Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.117 n.8 Johannesburg Aug. 2017
http://dx.doi.org/10.17159/2411-9717/2017/v117n8a3
HYDROMETALLURGY CONFERENCE 2016
The FLSmidth® Rapid Oxidative Leach (ROL) process: a mechano-chemical approach and industrial applications for rapid metal sulphide dissolution
M. Mulligan; D. Chaiko; F. Baczek; S. Rocks; C. Eyzaguirre; C. Dickinson; R. Klepper
FLSmidth, USA
SYNOPSIS
The leaching of primary copper concentrates using acidic ferric sulphate lixiviants is known to suffer from slow kinetics and poor copper recoveries. A number of processes have been developed over the years to improve the leach kinetics and copper recoveries, and while many are effective, they have high CAPEX and/or OPEX. FLSmidth is developing a mechano-chemical approach that confers significant process efficiencies by taking advantage of the enhanced reactivity of transitory, surface-defect structures generated during particle fracture. The patent-pending FLSmidth® Rapid Oxidative Leach (ROL) process uses a stirred media reactor (SMRt) to achieve copper recoveries of more than 97% in less than 6 hours under atmospheric conditions. The mechano-chemical approach overcomes many of the surface passivation problems that have hindered other atmospheric leach processes. Results of batch leach tests and new insights to leach mechanisms are presented. During the life-cycle of many orebodies, the mineralogy transitions from oxides to secondary and primary sulphides, ore grades decrease, and the ore become more difficult to process. Cost-efficient leach processes will be required to maintain copper production at existing mines or take advantage of new orebodies, particularly those containing arsenic-rich minerals like enargite. To date, the majority of the FLSmidth work has been on primary chalcopyrite concentrates, with a minor amount of work done on enargite and arsenopyrite concentrates. Brownfield and greenfield industry applications are reviewed for potential use of the FLSmidth® ROL process, primarily looking at copper recovery from chalcopyrite and enargite. We also include a discussion on applications to other metals, like refractory gold, nickel, and zinc.
Keywords: chalcopyrite, enargite, refractory gold, atmospheric leaching, stirred media reactor, mechano-chemical processing.
Introduction
The hydrometallurgical production of copper dates from the late 1960s, when copper-selective solvent extraction (SX) reagents were commercially introduced by BASF (General Mills at the time) in response to the need for a more economical and cost-efficient process to recover copper from oxide leach solutions other than cementation on scrap iron. With the progressive introduction of more stable and selective reagent/diluent systems, copper SX technology has matured to become a cost-effective, large-scale technology that is used throughout the world. It is now used to recover copper from pregnant leach solutions (PLS) containing 2-20 g/L copper produced by either heap leaching of oxide ores or atmospheric tank leaching of secondary sulphide ores. Currently, about 20% of the world's copper production uses the combination of SX and electrowinning (EW). Heap leaching and atmospheric tank leaching technologies are now accepted as state-of-the-art for processing oxides and secondary sulphides.
Pressure oxidation (POX) autoclave technology, operating at temperatures of 140-225°C, offers acceptable dissolution kinetics and recoveries for primary copper sulphide concentrates, but suffers from relatively high CAPEX and OPEX. At the higher operating temperatures, total oxidation of sulphide to sulphuric acid consumes oxygen in excess of that required for copper dissolution. In the absence of an accompanying oxide heap leach process, the relatively dilute acid produced by POX requires neutralization and disposal. Thus, unique project circumstances are necessary to produce acceptable economics.
The CESL process, owned by TECK, is a chloride-catalysed autoclave process that operates at 140°C with partial sulphur oxidation. The Salobo demonstration plant in Brazil, owned by Vale, was built and operated to demonstrate the CESL technology for copper recovery. The CESL process is currently under consideration for treating arsenic-rich copper concentrates.
