, and sulphuric acid heap leaching of low-grade copper ores. Currently, smelting remains the dominant approach for treatment of concentrates (Habashi, 2009), which are typically transported by road or rail to the nearest port for shipment to smelters worldwide. This global movement of millions of tons of concentrates annually has a detrimental impact on local communities and the environment and represents a large shift of employment prospects and economic value across international borders. Ore grades have been steadily decreasing, while the ore mineralogy of available resources is becoming more complex - with polymetallic ores becoming more prevalent as new resources (Adams, 2016). These factors have had an impact on the smeltability of concentrates due to the higher flotation mass pulls required from lower grade ores, resulting in lower concentrate grades. The direct result of this approach is an increase in gangue minerals and penalty elements such as arsenic, antimony, bismuth, chromite, mercury, and magnesium oxide, further constraining concentrate flotation recoveries and smelter payabilities. Arsenic is a problem for the economic and environmentally responsible processing of copper-gold and polymetallic concentrates as environmental regulations become globally more restrictive. China currently imposes a cut-off of 0.5% As in imported copper concentrate, beyond which it is not accepted by the smelters (AQSIQ, 2006). An increasing number of copper-gold and refractory gold concentrates have significantly higher arsenic contents, for example, at Cananea in Mexico, Chelopech in Bulgaria, Northparkes in Australia, Gortdrum in Ireland, Tampakan in the Philippines, Aljustrael and Neves-Corvo in the Iberian Belt in Portugal and Spain (Long, Peng, and Bradshaw, 2012; Lane et al., 2016). Similarly, chromite and magnesium oxide constraints on platinum group metal (PGM) concentrate smeltability impose restrictions on concentrate flotation recoveries and smelter payabilities in the PGM industry. In response to these constraints, Kell was initially conceived by one of the authors (KL) and subsequently further developed (Liddell et al., 2011; Liddell and Adams, 2012). Kell is unconstrained by concentrate grade and impurities, allowing the treatment of concentrate streams that may not be readily smeltable by primary smelters.
An additional environmental concern is the emission of sulphur dioxide by smelters and roasters directly into the atmosphere in some regions; emission requirements in more regulated jurisdictions necessitate the construction and operation of expensive wet sulphuric acid plants, which then require a market for the sulphuric acid. Co-location of Kell plants alongside smelter-refineries offers several potential synergies, including utilization of recovered acid from sulphur abatement systems at smelters and use of excess capacity in precious metal refineries.
The long-established cyanide leaching process for the extraction of gold and silver is also meeting increasing resistance from stakeholders, primarily due to the human and ecotoxicity of the cyanide reagent, exacerbated by recent tailings spills and wildlife death events (Greenwald and Bateman, 2016).
Current industry status is well expressed by Habashi (2014): 'A new era in pressure hydrometallurgy is marked when roasting-leaching-electrowinning in the zinc industry was replaced in the 1980s by direct pressure leaching of zinc sulfide concentrate. Since then, pressure leaching has received recognition as the technology of the future. Large autoclaves of unprecedented size have been manufactured and installed in a number of metallurgical plants for recovering copper and nickel from their concentrates or liberating gold from refractory ores'.
Kell as a step-change technology
Kell is a patented technology comprising four core sequential steps (depicted in Figure 1), all of which are well proven, commonly used in the metallurgical industry, and provide high recoveries of base and precious metals:
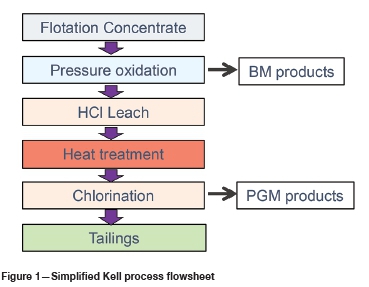
1. Aqueous pressure oxidation (POX) in an acidic sulphate medium to dissolve the sulphides and remove the base metals while minimizing dissolution of the precious metals. LME A Grade copper cathode is produced by solvent extraction and electrowinning (SX-EW). Excess iron in solution is removed by precipitation with limestone. Nickel and cobalt products are tailored to suit owner, site, and market conditions for the specific project, and may include mixed sulphide precipitate (MSP), high-purity nickel and cobalt metals, salts, or precursors. Conditions are similar to those used in industrial practice treating refractory gold or nickel concentrates targeting complete sulphur oxidation (typically 170-230°C with excess oxygen overpressure).
2. Atmospheric leaching for removal of soluble iron, and recovery of other HCl-soluble metals such as silver and residual base metals by means of scavenging sorbents and precipitation to secondary products suitable for further refining on site or externally.
3. Heat treatment of the solids residue to condition the mineral phases, rendering the material amenable to subsequent leaching. Typically PGM concentrates are heat treated at 500-1000°C using syngas.
4. Atmospheric oxidative leaching of the PGMs and/or gold in chloride/chlorine medium in a similar manner to that typically used in PGM refineries (50-90°C; 3-6 M HCl), with metal recovery by ion exchange and precipitation. Various selected products can be made using standard techniques to suit owner, site, and market conditions for the specific project, and may include intermediate reductive mixed precipitates, individual metals, salts or precursors.
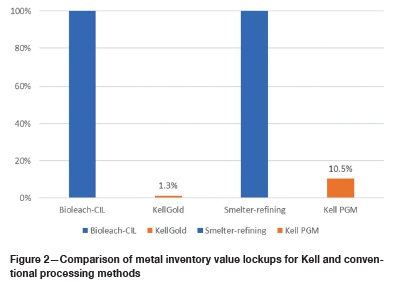
The separate leaching stages for the precious and base metals are designed to keep their respective chloride and sulphate chemistries disengaged, allowing for decoupled optimization of leach parameters and materials of construction for the two main value streams. Both base and precious metals leaching systems in Kell show very rapid kinetics - over 90% leach extraction in less than 60 minutes as shown in Kell test work leach rate data, compared, for example, with 24 hours typical for conventional gold cyanidation. For KellGold applications, the rapid leaching results in short pressure-oxidation autoclave residence times (RTs) of approximately 1 hour (approx. 1% of typical bacterial oxidation RTs of 5-6 days; (Miller and Brown, 2016). KellGold leach tank RTs are approximately 1 hour (4% of typical carbon-in-leach (CIL) RTs of about 24 hours). KellGold therefore offers proportionally lower lock-up of contained gold metal value as working capital.
