Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Journal of the Southern African Institute of Mining and Metallurgy
versão On-line ISSN 2411-9717
versão impressa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.112 spe Johannesburg Jul. 2012
JOURNAL PAPER
Safety considerations when handling metal powders
J.M. Benson
Materials Science and Manufacturing Unit, CSIR
SYNOPSIS
Metal powder compaction offers unique advantages in the manufacture of net-shape components using techniques such as laser sintering, conventional press and sintering, metal injection moulding, direct rolling, direct forging, and hot isostatic pressing. If the output from the primary metal production process is in powder form, then considerable cost and energy savings can be realized by direct conversion to semi-finished or final shapes. This possibility exists for titanium and possibly also for Ta, Zr, Hf, and Nb metals.
However, these attractive benefits are associated with some significant risks. The high surface-to-volume ratio of powder particles coupled with the reactive nature of these metals means that special care must be taken when handling them. Powder explosions are unfortunately still a regular occurrence internationally and these often result in serious injury and loss of life. Even seemingly 'safe' compounds such as sugar, flour, and grain can be extremely hazardous when handled or milled and dust clouds are produced. In addition, exposure to airborne particles can have adverse effects on the human body, especially when particles are inhaled on a regular basis. Furthermore, the medical consequences of these are not fully understood, especially in the case of nanoparticles. The impact is often not observed immediately and debilitating illnesses may emerge only years or decades later.
As far as is known, there are no South African guidelines for handling of metal powders.
This paper attempts to provide an awareness of the risks associated with metal powders (including those produced indirectly by other metalworking/finishing operations) as well as some guidelines for their safe handling, based on international best practices.
Keywords: Metal powders, safety.
Introduction
The powder metallurgy industry has grown significantly over the past few decades due to the economic benefits of manufacturing net- or near-net-shaped components compared with conventional metalworking methods, such as casting or forging. This is due to better material utilization (fewer losses by machining etc.) and lower overall energy consumption. Additionally, unique properties can be obtained that would not be possible by other techniques.
However, the large surface-to-volume ratio of powders means they are inherently more reactive than the bulk form. If these powders are inappropriately handled, through carelessness or ignorance, this can lead to potentially life-threatening fires and explosions. The incidence of fires and explosions due to powders (also often referred to as combustible dusts) is unfortunately a regular occurrence throughout the world, with many fatalities and injuries reported each year.
In 2006, the US Chemical Safety Board endeavoured to compile a report1on all the known incidences of dust-induced explosions (involving all types of powders) that had occurred in the USA between 1980 and 2005. They found that 281 events had occurred, leading to 119 deaths and 718 injuries. However, this was only a partial record as they excluded several sectors that historically have posed high risks for dust explosions (i.e. the grain industry, coal mining, transportation, and military and research institutions). In fact the real picture is likely to be considerably worse, as many cases are either not reported or are attributed to some other cause. One estimate2 suggests that about 4 000 incidents (12 per month) actually occurred between 1980 and 2005. Attempts to prevent these through awareness training and hefty fines has had some effect, but explosions still occur regularly in the USA (at least 2 have been reported between January and April 2011)3.
Statistics for elsewhere in the world are not readily available but there are indications that dust-related explosions are a common occurrence in most countries. It is estimated that about 2 000 dust explosions are recorded each year throughout Europe, with on average 1 per week in the UK alone4. Further afield, a recent explosion in China (May 2011) happened at one of Apple's main iPad 2 manufacturers, leaving three dead and fifteen injured5.
Although the incidents are a compilation of all types of dusts, the number due to metal powders is a significant proportion. The Chemical Safety Board's report showed that (albeit for a limited number of cases) metal dust accounted for 20 per cent of all explosions.
An example of one such incident was at Hayes Lemmerz International-Huntington's aluminium foundry in October 2003, and this gives some perspective on the immensity of the hazard. Aluminium dust particles produced during the diecasting process were collected by a dust extraction system for recycling and were stored in a trap outside the factory. A spark ignited an initial explosion in this trap, which then rapidly spread along the extraction piping (containing dust deposits) into the plant. The shock waves caused additional dust to be released into the atmosphere in the plant and this then exploded and destroyed a major part of the factory. It was fortunate that only a few people were present at the time, as the death toll could have been very highÂnevertheless one person died and six were injured. Figure 1 shows the damage to the foundry. The investigation report concluded that this was a preventable incident and was due to a combination of ignorance of the danger as well insufficient maintenance, inspection, and cleaning of the dust collection system6.