A partial list of processes that have been considered for leaching primary sulphide copper concentrates during the last 25 years is given below, along with the current owners:
► > Activox® process-Norilsk Nickel, formerly LionOre Mining International Ltd
► Albion™ process-XT of Glencore, formerly Xstrata Technologies
► CESL Process-TECK, formerly Cominco Engineering Services Ltd
► Dynatec-Sherritt International
► GalvanoxTM-University of British Columbia
► HydroCopper®-Outotec
► Placer Dome/Phelps Dodge-Freeport McMoRan.
Efforts have been made to commercialize all of these processes, but either process- or chemistry-related problems have been encountered that resulted in unacceptable technical risk and return on investment.
FLSmidth has identified a simple process that will leach copper sulphide concentrates, with chalcopyrite as the predominant copper-bearing mineral, at 80°C and under atmospheric pressure. Over the past two years, more than 150 batch leach tests have been completed in 14-litre reactors, using more than ten copper concentrates from different sources. The data confirms that the process is robust and can attain 97% to >99% copper dissolution in less than 6 hours. The rapid leach kinetics and high copper recoveries place the process economics on par with stirred-tank leaching of secondary copper sulphide concentrates. The key to process performance is the use of very low-energy, interstage and/or intrastage attrition/grinding to enhance the selective dissolution of copper-bearing minerals by a mechano-chemical process. In this paper we present representative results of batch leach tests and some new insights into potential industrial applications.
Methodology
Concentrate processing
Numerous copper concentrates, from different mine sites, were used in the leach testing. Chalcopyrite was either the major or the sole copper-bearing mineral. In all cases, pyrite and quartz comprised the bulk of the gangue mineralogy in the high-grade concentrates. A low-grade concentrate, containing 20% chalcopyrite and 7.6 wt% Cu was also tested. In this sample, the majority of the gangue minerals were layered silicates, pyrite, and quartz.
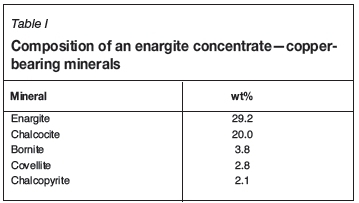
Also, three enargite-containing concentrates, which contained secondary copper sulphides and layered silicates, were used in a limited number of leach tests (see Table I for the copper mineralogy).
The as-received concentrates were slurry-split, and individual 1 kg samples were pressure filtered, then vacuum sealed under argon for later use in leach testing. In certain cases, chalcopyrite concentrates were fine-milled to a D80 of 17 μm using a FLSmidth VXP laboratory-scale fine grinding mill. The enargite concentrate was tested as-received with a D80of 47 μm. Concentrate samples were analysed for moisture, particle size distribution, Rietveld XRD mineralogy, and elemental composition by inductively coupled plasma (ICP). Mineralogy and elemental analysis were also determined on the leach residues.
Atmospheric leaching
A jacketed 14 L (operating at 10 L volume) glass leach vessel was coupled to an Eppendorf BioFlo 310 control unit, which enabled precise monitoring of temperature, pH, Eh, agitation rate, redox control via modulation of oxygen sparging, instantaneous gas flow, and cumulative gas flow.
Flotation concentrates were leached in a stirred reactor with the assistance of a stirred media reactor (SMRt), in acidic ferric sulphate media at 80°C. The slurry was continuously recirculated between the two reactor types as shown in Figure 1a. Oxygen was sparged into the stirred reactor to maintain a minimum redox set-point of 650 mV (SHE). The applied mixing power in the stirred reactor was approximately 1-5 kW/m3, and 15-20 kW/m3 in the SMRt reactor.
A larger, heat-blanketed 120 L stainless steel leach vessel was coupled to a LabVIEW® control unit, and the same SMRt reactor was used with the same methodology as with the 14 L unit. The 120 L vessel was operated with a 50 L volume.
Pressurized leaching
A limited number of pressurized leach tests were performed using a model 4520-2 L stainless steel Parr reactor fitted with a Teflon insert. A model 4848 Parr controller was used to adjust the temperature within ± 2°C accuracy, and a Parr magnetic drive, modified to house three impellers, was used to provide agitation. To simulate the mechano-chemical properties of the SMRt unit at higher temperatures and pressures, the Teflon insert contained ceramic media similar to that used in the SMRt reactor. Two of the impellers were located below the media level and the third impeller remained halfway up the remaining slurry level (Figure 1b).