Kell PGM plant design metal inventory pipeline lock-up times are estimated to be about 6 days for Pt and Pd and 4 days for Ni, Co, and Cu. A benchmark for overall conventional smelter-refining pipeline time for contained value metals may be estimated from reported segmental revenues and in-process metal inventory values (excluding refined metal) at Impala Refining Services (IRS), of 55 days and 60 days in 2017 and 2016, respectively (Impala Platinum, 2017). Hence, Kell pipeline times are estimated at approximately 10% of PGM smelter-refining pipeline times, with proportionally lower lock-up of contained metal value as working capital, a potential value release of some R800 million in the example cited.
The relative impacts of Kell pipeline time on metal inventory lock-up compared with conventional concentrate processing in the PGM and gold industries are illustrated in Figure 2.
Because sulphides and base metals as well as other acid-consumers are removed prior to chlorination, the benign gangue mineral particles pass almost intact through the Kell chlorination circuit, particularly so for chromite. The PGM or gold pregnant leaching solution (PLS) is clean, with low reagent consumption and amenable to production of low-impurity end products on site, such as 99.95% Pt, Pd, and Au sponges.
Environmental metrics
Kell has many environmental benefits over traditional technologies (Smith, Adams, and Liddell, 2019). This alleviates concerns with SO2 and CO2 emissions, arsenic control, solid waste storage, water contamination, and cyanide usage.
> SO2 in vent gases is very low because sulphur is quantitatively removed as sulphate in an environmentally responsible manner.
> Arsenic is quantitatively removed in the front-end leaching circuits and is stabilized in phases such as basic ferric arsenate and scorodite, a well-accepted route that is standard practice in the extractive metallurgical industry.
> No cyanide is used, avoiding the need for cyanide transportation, detoxification, management, monitoring, storage, and the risk of wildlife and human toxicity.
> Solid waste may be co-stored as a very small (typically 2-5%) component of current flotation tailings arisings, creating no additional storage construction or management requirements. Alternatively, the Kell residues can be dry-stacked.
> Water contamination risk is minimized as water is re-used in the process.
> Low electricity consumption - 54-87% less compared to smelting using a streamlined cradle-to-gate life cycle assessment boundary, depending mainly on factors such as concentrate mineralogy and onsite or offsite reagent production. This results in substantially lower CO2 emissions.
Smith, Adams, and Liddell (2019) provide additional data on these topics. Samples of combined tailings from a 9-week pilot-plant campaign for Kell processing of concentrate from Pilanesberg Platinum Mine (PPM) were subjected to geochemical and water extraction analysis, showing that waste type classification would remain unchanged (non-potentially acid generating, Type 3), and metals mobility from the tailings was determined to be very limited. To further quantify the environmental impacts and benefits associated with the Kell process, a preliminary streamlined environmental life cycle assessment (LCA) study was carried out by Energetics, supported by SysCAD mass-energy balance modelling by Simulus Engineers. The LCA compared Kell against traditional pyrometallurgical (smelting and refining) treatment of both high-sulphur and low-sulphur sulphide concentrates. Environmental performance was assessed in terms of impact categories, including:
> Greenhouse gas emissions into the atmosphere (expressed in kg CO2 equivalents)
> Depletion of non-renewable fossil fuels resources (expressed in MJ (net calorific value)
> Acidification (relative effect of total emissions of acidic gases, expressed in kg SO2 equivalents)
> Toxicity (consensus-based, chemical specific characterization factors in USEtox™ toxicity assessment models, expressed in comparative toxic units, CTU).
The outcomes of the LCA indicated that for each ton of concentrate processed, Kell (when compared against conventional smelting and refining process on an equal output-value basis) results in significantly lower environmental impacts, primarily due to the substantially lower electricity requirements of Kell. The LCA study determined that Kell provides measurable benefit in each of the performance indicators examined (Figure 3):
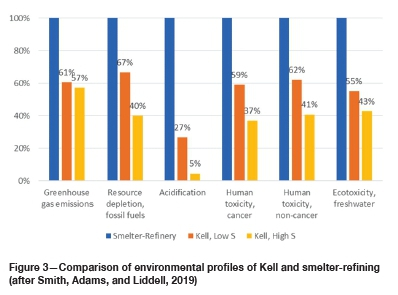
> Greenhouse gas emissions: 39-43% reduction
> Resource depletion (fossil fuels): 33-60% reduction
> Acidification: 73-95% reduction
> Human toxicity: 38-63% reduction
> Freshwater ecotoxicity: (45-57% reduction).
Economic metrics
Kell typically recovers a higher percentage of value metals (approx. 94-99% for Pt, Pd, Rh, Au, Ni, Co, Cu) than smelter-refining or cyanide leaching; hence, comparative discounted cash flow financial analysis is more appropriate than consideration of costs in isolation. Kell recoveries are generally higher than in other processes because (i) the POX step effectively liberates the PGM and gold particles from the encapsulating base metal sulphide matrix; (ii) PGM and other mineralogy is conditioned in the heat treatment step, producing readily leachable phases; (iii) chlorination conditions can be well controlled because the base metal sulphides and other ionic species have been removed from the system; (iv) specification of refined end products is integrated into Kell, is flexible, and may be selected to suit marketing and site location requirements; (v) relaxation of concentrate specifications can result in increased flotation recoveries.
Economic modelling based on comparative mass-energy balances carried out by Simulus Engineers shows that Kell outperforms existing pyrometallurgical processing for both capital and operating costs, for two different concentrate types (low S - approx. 1%; high S - approx. 6%), as illustrated in Figure 4. These outcomes are based on Kell design data from scoping or feasibility studies, with firm quotes for major equipment items, as well as mass-energy balances for Kell and smelter-refining compiled by Simulus Engineers. Smelter capital cost estimates were derived from prefeasibility study (PFS) data for a PGM concentrate containing approximately 8% S at 140 kt/a. Base metal refinery (BMR) capital cost estimates were derived from published costs for a previously considered BMR refurbishment at Zimplats' Selous complex (Mandizha, 2015).