Apart from the risk of fire or explosion, powder particles can also have a serious impact on health, especially when inhaled. A concern is that the effects on the human body may take time to develop and the consequences of long-term exposure may be evident only many years later. In the case of nano-scale particles, absorption can also occur through the skin. How these particles impact on cell and organ function is not fully known, but effects range from mild irritation to auto-immune diseases and cancers?.
This paper will outline the hazards of metal powders in general but will also make specific reference to the metals of interest to this conference; namely, zirconium, tantalum, hafnium and niobium.
Although the emphasis is on powder metallurgy related activities, this information is also relevant for facilities using other metalworking operations (e.g. casting, grinding, machining, and welding) as these can generate a significant amount of dust deposits within a work area over time. A lack of awareness coupled with inappropriate cleaning methods (i.e. using compressed air) can create high-risk conditions.
Fire and explosion
The conditions required for a fire or an explosion are shown schematically in Figure 2.
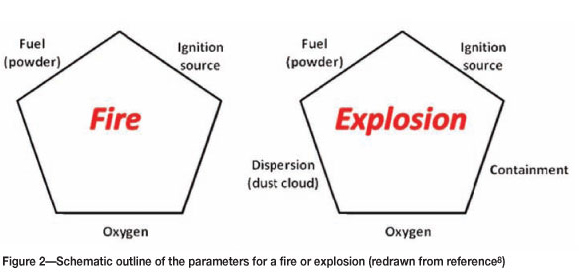
In the case of a fire, three components are needed: a fuel (powder), sufficient oxygen in the atmosphere, and a spark or other source of ignition.
An explosion on the other hand requires the powder be present as a dust cloud. The severity of the explosion can be increased if these elements are constrained within some form of enclosure. In this case the explosion may escalate from a deflagration (subsonic wavefront) to a detonation (supersonic wavefront).
A dust/powder explosion in a factory or facility typically occur in two stages: the first being the initial ignition and explosion of a quantity of powder. This is usually of limited size (depending on the amount of powder involved), with the damage to buildings and personnel being relatively low. However, the shock waves created by this can cause powder deposits within the facility (on ledges, beams, tops of equipment, on the floor etc.) to be released into the air and this provides a ready source of fuel for the initial flame front to consume, resulting in the second and far more devastating stage. This latter explosion or series of explosions can rapidly progress throughout the factory, with catastrophic consequences.
The powder particle size is an important factor, and it is generally considered that this must be less than 420 ìm (i.e. will pass through an US #40 sieve) for an explosion to be initiated^. If the size is larger than 420 ìm the mass becomes sufficient to act as a heat sink and it will be difficult to cause ignition. However, once an explosion is in progression, larger particles will be readily consumed.
As would be expected, the composition of the powder particles is a strong determinant of the amount of energy released during an explosion. Figure 3 shows explosion test results for a range of materials under similar conditions.
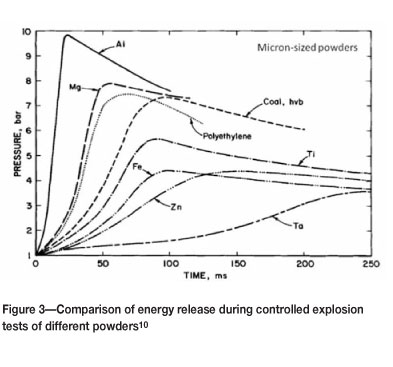
Aluminium can be seen to be the most reactive, followed by magnesium.
Unfortunately similar data for Zr, Ta, Hf, and Nb could not be found. However, comparison of maximum combustion enthalpies (Figure 4) would suggest that zirconium is slightly less reactive than titanium11. Conversely, the flame speed is much faster than even aluminium (Figure 5), despite zirconium having a coarser particle size12.