The pulp density in the reactor was typically 7 wt% solids in a solution of sulphuric acid and iron sulphate salts. The system was then closed and brought to temperature. Pure oxygen gas was allowed to freely flow into the reactor as needed to maintain the targeted oxygen partial pressure. The flow of oxygen and total oxygen consumption were measured by an Aalborg gas flow unit during leach testing.
Results and discussion
The FLSmidth® Rapid Oxidative Leach (ROL) process-14 L vessel
The FLSmidth® Rapid Oxidative Leach (ROL) process utilizes a series of stirred media reactors (SMRts) placed in tandem with conventional, stirred leach tanks to achieve copper recoveries of 97% to >99% in 6 hours or less from primary sulphide concentrates. The interstage or intrastage placement of the SMRts, shown in Figures 2a and 2b, gives the advantage of mechano-chemical activation processes with only a minimal addition to baseline processing costs. Processing costs are further reduced by optimizing the volume ratio between the stirred leach tanks and the SMRts.
As a point of reference, the full-scale ROL processing costs are estimated to be only marginally above those for the stirred-tank leaching of secondary copper sulphides. Minimum concentrate grades of approximately 7 wt% copper would be economically viable.
The concept of combining mechanical and chemical processes has received significant interest within the chemical process industry (e.g. Hickenboth et al., 2007; Stellacci et al., 2009), and has been shown to offer potential benefits to hydrometallurgical applications (e.g. Balaz and Achimovicova, 2006; Cobble, Jordan, and Rice, 1993). However, the concept has not caught on commercially due to either the prohibitively high mixing energies that have been employed or the use of equipment not amenable to scaling to reach commercially relevant throughputs for copper production. In addition, the leaching of metal sulphides is a special application as the sulphur product layer can complicate the process if not accounted for in mechano-chemical reactor designs. In its current configuration, the FLSmidth® SMRt is purposefully tuned for metal sulphide leaching, and is designed to promote mechano-chemical activation with specific power densities much lower than previous systems (discussed in more detail below).
Atmospheric chalcopyrite leaching
Representative copper recovery curves for low- and high-grade chalcopyrite concentrates (7.6 and 22 wt% Cu, respectively) using the FLSmidth® ROL process under batch conditions, are presented in Figure 3a. The process is capable of leaching chalcopyrite concentrates within 6 hours, regardless of grade, and readily handles common gangue materials such as layered silicates without adverse effects on copper recoveries. While the initial and final leach rates are virtually identical, the low-grade concentrate actually responds better than the high-grade concentrate.
Leach data from two low-grade (7.6 wt% Cu) chalcopyrite concentrates differing only in particle size distribution are presented in Figure 3b. As expected, the smaller particle size led to a faster initial leach rate. However, both concentrates leached within approximately 5.5 hours despite the large difference in particle size distributions. It is hypothesised that the removal of passivating product layers and the generation of active species on the mineral surfaces within the SMRt enables the rapid leaching of chalcopyrite concentrates. Thus, rapid leach rates and high metal recoveries are possible even for low-grade concentrates and without fine grinding.
Figure 3a also shows the effect of specific mixing power on chalcopyrite dissolution. In the ROL system, the time to reach 80% copper recovery was unaffected by reducing the specific power in the SMRt from 20 to 11.4 kW/m3. During the early 1990s, studies were conducted by the US Bureau of Mines (USBM) on the effects of turbomilling during ferric sulphate leaching of chalcopyrite. In that system (the optimum leach recovery value is denoted by ▲ in Figure 3a), leaching was confined exclusively to the turbomill. Thus, while the optimum specific power density was fairly low in batch leach tests (e.g., 1048 kWh/t Cu leached at 80% Cu recovery), inefficiencies inherent to the configuration of the USBM leach circuit resulted in the consumption of over 1800 kWh/t Cu leached at 90% copper recovery. Significantly more energy would have been required to reach 95-98% recoveries, making the approach of using only attrition mills in the leach process uneconomical. In contrast, the reactor configuration of the ROL process (Figure 2) results in an estimated total mixing energy for the combination of all SMRts and CSTRs of about 550 kWh/t Cu leached. Of this total, the contribution from the SMRt reactors is approximately 130 kWh/t Cu leached.