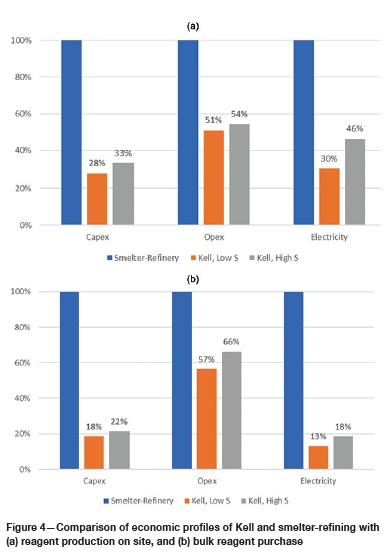
These results are consistent with those from an independent comparative scoping-level options study undertaken by an international engineering company in 2012, as well as those from an investment case study conducted by an international consulting metals and commodities analyst company in 2018. Kell capex benefits arise from: (i) modular design that cements capex as part of quoted fabrication cost, avoiding capital blowout due to construction delays; (ii) short leach residence times, resulting in a relatively small plant footprint and tankages. Kell plant designs are scalable and have ranged in capacity (including refining) from 50 000 to 2 million ounces of precious metals per annum.
Power consumption in Kell is low compared to the power required to melt the entire concentrate feed, including gangue minerals and fluxes, in conventional smelting. Chlorine consumption is low because chlorine consumers are removed in prior Kell process steps. Hydrochloric acid is recovered and recycled and is typically a nil operating consumption. On a case-by-case basis recovery and re-use of sulphuric acid may also apply. Generally, limestone sources near the mine site are available for any neutralization requirements.
The chlorination leaching system provides fast leaching rates (Aylmore, 2016), although halogens are reactive with other ore minerals, especially sulphides. Hence, under typical chloride leaching conditions, reagent consumptions are high if the ore or concentrate contains significant amounts of sulphide minerals (Aylmore, 2016; Mpinga, 2015). Kell eliminates this problem of reaction with sulphides, base metals, and other species because these are removed in the POX and atmospheric leach stages, leaving a residue that does not consume chlorine to any significant extent. The PLS is consequently quite low in impurities, enabling the recovery of precious metals to relatively pure products without excessive competition from other metals. Reducing minerals such as sulphides are removed prior to chlorination leaching and therefore the metal-chloride complexes tend to remain stable in solution under Kell chlorination leach conditions.
PGM concentrates
Planned expansions and developments of southern African PGM concentrate production from the Platreef present operators with a potentially high capital and operating cost for new smelting-refining capacity compared with the cost of a Kell plant, as illuminated above. A potential major expansion at Mogalakwena has been considered, which would involve building a third concentrator and would increase palladium output by 270 000 ounces per annum and platinum by 250 000 ounces per annum, on top of the approximately 500 000 ounces per annum of each metal currently produced (Seccombe, 2018). Platinum Group Metals reports that the definitive feasibility study (DFS) for the Waterberg project is due for completion in early 2019; the 2016 PFS considered a large-scale 600 000 t/month mine producing 744 000 ounces of 3PGM+Au per annum (Platinum Group Metals, 2018). Ivanplats delivered a DFS in July 2017 for a mine producing 476 000 ounces of 3PGM+Au per annum (Ivanhoe Mines, 2018). The net additional production of some 2 million ounces of 3PGM+Au per annum from the Platreef deposit will require additional concentrate processing and refining capacity.
Currently there is a lack of electricity supply availability and certainty in both South Africa and Zimbabwe; the low electricity consumption in Kell processing therefore presents a further advantage.
Sulphur dioxide emissions abatement is an increasingly important factor. Kell converts sulphur-containing compounds to stable insoluble residues, eliminating gaseous emissions. Sulphur abatement systems are currently not required for smelters in Zimbabwe (Gwimbi, 2017) and are installed at only some South African PGM smelters (Jones, 2005). Anglo American Platinum has announced a new R1.576 billion smelter abatement project at its Polokwane smelter (Anglo American Platinum, 2018) to meet South African legislation changes regarding new minimum emissions standards for SO2 stack emissions (DEA, 2013). Total capital expenditure on SO2 abatement technology is R2.5 billion - equal to Anglo American Platinum's total profit for the 2017 financial year (Anglo American Platinum, 2018).
Gold and polymetallic concentrates
Some 38% of the top 20 producers' gold production in 2012 was from refractory sources, with an additional 14% in concentrate and 48% by free milling or heap leach cyanidation (Adams, 2016). Therefore, about half of current gold production is likely to be amenable to KellGold processing. Gold production trends are increasingly towards refractory and polymetallic feeds, with decreasing ore grades and more restrictions on cyanide use, effluent emissions, and increasing intensity of environmental activism. Traditional refractory gold roasters have been taken offline for environmental reasons; for example, Barrick and Newmont Mining's KCGM Gidji circulating fluid bed (CFB) roaster in Western Australia was permanently shut down in June 2015 to eliminate atmospheric stack emissions. The Gidji roaster had previously accounted for around 99% and 90% of Newmont's total annual sulphur dioxide and mercury emissions, respectively (Newmont, 2015).
Cyanide-free KellGold treatment of various ores and concentrates has shown potential for application to a range of feedstocks (Adams, Liddell, and Smith, 2015), and more detailed batch testing and comparative economic assessments have since been completed (Adams, Smith, and Liddell, 2019), summarized in the following section. High metal recoveries of value elements into solution have been achieved from a variety of concentrates from batch tests (>95% of Au, Ag, Cu, Ni, Co, Zn, Pb, and Sb). Kell presents a substantial value recovery advantage over conventional alkaline cyanide processing, which does not readily recover silver from argentojarosites and other phases, for example. Rapid leaching results in small-size equipment units with substantially lower gold inventory lock-up compared with bioleaching-CIL. Considering overall residence times for bacterial oxidation (Miller and Brown, 2016), in the majority of cases a high level of oxidation (5-6 days) is required, and cyanide leaching of bioleaching residues requires 24-48 hours' residence time. As shown in Figure 2, Kell requires about 1% of the bioleaching-CIL residence time, and hence gold lock-up, pipeline time, and tankage volumes for KellGold are much lower than for bioleaching. In several case studies (Adams, Smith, and Liddell, 2019), these factors, along with the typically higher metal recoveries, constitute a positive business case for Kell processing of many copper-gold, refractory gold, and polymetallic concentrates.
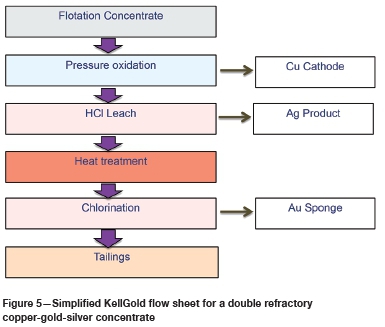
Several scoping engineering studies have now been carried out for KellGold applications. The example shown in Figure 5 of a double refractory copper-gold-silver concentrate aims to produce refined 99.99% Au and Cu products on site, along with a silver product. KellGold eliminates smelter emissions of SO2 and As2O3 and no cyanide management, detoxification, or monitoring costs would be incurred in this cyanide-free application. KellGold represents a potential step-change in the gold industry.