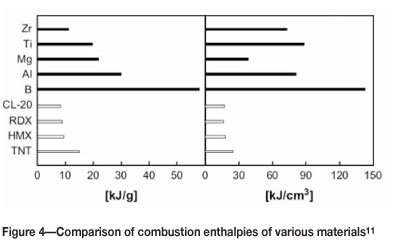
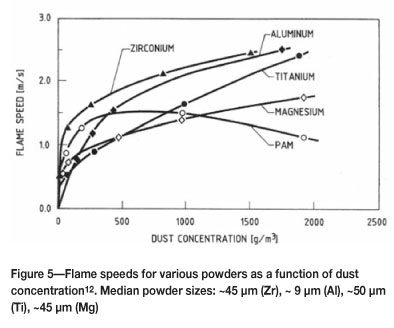
Hafnium appears to be more reactive than both zirconium and titaniumi3, but less so than magnesium14
Niobium is probably similar in reactivity to tantalum14
Powder characterization
Characterizing the powder that is being used is an important tool in understanding the potential fire or explosion risks. Various parameters have been explored in the literature, but the most important are the particle size range, the minimum explosion concentration, the minimum ignition energy, the minimum ignition temperature, the minimum auto-ignition temperature, and the dust deflagration index. Standard methods for determining these values have been established12,15-20. These parameters are discussed in the following sections.
Particle size
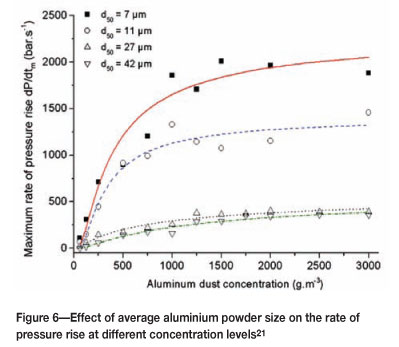
As mentioned earlier, there is a practical guideline that states the maximum particle size that will initiate an explosion is 420 ìm However as this size is reduced the reactivity increases markedly, and this is clearly shown in experimental work done on aluminium powder21,22.
Figure 6 indicates that as the average particle size decreases, the energy released (shown by the maximum rate of pressure rise) increases. A further factor is that less powder is required for a significant pressure rise to take place.
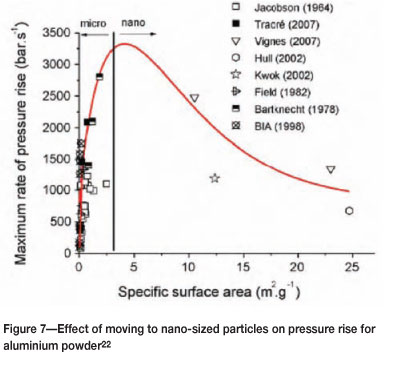
When the size is reduced further into the nanoscale range, there is a continued increase in the rate of pressure rise, but this then appears to reach a maximum value and subsequently decreases (Figure 7). The difficulty in measuring the actual effect of size in the nanometre range is the propensity for the particles to agglomerate, and this may be part of the reason for the decrease observed in Figure 7. Another reason may be the higher ratio of oxide to metal in the smaller particles, which would also reduce the reactivity.
As will be seen in the following sections, the powder particle size has a strong influence on all the other parameters.
Minimum explosion concentration
The minimum explosion concentration (MEC) is defined as the smallest amount of dust suspended in air, under a specific set of test conditions^, that will initiate an explosion. It is also referred to as the lowest explosibility limit (LEL).
Data for a variety of metal powders is given in Table I.
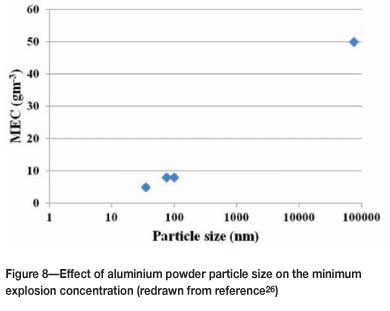
Significant variations in the minimum concentration limits are reported for the same metal. This can be due to a variety of reasons, e.g. different particle sizes, moisture levels, and degree of oxidation of the particles. The effect of particle size on the minimum concentration of aluminium can be seen in Figure 8.