In addition to controlling the power density of the SMRt, further gains in process efficiency can be realized by varying the volumetric ratio between the CSTRs and the SMRts (CSTR:SMRt). A reactor volume ratio of between 40:1 and 80:1 represents a good balance between the mean residence times within the two reactor types.
Atmospheric enargite leaching
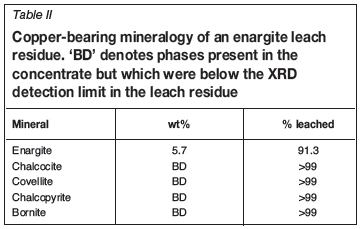
While the primary focus has been on the leaching of chalcopyrite concentrates, preliminary leach results from an arsenic-rich concentrate are presented to illustrate the process's ability to handle a wide range of refractory minerals (see Table I for the feed Cu mineralogy). Leach conditions were identical to those used in leaching chalcopyrite, and it should be understood that further process optimization is needed. The leach was conducted at 80°C, with 30 g/L initial Fe, 50 g/L initial H2SO4, and with continuous slurry recirculation between a SMRt and a stirred tank reactor. A summary of the leach results is provided in Table II. The total copper recovery reached 97% within 12 hours, and complete dissolution of all secondary sulphides and chalcopyrite was noted. The leach resulted in a 55% mass loss due to dissolution of the copper and iron sulphides. While the enargite concentration in the leach residue was 5.7 wt%, this equates to only 9% of the initial enargite remaining unleached. Arsenic remained soluble during the test and there was no evidence of scorodite formation, although it should be noted that no attempt was made to produce scorodite during the leach test. An advantage of producing soluble arsenic during the copper leach is the opportunity of selectively removing arsenic from the PLS and the leach residue.
Pressurized ROL leach
Heat and mass balance models of a continuous, multi-stage ROL process indicate that autogenous heating of the slurry to slightly above 100°C can be expected in the initial leach stages of a full-scale system. In addition, the advantages of operating a leach process at moderate temperatures (80-105°C) with above-atmospheric O2 pressures (200-500 kPa) have been noted (Richmond and Dreisinger, 2003). These potential advantages include somewhat faster Cu dissolution kinetics; more efficient oxidation of ferrous to ferric-thereby ensuring that the required redox potential is readily maintained during the leach process; and higher oxygen utilization efficiencies. Normally, mechano-chemical processes would not be expected to show temperature sensitivity, as the mechanical action supplies the necessary activation energy to drive a chemical reaction (Ma et al., 2014). To determine if the ROL process would benefit from operating under slightly higher temperatures and pressures, a limited number of leach tests were conducted in a pressurized SMRt at 105°C and 140°C. Because of the complications of leaching at or above the melt temperature of elemental sulphur (Marsden, Wilmot, and Hazen, 2007), we report here a representative summary of leach results for 105°C only.
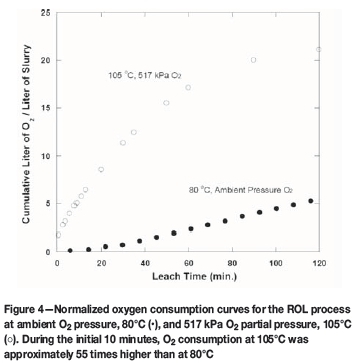
Interestingly, and contrary to expectations, the combination of slightly higher leach temperature and elevated O2 pressure dramatically altered metal sulphide leach behaviour. Increasing the temperature from 80 to 105°C (517 kPa O2 overpressure) substantially enhanced O2 mass transfer by approximately 50-55 times during the initial 15 minutes (see Figure 4). Not surprisingly, the higher O2 overpressure resulted in a slightly higher redox potential. The higher O2 mass transfer was accompanied by enhanced chalcopyrite and pyrite dissolution (see Table III). At the end of 2 hours, the Cu extraction yield from chalcopyrite was 90%, compared to 59% for the same period at 80°C. While the Cu leach rate was faster, chalcopyrite dissolution rates at 80°C are, nevertheless, quite acceptable.