Kell process test work
Batch amenability test work
Concentrate samples of 5-15 kg are subjected to a standard batch amenability test using bench-scale equipment. The work, including inductively coupled plasma-optical emission spectrometry (ICP-OES) and inductively coupled plasma-mass spectrometry (ICP-MS) assay suites, is mainly carried out at Simulus Laboratories in Perth, Australia, supported by specialist assays and mineralogical investigations from other providers.
The main Kell leaching and heat treatment unit operations are employed sequentially to assess (i) ultimate overall recoveries into solution and (ii) stagewise amenability to processing. Very high extractions of value metals have been achieved from a wide variety of PGM concentrates (listed in Table I), including UG2, Merensky, Platreef, Great Lakes, Great Dyke. and blends, indicating the robustness of the technology. To date, all PGM concentrates tested have been amenable to Kell processing, as illustrated in Table I. More extensive optimization and locked-cycle test work on each unit operation in the integrated Kell process has also been completed for several PGM concentrates.
According to Mpinga (2015), the complexity of PGM mineralogy makes for a complex metallurgical scenario where a priori prediction of feasible processes is difficult. This is certainly the case for PGM treatment technologies that do not address the mineralogical complexity. In Kell processing, however, the complex PGM mineral phases liberated in the POX stage are converted to simple leachable metallic phases in the heat treatment step, eliminating the complexity issue completely. Moreover, Kell removes sulphur, base metals, amphoterics, and iron in the leaching stages prior to chlorination, resulting in a clean chlorination leach solution. Leaching of metallic PGMs in the Cl2/HCl system is well-established chemistry, readily described by simple equilibrium potential-pH diagrams (Pourbaix, Van Muylder, and de Zoubov, 1959) and with rapid leach rates (Landsberg and Schaller, 1971).
Several refractory gold and polymetallic concentrates have been subjected to KellGold amenability testing, as shown in Table II. Additional leaching and metals recovery test work has also been completed on several of these concentrates.
Integrated pilot-plant operation
Several pilot-plant campaigns were carried out at commercial metallurgical testing laboratories between 2011 and 2016 to test Kell for the processing of PGM polymetallic concentrates for recovery of Pt, Pd, Au, Rh, Ni, Cu, and Co. The most comprehensive campaign was carried out at Simulus Laboratories in Perth, Australia in 2016 on concentrate from PPM, and ran for 9 weeks as a decoupled integrated circuit at approximately 1000 scale-up ratio (illustrated in Figure 6). An industry norm of about 8000-15 000 is typical for hydrometallurgical plants scaled up from pilot to eventual commercial scale (Adams et al., 2004). An informal survey of seven autoclave-based nickel hydrometallurgy projects that were piloted since 2000 and culminated in operating plants showed that six of the seven (Ravensthorpe, Ambatovy, Rio Tuba, Ramu, Taganito, Gordes) scaled up from pilot to full scale at scale-up ratios typically of >10 000 (Adams et al., 2004; Collins et al., 2005; Collins and Vardill, 2005; Valle et al., 2016; Wilkinson, 2006; Tsuchida, 2015; Yesil and Iplikcioglu, 2015). One operation (Goro) built and operated a demonstration-scale plant at a scale-up ratio of 1000. Therefore, the Kell PPM campaign was considered well within industry norms.

The overall Kell PPM test work programme included the following components:
> Batch and pilot plant tests were completed in 2013 and 2016 on bulk samples of PPM concentrate (UG2-Merensky blends, approximately 95 g/t and 75 g/t 3PGM+Au, respectively).
> Monthly variability test work was completed in 2014 on 10 monthly mine production sample batches (with four duplicates) using Kell design operating conditions.
> Variability test work was completed in 2015 on five UG2/ Merensky blended sample batches using Kell design operating conditions. A 50:50 UG2:Merensky blend was selected for the 2016 pilot plant.
A summary of recovery of value from concentrate (an expression of metal extraction from concentrate using 2016 Q4 metal prices) through the various stages of the overall programme is shown in Table III. It is noteworthy that the head grade was sequentially lowered during the stages of the programme, demonstrating consistent value recovery on lowering head grade from 110 g/t 3PGM+Au in 2014 to 73 g/t 3PGM+Au in 2016. Smelter feed grades are typically higher, at 130-340 g/t 3PGM+Au (Crundwell, 2011). This demonstrates the potential for Kell to treat higher mass pull concentrates by removing the smelter feed constraints, resulting in substantially higher flotation recoveries.
Long-term testing of materials of construction
A 100 L continuously-agitated sealed tank system under chlorination conditions was operated continuously at Simulus Laboratories for 7 months to test design-specified materials of construction for the chlorination circuit under operating conditions (shown in centre foreground in Figure 6). No wear of the tank or agitator impeller was observed during the trial and the materials were therefore considered suitable for use. The agitator seal and drive also performed well. No emissions triggers were experienced during the 7 months of operation.
Environmental testing
Final neutralized Kell tails may either be dry-stacked or comingled with flotation tails and stored in the tailings storage facility (TSF). This allows for: (i) utilization of acid-consuming components of the flotation tailings (net acid-consuming); (ii) elimination of the need for separate lined dams for storage, thereby decreasing cost and eliminating environmental issues. Combined tailings from the pilot plant campaign described above were subjected to geochemical and water extraction analysis by an independent service provider, concluding that waste type classification would remain unchanged (non-potentially acid generating, Type 3), and that metals mobility from the tailings was very limited (Smith, Adams, and Liddell, 2019).
Kell process engineering
Engineering studies at scoping, PFS, and DFS level have been carried out for a range of Kell applications by Simulus Engineers, an engineering company in Perth, Australia specializing in hydrometallurgy, supported by targeted test work conducted by Simulus Laboratories on the same premises.
For a given project, preliminary process design criteria (PDC) are prepared based on the client's product requirements, test work results, prior work, and Kell process experience. Mass and energy balance outputs are generated for each case under consideration (e.g. throughput, grade, or concentrate type), using inputs from the PDC. The model outputs are used to size mechanical equipment, electrical loads, and motor selections.