Minimum ignition energy
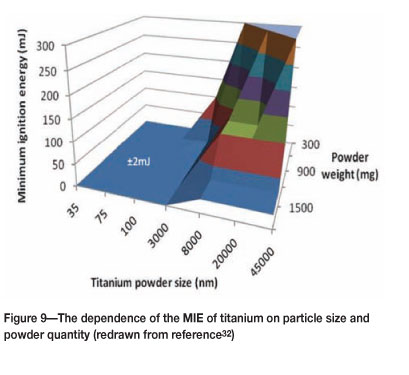
The minimum ignition energy (MIE) measures the ease of ignition of a dust cloud by electrical and electrostatic discharges. The test should be conducted under specific conditions that are detailed in the relevant standard17y MIE values for various metal powders are listed in Table II. Two of the key factors that determine the ignition energy of a dust cloud are the particle size and the quantity of powder present. This is illustrated for the case of titanium in Figure 9, where sizes of 3 ìm and less have a value of about 2 mJ. Large quantities of powder also have a significantly lower MIE.
Minimum ignition and auto-ignition temperature
The minimum ignition temperature (MIT) is the lowest surface temperature capable of igniting a powder or dust dispersed in the form of a dust cloud. This is relevant for defining the maximum operating temperature for electrical and mechanical equipment used in dusty environments. Some metal powders, however, are capable of self-ignition (auto-ignition), and it is obviously important to know the lowest temperature at which this occurs.
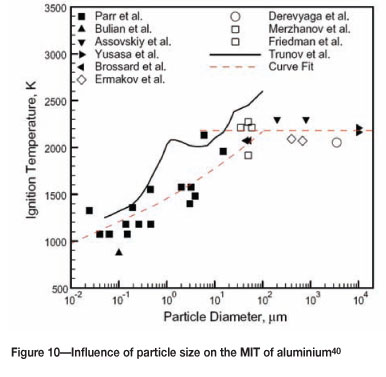
Table III lists these values for the metals of interest.
Again, reducing the particle size will increase the reactivity of the powder and consequently lower the MIT. Data for aluminium is shown in Figure 10.
Dust deflagration index
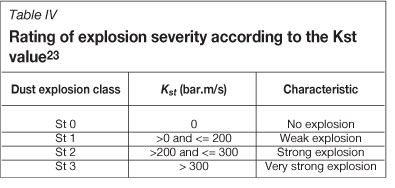
The Deflagration Index (KSt) is essentially the maximum rate of pressure rise generated when dust is tested in a confined enclosure, and provides an estimate of the anticipated violence of a dust explosion.
The formula used to calculate this parameter is21:
Kst= (dP/dt)max ÷ W where (dP/dt)max is the maximum rate of pressure rise (bar.s-i) and V is the volume of the testing chamber (m3). A 20-litre chamber is typically used as this gives comparable results to much larger chamber volumes that would occur in practice. However, these need to be geometrically similar for this index to be valid41.
The classification of explosion severity is according to the Table IV.
The dust deflagration index values for some metal powders are listed in Table V.
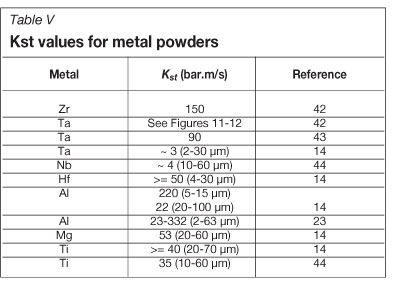
Table V shows some variation for particular metals and this could be due to a combination of factors that are known to influence the index value: the size of the particles, the quantity of powder, moisture level, initial powder temperature, igniter energy, and degree of particle oxidation44. Considerable variations in results are also known to occur when different test method are used41.
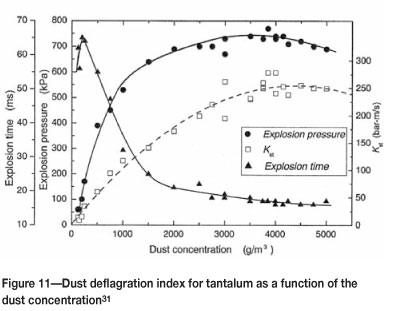
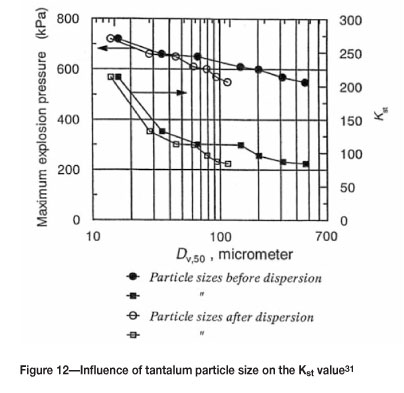
The effects of dust concentration and particle size on the values for tantalum are shown in Figures 11-12. Clearly the amount of powder present and the particle size have a large impact on the severity of the explosion.