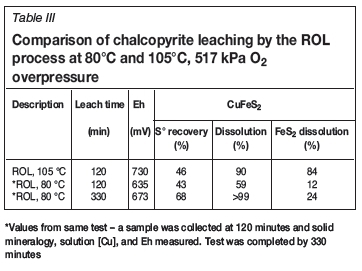
Operating the mechano-chemical leach process above 100°C, but below the melting temperature of elemental sulphur, significantly enhanced the dissolution of pyrite. X-ray analysis of the leach residues indicated that 84% of the pyrite was leached at 105°C, compared with only 24% at 80°C. High pyrite oxidation yields are expected to occur in autoclaves at much higher temperatures (e.g. 190-230°C), as routinely observed in the pretreatment of refractory gold ores (Rusanen, Aromaa, and Forsen, 2013). That this level of pyrite reactivity was observed at only 105°C is unique and may open new opportunities in precious metals recovery. One of the disadvantages of high-temperature POX is the high oxygen consumption which accompanies the near-complete oxidation of sulphide to sulphuric acid (McDonald and Muir, 2007). An advantage of both the low- and high-temperature ROL processes is the fact that the oxidation of sulphide to sulphate during metal dissolution is limited. Elemental sulphur recoveries were approximately 68% at 80°C and 46% at 105°C.
While the atmospheric ROL process is fully capable of leaching chalcopyrite, pressurized ROL leaching offers the advantage of simultaneously generating acid and iron from pyrite. The lower operating temperatures provide an advantage over POX autoclaves for those instances where a source of iron is needed, as for example in the leaching of secondary copper sulphides and copper arsenic sulphides (e.g. enargite).
Scale-up of the FLSmidth® Rapid Oxidative Leach (ROL) Process
50 L batch leaching
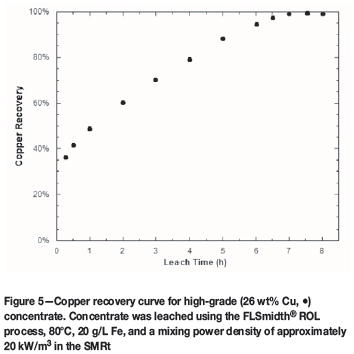
A few leach tests have been performed with a concentrate containing 26 wt% copper in a scaled-up 50 L system using the FLSmidth® ROL process, and included an activation step of 4% chalcopyrite conversion. The activation step is a rapid pretreatment of chalcopyrite with soluble copper that improves copper leach kinetics by producing a lattice-strained, iron- and sulphide-depleted copper sulphate (Chaiko et al, 2015a, 2015b). The leach was conducted at 80°C, 20 g/L Fe, and a mixing power density of approximately 20 kW/m3 in the SMRt. Figure 5 shows that copper recoveries of greater than 95% were achieved in 6 hours' residence time, and greater than 99% in 7 hours' residence time. It is believed that following some minor improvements to the system, the leach results will be on a par with those from the 14 L tests.
Continuous pilot plant
A pilot plant facility (Figure 6) has been constructed at FLSmidth's technology centre in Salt Lake City, USA and was commissioned in Q2 2016. It includes four 14 L reactors and four 50 L reactors, and a number of SMRts that will be able to operate in interstage and/or intrastage arrangements. Each reactor is instrumented for measurement and control of temperature, pH, Eh, and oxygen addition, and is linked to a data recording system. This pilot plant facility will enable continuous operation of the ROL process on a larger scale. Following the demonstration of the FLSmidth® ROL process in the pilot plant, countercurrent decantation (CCD), solvent extraction (SX), electrowinning (EW), and impurity removal stages will be added to demonstrate a fully integrated circuit.