The capital cost estimate is built up from supplier quotations for major equipment items. For scoping-level studies only, earthworks, civil, structural, electrical, instrumentation, and piping costs are factored from previous Kell process studies and feasibility level designs. Installation costs for mechanical equipment and each of the disciplines are calculated from an estimate of installation hours and local costs. The operating cost estimates are developed from first principles. Reagent consumption rates are taken from the mass balance outputs. Unit costs are based on local supplier quotes provided by the client and vendors during recent studies. Labour requirements are estimated based on employee costs for site labour as supplied by the mine site. Energy consumption is built up from the equipment list and from site power costs. The target accuracies for the capital and operating costs are aligned with the AusIMM standard as stated in their Monograph 27, Cost Estimation Handbook, 2nd Edition (AusIMM, 2012). Trade-off studies for site-specific criteria, such as on-site reagent production, are included where warranted.
The design methodology is modular fabrication and supply. Most of the process equipment, piping, valving, and instrumentation is pre-installed on skids or modules for transport to site. A small number of larger equipment items are installed directly on site.
Engineering design status
Over 15 scoping and prefeasibility studies and preliminary economic assessments have been completed for Kell applications to PGM, gold, and polymetallic concentrates, all showing attractive returns, particularly when Kell replaces smelting/ refining offtake contracts.
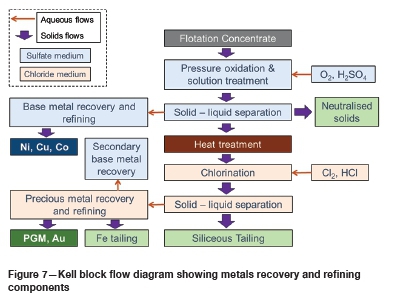
A DFS, supported by batch optimization and variability and pilot-scale test work, was completed in 2013 for installation of a 280 000 ounce per annum Kell plant at PPM's mine site. This was extended and upgraded, supported by circuit and set-point optimizations, additional bench-scale locked-cycle test work, and more extensive integrated piloting, resulting in a bankable feasibility study completed in 2016 (the block flow sheet is depicted in Figure 7). The overall body of work includes specific throughput studies, targeted test work, and Monte Carlo sensitivity analyses. Engineering and process simulation has been completed at construction design level.
Industrial applications of Kell unit operations
Unit operations in the Kell flow sheet are applied industrially under similar conditions in the extractive metallurgical and other industries. This body of experience and knowledge informs the design and materials selection of Kell unit operations and de-risks the engineering accordingly. A few examples from the database are given below to illustrate this point.
Pressure oxidation and base metals recovery
Pressure hydrometallurgy is well established in the processing of nickel sulphides and laterites, refractory gold, and some copper concentrates (Habashi, 2014; Thomas and Pearson, 2016). Kell POX conditions are selected to be similar to industrially proven processes in gold and base metals processing, targeting full oxidation of sulphides to sulphate, thus avoiding any complications of elemental sulphur formation. Base metal recovery methods follow established practice at primary base metal hydrometallurgical plants. Therefore, the process and materials performance and operability of the sulphate circuit are well established.
Heat treatment
Flash dryer technology is already well utilized in the PGM industry on wet concentrate to produce smelter feed (Van Manen, 2006).
Kell kiln conditions are similar to those used in industrially applied processes in other sectors, such as:
> Rotary kiln-electric furnace (RKEF) plants for smelting of nickel laterites to ferronickel (Stober et al., 2008)
> Calcination of kaolin for subsequent alumina leaching (Turner et al., 1982)
> Direct reduced iron production with coal-based rotary kilns (Yong-He and Sheng-Hui, 1999)
> Ilmenite reduction in a rotary kiln to allow iron removal by leaching and TiO2 production (Grey and Reid, 1973)
> Vanadium salt-roast and iron rotary-kiln reduction at Highveld Steel (Rohrmann, 1985)
> Conversion of spodumene to leachable form by heat treatment (Peltosaari et al., 2016), and
> Cassiterite reduction under CO-CO2 atmosphere (Su et al., 2016; Zhang et al., 2016).
Mineralogy in the Kell heat treatment step is benign because sulphur in the kiln feed has been removed, in contrast to direct roasting of sulphide concentrates where bulk sulphur dioxide is produced. The conditions are non-oxidizing and non-corrosive, and materials performance is well established.
Chlorination and precious metals recovery
Chlorination leaching technology has been applied in all PGM refineries since the advent of modern primary platinum ore production in the mid-1900s (Crundwell, 2011). Chlorination was first used in the recovery of gold from its ores in the Plattner, Deetken, Mears, and Newbery-Vautin processes in the mid- to late 1800s, before being displaced by cyanidation, primarily due to the high reagent consumptions incurred with chlorine when treating ores containing high sulphide grades (Adams, 2016). In Kell processing, these sulphides and other reagent consumers are removed prior to chlorination, resulting in low chlorination reagent consumptions.
Criticisms of Kell include, for example, the misapprehension that 'Cl2 gas is highly corrosive requiring special materials of construction and therefore results in both high capital and operating costs if such technology is adopted' (Mpinga, 2015). Kell plant design incorporates modern high-temperature and abrasion-resistant, low-cost dual-laminate composite plastic materials such as thermoplastic-lined fibre-reinforced plastic (FRP). These materials have been well proven for similar duties in other industries such as steel pickling and gas scrubbing (OxyChem, 2013) and in some PGM refineries (Dunn, 2018).
Kell process implementation
Comparison of Kell with smelter-refining
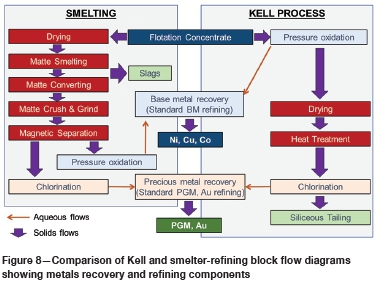
Potential synergies in the co-location of Kell within an existing smelter complex have been described earlier in this paper. Consideration of new concentrate treatment capacity at a greenfield site is also useful. Smelting melts the whole concentrate to recover the PGMs, Au and base metals - 80-95% of smelter feed is gangue. In contrast, Kell dissolves the base metal sulphides and precious metals, leaving most of the gangue minerals in their original form. The high energy input required to melt the gangue minerals is dictated by their heat capacities and latent melting heats, and this is the main reason for the much higher energy consumption in smelting than in Kell processing. A schematic comparison of Kell with smelter-refining of PGM concentrates is shown in Figure 8, which illustrates the main differences, particularly as regards elimination of the pyrometallurgical steps when adopting Kell processing.