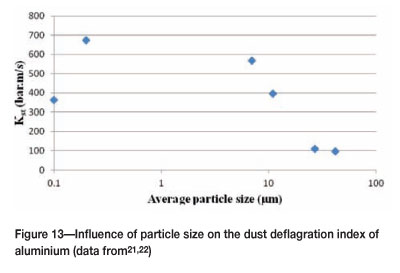
Reducing the particle size into the nanometre range appears to lower the Kst value, as shown for aluminium (Figure 13). This may be due to the agglomeration effect that was noted earlier.
Influence of moisture
The presence of moisture can increase the MIT, as shown for zirconium (Figure 14).
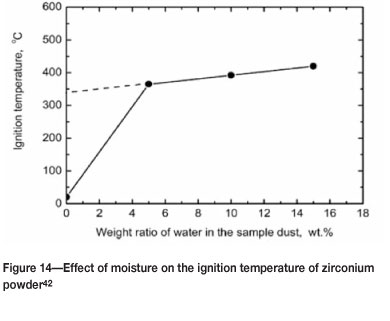
In view of this, safety guidelines for zirconium and hafnium stipulate that these powders should not be handled when dry or when the moisture content is less than 10-25 per cent by weight34,45.
Influence of inert elements
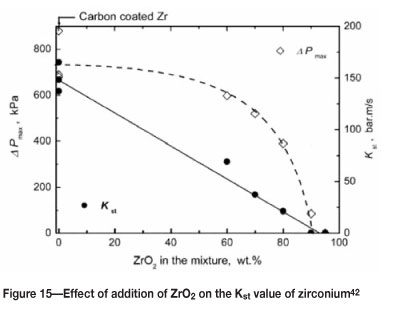
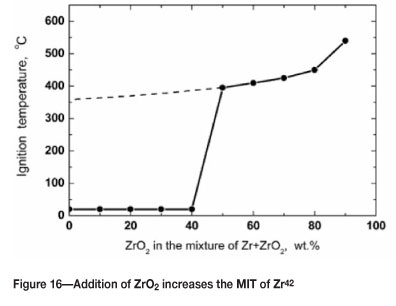
The addition of an inert element or compound can significantly reduce the reactivity of a metal powder. This may not be a feasible option during the processing of powders, but could be a consideration when disposing of powder waste. As noted earlier, the oxidation of powder particles can cause an inerting effect, particularly when the particle size is small. Figures 15-16 show the effect of adding ZrO2 to zirconium on the Kst and MIT values.
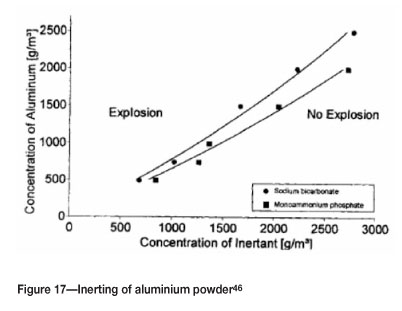
A further example is for aluminium, where additions of sodium bicarbonate and monoammonium phosphate increase the MEC (Figure 17).
The characterization parameters that have been discussed are vitally important for assessing the risk of fire or explosion. However, reliance on data from the literature is not recommended as there can be considerable variance in the reported test values, as has been seen. Furthermore, there may be little correlation between laboratory test conditions and the actual situation in a facility or factory. It is therefore essential to conduct tests on the actual powder (or powder combinations) being processed as well as simulate, as closely as possible, the real environment that is experienced (i.e. temperature, humidity, turbulence etc) in the working area. Having realistic parameter values is obviously important when suitable venting and containment systems need to be designed.
Health risks
Exposure to metal powders can have an impact on the health of personnel and is of particular concern when exposure is over a long term. However, understanding the influences of a particular powder on health is problematic, as individual sensitivities vary and predispositions for a particular illness can be triggered in one person while others are unaffected. Furthermore, personnel in industry are usually exposed to a multitude of dusts and other chemicals, and it may not be clear what has caused a particular effect.