Potential industrial applications
Copper industry
Sustained SX-EW copper production - oxide to sulphide transition
There are a number of copper hydrometallurgical facilities around the world with SX-EW plants where the oxide orebodies are being depleted, and the mines will start moving into transitional and sulphide zones. These transitional zones are either secondary sulphides with underlying primary sulphides, or a mix of oxide and primary sulphides. This is resulting in a drop in heap leach recoveries and thus a lower PLS copper tenor, leading to lower copper cathode production.
With the FLSmidth® ROL process, a grinding circuit with bulk flotation can be added upstream of the existing SX-EW plant. A bulk concentrate can be leached using the FLSmidth® ROL process, followed by CCD with the resulting PLS fed to the existing SX-EW facility. The higher copper tenor PLS can either (a) be blended with the lower copper tenor PLS from the existing heap leach facility; or (b) be fed directly into the existing SX-EW plant.
Due to the brownfield nature of the transitional zone and the subsequent move into the primary sulphide zone, scenarios need to be carefully studied for each plant to ensure that the existing SX-EW facility is properly utilized to sustain copper cathode production, and in some cases additional EW capacity would need to be added.
Low- to medium-grade concentrates
Some copper concentrator plants have to process feed with decreasing copper grades, resulting in concentrates with a low copper grade. This leads to high penalties charged by the smelter, which significantly impact the profitability of the mine. In some cases, the copper mine becomes uneconomic and is either put on care and maintenance or permanently closed.
The FLSmidth® ROL process can be used in a number of scenarios for these low- to medium-grade concentrates.
► Where pockets of low- to medium-grade feed material are mined, the ROL process can be used to selectively leach copper concentrate that does not meet acceptable smelter grades, and produce copper cathode from a new SX-EW facility. When high-grade concentrate is produced, this is still sold to the smelter with no penalty charges
► If the feed grade is consistently low, the ROL process can be used to leach all of the concentrate, and copper cathode produced in a new SX-EW facility. Either a rougher bulk concentrate, with the additional benefit of higher overall copper recovery, or a cleaner concentrate can be leached using the ROL process, and analysis of the particular project would be required to assess economics
►Some historical greenfield projects have not progressed due to the concentrate being too low a grade to sustain the project. Some of these projects could become economically viable with the FLSmidth® ROL process, and should be studied to assess the current economics.
Arsenic-rich concentrates
The FLSmidth® ROL process makes it possible to develop mineral deposits containing arsenic (e.g. enargite) for recovery of copper, gold, and silver, while complying with stringent environmental regulations. As the FLSmidth® ROL process operates at atmospheric pressure, a concentrate can be treated at the mine site with complete control over the arsenic-bearing leach residues. This makes it possible to avoid the potential of arsenic contamination of the environment while transporting concentrate from the mine to the smelter.
Many existing mines have stockpiles of copper concentrate containing more than 0.5% arsenic, which is too high for smelting. The FLSmidth® ROL process can be used to treat these high-arsenic concentrates, and even makes it possible to develop new mineral deposits high in arsenic.
Electrorefinery and smelter materials
The FLSmidth® ROL technology can integrate within existing electrorefinery and smelter operations to supplement and/or increase copper cathode production. The enabling feature of the technology is the use of an FLSmidth® SMRt in which metallic materials such as anode scrap and blister copper are rapidly and selectively dissolved into dilute sulphuric acid (1-2 M H2SO4). The dissolution process is exothermic and produces a concentrated copper sulphate electrolyte that can be used to increase existing electrorefinery output while simultaneously eliminating the need to recycle anode scrap back to the smelter. Alternatively, the process has the potential to eliminate the pyrometallurgical refining step altogether by processing blister copper directly for cathode production by electrowinning.