Kell processing comparisons with smelter-refining of PGM concentrates in this work and elsewhere (Smith, Adams and Liddell, 2019) are listed below. Similar outcomes would likely apply to copper-gold or refractory gold concentrates.
> Capital costs: 18-33% of smelting and refining (depending on concentrate mineralogy; on-site reagent production or bulk purchase)
> Operating costs: 51-66% of smelting and refining (depending on concentrate mineralogy; on-site reagent production or bulk purchase)
> Electrical energy consumption: 13-46% of smelting (depending on concentrate mineralogy; on-site reagent production or bulk purchase)
> CO2 equivalent emissions: 57-61% of smelting
> Shorter time to metal payment: <2 weeks rather than >5 weeks
> Metal recoveries comparable to smelting (approx. 96% value recovery)
> Processes lower grade, higher impurity concentrates - allows for increased concentrator recoveries
> No practical constraints on smelter grade or impurities such as As2O3, Cr2O3, and MgO
> Allows for higher concentrator mass pulls to lower grade, higher impurity concentrates
> May result in additional concentrator recovery compared with smelting.
Kell implementation initiatives
Pilanesberg Platinum Mine
PPM is the wholly-owned operating subsidiary of Sedibelo Platinum Mines (SPM), which has also invested directly in the Kell licence for the SADC region. The South African Industrial Development Corporation (IDC) has further invested directly in the South African region Kell licence. The local Batkatla-ba-Kgafela community is the largest shareholder in SPM and benefits from employment and training opportunities, and regional and social development. The project (Sedibelo Platinum Mines, 2018) has current overall resources for some 50 years of mining and milling 220 kt/month Merensky ore and 65 kt/month UG2 ore, producing 15 000 ounces of 3PGM+Au per month plus Cu, Ni, and Co in concentrate. The comprehensive body of Kell test work supports a positive bankable feasibility study for a 110 000 t/a (280 000 ounces per annum) plant treating a UG2-Merensky concentrate at PPM, with the following features:
> Feed concentrate: UG2 plus Merensky blend
> Design/construction: 21 months
> Commissioning: 3 months
> Ramp-up:18 months
> Construction phase: about 300 jobs
> Operations phase: 144 jobs
> Refining to Pt and Pd metals and added value products on site for direct end-user sale, or intermediate products
> Kell tailings co-deposited with flotation tailings, meeting regulatory and permitting requirements as shown by environmental test work (Smith, Adams, and Liddell, 2019)
> Potential for toll treatment of third-party concentrates.
A 280 000 ounces per annum Kell plant has been approved for construction at PPM, subject to financial closure and forward mine planning.
Zimbabwe platinum producers
Additional advances have been made in Zimbabwe, the world's third largest platinum-producing country. The Zimbabwean government, represented by the Zimbabwean Mining Development Corporation (ZMDC), signed a Memorandum of Agreement (MOA) with KellTech Ltd. for a centralized metal concentrate processing plant. KellTech is actively engaging with the Zimbabwe Platinum Producers Association (ZPPA), as well as individual producers and owners, towards crystallization of this initiative. Test work and scoping-level engineering studies have been carried out for several Kell applications for in-country processing of various Zimbabwean PGM and gold concentrates, showing high value recoveries (>95%), and comparatively low capital and operating costs.
Additional Kell implementation initiatives
Further test work and engineering studies for Kell applications at several other producers and development projects are being progressed for a range of concentrates, including PGMs (Platreef, UG2, Merensky, Great Dyke and elsewhere), refractory gold and silver, copper-gold, and polymetallic. In addition, Kell philosophy is being applied to the treatment and recovery of lithium (from brines, clays, and hard rock concentrates) and other materials.
Conclusion
Kell is a potential step-change in the low-emissions, cyanide-free hydrometallurgical recovery of metals from concentrates, with particular application to primary PGM, refractory gold, copper-gold, and polymetallic concentrates. Capital and operating costs and other economic drivers such as payment pipelines and working capital metal lock-up are a fraction of those for smelter-refining or oxidation-cyanidation, as are environmental impacts such as CO2, SO2, and cyanide emissions. Kell shows high metal recoveries across a broad span of concentrates and has been demonstrated in a 9-week integrated pilot-plant campaign and a 7-month large pilot-scale materials testing regime. This has culminated in delivery of some 15 scoping and prefeasibility studies for various concentrates, and a bankable feasibility study for a 110 000 t/a plant treating a UG2-Merensky concentrate at Pilanesberg Platinum Mine, for which construction is approved subject to financial closure and forward mine planning. The Zimbabwean Mining Development Corporation (ZMDC) signed a Memorandum of Agreement (MOA) with KellTech for a centralized PGM concentrate processing plant, towards which KellTech is actively engaging with the Zimbabwe Platinum Producers Association (ZPPA) and the mining industry.
Acknowledgements
Industry partners who have provided samples and sponsorship, as well as fruitful discussions, are gratefully acknowledged. Sedibelo Platinum Limited is particularly thanked for their substantive financial, technical, and logistical support for Kell, and for permission to utilize specific operational and smelter PFS data. The support and investment of the Industrial Development Corporation of South Africa is acknowledged. The authors appreciate the excellent technical and engineering effort of staff at The Simulus Group, particularly Simon Willis, Brett Lawson, Duncan Cumming, Mike Storey, and Clint Perkis. Energetics personnel are thanked for their comparative life cycle assessment work on Kell and smelter-refining. The commercial skills of KellTech's Chris Showalter are also well recognized.
References
Adams, M.D. (ed.), 2016. Gold Ore Processing: Project Development and Operations, 2nd edn. Elsevier, Amsterdam. 1040 pp. [ Links ]
Adams, M.D., Liddell, K.S., and Smith, L.A. 2015. The KellGold hydrometallurgical process for cyanide-free extraction of gold from refractory concentrates and feedstocks - a preliminary assessment. Proceedings of World Gold Conference 2015. Southern African Institute of Mining and Metallurgy, Johannesburg. pp. 141-154. [ Links ]
Adams, M.D., Smith, L.A., and Liddell, K.S. 2019. Application of KellGold process to the cyanide-free low emissions recovery of gold, silver and copper. World Gold 2019, Perth, Australia, 11-13 September 2019. In press. [ Links ]
Adams, M.D., van der Meülen, D.E., Lünt, D.J., and Anderson, P. 2004. Selection of piloting parameters in pressure hydrometallurgy. Metallurgical Plant Design and Operating Strategies. Australasian Institute of Mining and Metallurgy, Melbourne. pp. 543-555. [ Links ]
Adams, M.D., van der Meülen, D.E., Czerny, C., Adamini, P., Türner, J., Jayasekera, S., Amaranti, J., Mosher, J., Miller, M., White, D., and Miller, G. 2004. Piloting of the Beneficiation and EPAL® Circuits for Ravensthorpe Nickel Operations. Proceedings of the International Laterite Nickel Conference. The Minerals, Metal and Materials Society, Warrendale, PA. pp. 193-202. [ Links ]
Anglo American Platinum. 2018. Why Anglo American Platinum is implementing new SO2 abatement technology. Fact Sheet. 2 pp. http://www.angloamericanplatinum.com/media/press-releases/2018/16-08-2018.aspx [accessed 11 September 2018]. [ Links ]
AOSIO. 2006. Announcement on releasing the content ceilings of arsenic and other harmful elements in imported copper concentrates, No. 49, 2006, General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China, 5 April 2006. http://english.aqsiq.gov.cn/PolicyRelease/LatestPolicies/200907/t20090708_121104.htm [accessed 21 November 2018]. [ Links ]
AusIMM. 2012. Cost Estimation Handbook. Monograph 27, 2nd edn. Australasian Institute of Mining and Metallurgy, Melbourne. 527 pp. [ Links ]
Collins, M.J. and Vardill, W.D. 2005. Introduction to the Ambatovy Nickel Project. Proceedings of ALTA 2005Nickel-Cobalt Conference. ALTA Metallurgical Services, Melbourne. 16 pp. [ Links ]
Collins, M.J., Barta, L.A., Buban, K.R., Kalanchey, R., Owusu, G., Raudsepp, R., Stiksma J., and Masters I.M. 2005. Process development by Dynatec for the Ambatovy Nickel Project, CIM Bulletin, Sep/Oct 2005. Paper 35, , 7 pp. [Paper presented at Pressure Hydrometallurgy 2004, Banff, Alberta, 23-27 October 2004]. [ Links ]
Crundwell, F.K. 2011. Refining of the platinum-group metals. Extractive Metallurgy of Nickel, Cobalt and Platinum-Group Metals, Elsevier, Amsterdam. pp. 489-534. [ Links ]
DEA 2013. National Environmental Management: Air Quality Act, 2004 (Act No. 39 Of 2004), revised 2012, finalised 2013. South African Department of Environmental Affairs, Pretoria. [ Links ]
Dunn, G. 2018. Private communication, Hydromet Pty Ltd. [ Links ]
Greenwald, N. and Bateman, P. 2016. The International Cyanide Management Code: Ensuring best practice in the gold industry. Gold Ore Processing: Project Development and Operations. 2nd edn. Adams, M.D. (ed.). Elsevier, Amsterdam. Chapter 12, pp. 191-206. [ Links ]
Grey, I.E. and Reid, A.F. 1973. Reduction of ilmenite in a commercial rotary kiln - an X-ray diffraction study. Proceedings of the AusIMM Conference, Western Australia, May 1973. Australasian Institute of Mining and Metallurgy, Melbourne. pp. 583-604. [ Links ]
Gwimbi, P. 2017. Monitoring SO2 emission trends and residents' perceived health risks from PGM smelting at Selous Metallurgical Complex in Zimbabwe. InternationalJournalforEquity in Health, vol. 16, no. 200. 11 pp. [ Links ]
Habashi, F. 2009. Recent trends in extractive metallurgy. Journal of Mining and Metallurgy, vol. 45B, no. 1. pp. 1-13. [ Links ]
Habashi, F. 2014. A new era in pressure hydrometallurgy. Metall., vol. 68. pp. 27-34. [ Links ]
Impala Platinum Ltd. 2017. Consolidated Annual Results 2017. http://implats-reports.co.za/results/annual-results-2017/pdf/booklet.pdf [accessed 25 October 2018]. [ Links ]
Ivanhoe Mines. 2018. Shaft 1 reaches the top of Platreefs "Flatreef" Deposit at 780 metres below surface at Ivanhoe's platinum-group metals, nickel, copper and gold project in South Africa. Press Release, 8 October 2018. https://www.ivanhoemines.com/news/2018/shaft-1-reaches-the-top-of-platreefs-flatreef-deposit-at-780-metres-below-surface-at-ivanhoes-platinum-group-metals-nickel/ [accessed 25 October 2018]. [ Links ]
Jones, R.T. 2005. An overview of Southern African PGM smelting. Proceedings of Nickel and Cobalt2005: Challenges in Extraction and Production, The 44th Annual Conference of Metallurgists, Calgary, Alberta, Canada, 21-24 August 2005, Canadian Institute of Mining, Metallurgy and Petroleum, Montreal. pp.147-178. [ Links ]
Landsberg, A. and schaller, J.L. 1971. The kinetics and equilibria of the platinum-chlorine system. Journal of the Less-Common Metals, vol. 23. pp. 195-202. [ Links ]
Lane, D.J., Cook, N.J., Grano, s.R., and Ehrig, K. 2016. Selective leaching of penalty elements from copper concentrates: A review. Minerals Engineering, vol. 98. pp.110-121. [ Links ]
Liddell, K.s. and Adams, M.D. 2012. Kell hydrometallurgical process for extraction of platinum group metals and base metals from flotation concentrates. Journal of the Southern African Institute ofMining and Metallurgy, vol. 112, no. 1. pp. 31-36. [ Links ]