Other mineral sectors
While the copper industry has been the main area of focus for the FLSmidth® ROL technology, there are other industries that could also use the ROL technology. No FLSmidth® ROL leach test work has been performed in any of these other sectors; however, they are perceived as constituting potential areas for application.
Gold industry
Refractory gold ores yield poor gold recoveries in conventional cyanide leaching circuits, even if the ore is finely ground. This can be either due to the gold being finely disseminated and physically 'locked' in the sulphide minerals, or to the presence of naturally occurring preg-robbing carbonaceous materials. The finely disseminated gold is often enclosed within pyrite and arsenopyrite (FeAsS), and rimming of pyrite with arsenic-rich assemblages as well as arsenopyrite-marcasite-pyrite associations is common (e.g. Aylmore and Jaffer, 2012). Preliminary studies presented in this paper indicate that pyrite can be readily leached mechano-chemically at more moderate temperatures and pressures than those used in existing refractory ore treatment options.
Nickel industry
Nickel sulphides are processed via a variety of routes, for example pyrometallurgical, high-pressure acid leach (HPAL), and the Caron process. A number of atmospheric leach technologies have been tested, such as AlbionTM and Activox®, but none have been applied commercially at a large scale. The FLSmidth® ROL technology is another atmospheric leaching technology that has potential to be used in the nickel industry.
Zinc industry
Zinc is often separated from polymetallic ores via flotation processes. The FLSmidth® ROL technology could rapidly leach sphalerite, thus providing an alternative processing route to zinc smelting. The zinc PLS solution can then be further processed via SX-EW, which is well-proven hydrometallurgical technology.
Conclusions
The FLSmidth® Rapid Oxidative Leach (ROL) process provides chalcopyrite leach rates and Cu recoveries that are comparable to secondary copper sulphide leach processes operated under atmospheric conditions. The key to achieving this performance is the coupling of stirred media reactors (SMRts) in tandem with continuous stirred tank reactors (CSTRs). The concept is equally applicable for retrofitting to existing secondary copper sulphide tank leach circuits. The power density of the SMRts is controlled to balance the rates of mechanical and chemical processes within the reactors. Incremental mixing costs related to the use of the SMRts are further minimized by specifying reactor volume ratios within the range of 40:1 to 80:1. For mineral systems with slow leach kinetics, such as enargite, the reactor volume ratio can be advantageously increased to within the range of 50:1 to 100:1, thereby further reducing mixing costs. Leach times as low as 6 hours are sufficient to reach Cu recoveries from 97% to > 99% from chalcopyrite concentrates. The process can also be readily adapted to operate at temperatures close to the melting point of elemental sulphur. This has the added advantage of using pyrite as a source of iron for the leaching of secondary sulphides and enargite, thereby eliminating the need for high-temperature POX autoclaves.
There are many potential industrial applications for the FLSmidth® ROL technology. The copper industry is the current focus for implementation, and the technology has progressed from 14 L leach reactor to a 50 L leach reactor tests. A continuous pilot plant was constructed to prove the technology on a larger, continuous level, following which a fully integrated pilot plant will be completed with a CCD, SX-EW, and waste treatment sections.
A short- to medium-term application of the technology is the sustaining of copper SX-EW facilities that are transitioning from oxide orebodies to sulphide orebodies. This will allow existing facilities to continue operating with minimal CAPEX investment, and to continue using existing SX-EW facilities to produce copper cathode. The ability to process low- to medium-grade concentrates has the potential to significantly improve the economics of existing operations where concentrate grades are falling, and also for new greenfield projects that will be more viable if the economics can be improved. Arsenic-rich concentrates are continually becoming a greater problem, particularly in countries like Chile, and the FLSmidth® ROL technology has the potential to process these concentrates economically and in an environmentally acceptable manner. A newer potential area of application is in the use of ROL to process metallic materials, such as anode scrap and blister copper, to produce a concentrated copper sulphate electrolyte stream to increase existing electrorefinery output while simultaneously eliminating the need to recycle anode scrap back to the pyrometallurgical refiner.