Liddell, K.s., Newton, T., Adams, M.D., and Muller, B. 2011. Energy consumptions for Kell hydrometallurgical refining versus conventional pyrometallurgical smelting and refining of PGM concentrates, Proceedings of the 4th International Platinum Conference, Platinum in Transition 'Boom or Bust, Sun City, South Africa, October 2010. Southern African Institute of Mining and Metallurgy, Johannesburg. pp. 181-186. Journal of the Southern African Institute ofMining and Metallurgy, vol. 111, no. 2. pp. 127-132. [ Links ]
Long, G., Peng, Υ., and Bradshaw, D. 2012. A review of copper-arsenic mineral removal from copper concentrates. Minerals Engineering, vol. 36-38. pp.179-186. [ Links ]
Mandizha, T. 2015. Zimplats to refurbish refinery, The Standard, 31 May 2015. https://www.thestandard.co.zw/2015/05/31/zimplats-to-refurbish-refinery/ [accessed 20 October 2018]. [ Links ]
Miller, P. and Brown, A.R.G. 2016. Bacterial oxidation of refractory gold concentrates. Gold Ore Processing: Project Development and Operations. 2nd edn. Adams, M.D. (ed.). Elsevier, Amsterdam. Chapter 22, pp. 359-372. [ Links ]
Mpinga, C. N., Eksteen, J. J., Aldrich, C., and Dyer, L. 2015. Direct leach approaches to platinum group metal (PGM) ores and concentrates: A review. Minerals Engineering, vol. 78. pp. 93-113. [ Links ]
Newmont Mining. 2015. Beyond the Mine: Our 2015 social and environmental performance. https://sustainabilityreport.newimont.com/2015/case/19/new-mill-eliminates-stack-emissions-at-kcgm [accessed 11 September 2018]. [ Links ]
Peltosaari, o., Tanskanen, P., Hautala, s., Heikkinen, E.P., and Fabritius, T. 2016. Mechanical enrichment of converted spodumene by selective sieving. Minerals Engineering, vol. 98. pp. 30-39. [ Links ]
Platinum Group Metals Ltd. 2018. Platinum Group Metals Ltd announces increased confidence in large scale, palladium dominant deposit with strong gold credit at Waterberg JV South Africa, Press Release, 25 October 2018. http://www.platinumgroupmetals.net/investor-relations/news/press-releases/press-releases-details/2018/Platinum-Group-Metals-Ltd-Announces-Increased-Confidence-in-Large-Scale-Palladium-Dominant-Deposit-with-Strong-Gold-Credit-at-Waterberg-JV-South-Africa/default.aspx [accessed 25 October 2018]. [ Links ]
OxyChem. 2013. Hydrochloric acid handbook, 06/2013. Occidental Chemical Corporation, Dallas, TX. 51 pp. [ Links ]
Pourbaix, M.J.N., Van Muylder, J., and de Zoubov, N. 1959. Electrochemical properties of the platinum metals. Platinum Metals Review, vol. 3, no. 2, pp. 47-53; vol. 3, no. 3. pp. 100-106. [ Links ]
Rohrmann, Β. 1985. Vanadium in South Africa. Journal of the Southern African Institute ofMining and Metallurgy, vol. 85, no. 5, May 1985. pp. 141-150. [ Links ]
seccombe, A. 2018. Unlike peers, cash-flush Amplats is ready for growth again. Business Day, 24 July 2018. https://www.businesslive.co.za/bd/companies/mining/2018-07-24-unlike-peers-cash-flush-amplats-is-ready-for-growth-again/ [accessed 25 October 2018]. [ Links ]
sedibelo Platinum Mines. 2018. Resources and Reserves. http://www.sedibeloplatinum.com/assets/reserves-resources [accessed 21 September 2018]. [ Links ]
smith, L.A., Adams, M.D., and Liddell, K.s. 2019. The Kell Process: Efficient, low-energy metals recovery with low environmental impact. Sustainable Mining 2019 - Proceedings of the 6th International Conference of Environment and Social Responsibility in Mining, Santiago, Chile, 4-6 September 2019. In press. [ Links ]
su, Z., Zhang, Υ., Liu, Β., Zhou, Υ., Jiang, T., and Li, G. 2016. Reduction behavior of SnO2 in the tin-bearing iron concentrates under CO-CO2 atmosphere. Part I: Effect of magnetite. Powder Technology, vol. 292. pp. 251-259. [ Links ]
Thomas, K.G. and Pearson, M.s. 2016. Pressure oxidation overview. Gold Ore Processing: Project Development and Operations. 2nd edn. Adams, M.D. (ed.). Elsevier, Amsterdam. Chapter 21, pp. 341-358. [ Links ]
Tsuchida, N. 2015. HPAL in past, present and future. Proceedings of ALTA 2015 Nickel-Copper-Cobalt Conference. ALTA Metallurgical Services, Melbourne. 8 pp. [ Links ]
Valle, L., Benz, M., Chalkley, M., Collins, M., Dobson, T., Holmwood, R., Malevich, A., Tuffrey, N., and Vrolson, R. 2016. Completing the Ambatovy ramp-up: the road to successful financial completion. Proceedings of ALTA 2016 Nickel-Copper-Cobalt Conference. ALTA Metallurgical Services, Melbourne. 19 pp. [ Links ]
Van Manen, P.K. 2006. Process description and optimization of the flash dryers at Polokwane Smelter. Proceedings of the International Platinum Conference 'Platinum Surges Ahead'. Southern African Institute of Mining and Metallurgy, Johannesburg. pp. 253-258. [ Links ]
Wilkinson, T. 2006. Engineering of Coral Bay (Rio Tuba) nickel project: second generation HPAL? Proceedings of ALTA 2006 Nickel-Cobalt Conference. ALTA Metallurgical Services, Melbourne. 24 pp. [ Links ]
Yesil, M.M. and Iplikcioglu, A.s. 2015. Gördes nickel cobalt HPAL project in Turkey. Proceedings of ALTA 2015 Nickel-Copper-Cobalt Conference. ALTA Metallurgical Services, Melbourne. 15 pp. [ Links ]
Yong-He, H. and sheng-Hui, X. 1999. CRIMM - a new process for production of directly reduced iron with coal-based rotary kiln. ICARISM - International conference on alternative routes of iron and steelmaking, Perth, Australia, 15-17 September 1999. Misra, V.N. and Holmes, R.J. (eds.). Australasian Institute of Mining and Metallurgy, Melbourne. pp. 223-228. [ Links ]
Zhang, Υ., su, Z., Liu, Β., Zhou, Υ., Jiang, T., and Li, G. 2016. Reduction behavior of SnO2 in the tin-bearing iron concentrates under CO-CO2 atmosphere. Part II: Effect of quartz. Powder Technology, vol. 291. pp. 337-343. [ Links ]
 Correspondence:
Correspondence:
K.S. Liddell
Email: keith@kellprocess.com
Received: 23 Jan. 2019
Revised: 27 Mar. 2019
Accepted: 3 Apr. 2019
Published: June 2019