Acknowledgements
The authors wish to thank the staff of FLSmidth's analytical services laboratory for XRD and elemental analysis of process samples. We would also like to thank the staff of FLSmidth's Dawson Laboratory for processing the flotation concentrates used in the leach studies.
References
Aylmore, M. and Jaffer, A. 2012. Evaluating process options for treating some refractory ores. Proceedings of the ALTA 2012 International Gold Conference, Perth, Australia, 31 May-1 June 2012. [ Links ]
Baláž, P. and Achimovičová, M. 2006. Mechano-chemical leaching in hydrometallurgy of complex sulphides. Hydrometallurgy, vol. 84. pp. 60-68. [ Links ]
Chaiko, D.J., Baczek, F., Rocks, S.S., Walters, T. and Klepper, R. 2015a. The FLSmidth® Rapid Oxidative Leach (ROL) process. Part I: mechano-chemical process for treating chalcopyrite', Proceedings of the Conference of Metallurgists, Hydrometallurgical Processing and Technologies, Lucy Rosato Memorial Symposium, Toronto, Canada, 23-26 August. [ Links ]
Chaiko, D.J., Rocks, S.S., Walters, T., Asihene, S., Klepper, R., Baczek, F., and McMahon, G. 2015b. The FLSmidth® Rapid Oxidative Leach (ROL) process. Part II: a new chemical activation process for chalcopyrite. Proceedings of the Conference of Metallurgists, Hydrometallurgical Processing and Technologies, Lucy Rosato Memorial Symposium, Toronto, Canada, 23-26 August. [ Links ]
Cobble, J.R., Jordan, C.E., and Rice, D.A. 1993. Hydrometallurgical production of copper from flotation concentrates., Report of Investigations RI 9472. US Bureau of Mines. [ Links ]
Eyzaguirre C., Rocks S., Klepper R., Baczek F., and Chaiko, D. 2015. The FLSmidth® Rapid Oxidative Leach (ROL) Process: A mechano-chemical approach for rapid metal sulfide dissolution. Proceedings of Hydroprocess 2015, Antofagasta, Chile, 22-24 July. Gecamin Ltda, Santiago. [ Links ]
Hickenboth, C.R., Moore, J.S., White, S.R., Sottos, N.R., Baudry, J., and Wilson, S.R. 2007. Biasing reaction pathways with mechanical force. Nature, vol. 446. pp. 423-427. [ Links ]
Ma, X., Yuan, W., Bell, S.E.J., and James, S.L. 2014. Better understanding of mechanochemical reactions: Raman monitoring reveals surprisingly simple 'pseudo-fluid' model for a ball milling reaction. Chemical Communications, vol. 50. pp. 1585-1587. [ Links ]
McDonald, R.G. and Muir, D.M. 2007. Pressure oxidation of chalcopyrite. Part I. Comparison of high and low temperature reaction kinetics and products. Hydrometallurgy, vol. 86. pp. 191-205. [ Links ]
Marsden, J.O., Wilmot, J.C., and Hazen, N. 2007. Medium-temperature pressure leaching of copper concentrates - Part I: Chemistry and initial process development'. Preprint MMP-07-027. Society for Mining, Metallurgy and Exploration Inc., Littleton, CO. [ Links ]
Richmond, G. and Dreisinger, D.B. 2003. Processing copper sulphide ores. US patent 6,537,440. [ Links ]
Rusanen, L., Aromaa, J., and Forsen, O. 2013. Pressure oxidation of pyrite-arsenopyrite refractory gold concentrate. Physicochemical Problems of Mineral Processing, vol. 49, no. 1. pp. 101-109. [ Links ]
Stellacci, P., Liberti, L., Notarnicola, M., and Bishop, P.L. 2009. Valorization of coal fly ash by mechano-chemical activation Part II. Enhancing pozzolanic reactivity. Chemical Engineering Journal, vol. 149. pp. 19-24. [ Links ]