Detailed toxicological investigations have not been conducted on all metal powders, and thus data is often limited to various ailments that have been reported for people working with that particular powder (amongs other things).
There are three ways that powders can interact with the body:
![]() Skin contact, which may lead to some form of irritation or allergic reaction (e.g. dermatitis). In the case of nano-powders, the particles can penetrate the skin and become absorbed into cells in various parts of the body, including the brain
Skin contact, which may lead to some form of irritation or allergic reaction (e.g. dermatitis). In the case of nano-powders, the particles can penetrate the skin and become absorbed into cells in various parts of the body, including the brain
![]() Eye contact, resulting in a mechanical irritation and cause damage
Eye contact, resulting in a mechanical irritation and cause damage
![]() Inhalation and either ingestion by swallowing or entering the lungs, resulting in possible breathing problems.
Inhalation and either ingestion by swallowing or entering the lungs, resulting in possible breathing problems.
In the case of inhalation, the size of the particles determines the level of penetration into the lungs (Figure 18).
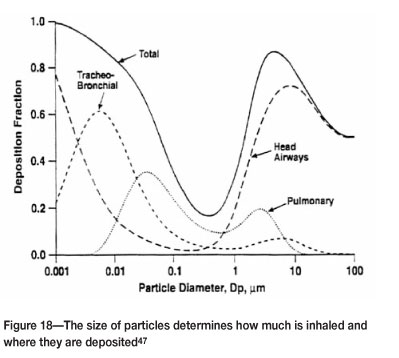
Nanometre sized particles are particularly concerning due to their ability to penetrate the skin and internal membranes, with the consequent potential to interfere with cellular activity. The realm of nanotechnology is still relatively new and it is too early to really know what the long-term effects will be. It appears that at this size range differences in powder composition may be of less importance, and that it is rather their presence as foreign objects in cells that causes the problem. However, more research needs to be done to understand the actual mechanisms involved. Nevertheless, there is some indication that nanoparticles (in general) can cause a variety of ailments, and this is summarized in Figure 19.
The following sections outline the known health aspects of the metal powders (in the micrometre scale) that are of interest to this conference.
Zirconium
Zirconium has no known biological role, and zirconium compounds are of low toxicity. Short-term exposure to zirconium powder can cause irritation, but only contact with the eyes requires medical attention. Inhalation of zirconium compounds can cause skin and lung granulomas. Zirconium aerosols can cause pulmonary granulomas49,50.
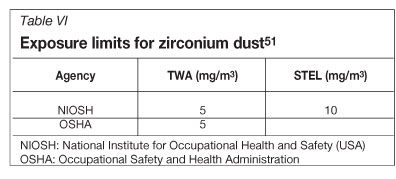
The exposure limits to dusts are usually specified in terms of a time-weighted average concentration for up to a 10-hour workday during a 40-hour workweek (TWA) and a short-term exposure limit (STEL). The values that have been set for zirconium are given in Table VI.
Tantalum
This is generally not considered toxic however one report indicates tantalum may be a non-specific, surface carcinogen (based on animal studies)35,52.
Repeated or prolonged exposure to tantalum alloy dust may lead to mild fibrosis and chronic rhinitis, and it may also play a role in producing 'hard metal pneumoconiosis'. Irritations to the eyes and skin have been observed.
The exposure limits for the USA and other countries are shown in Table VII.
Hafnium
As far as is known, the chemical, physical, and toxicological properties of hafnium metal have not been thoroughly investigated. Hafnium is a poison by unspecified route, but due to its low solubility in water it is not absorbed efficiently by ingestion. However many hafnium compounds are known to be poisonous54.
The following effects of exposure to hafnium have been noted37:
![]() Toxic by inhalation and may cause irritation to the nose, throat and mucous membranes
Toxic by inhalation and may cause irritation to the nose, throat and mucous membranes
![]() May cause irritation to the skin
May cause irritation to the skin
![]() May cause irritation to the eyes
May cause irritation to the eyes
![]() May cause damage to the liver.
May cause damage to the liver.
The exposure limits are given in Table VIII.

Niobium
The following effects have been reported55:
![]() On inhalation it may cause irritation of the mucous membranes. Inhaled particles may be retained in the lungs
On inhalation it may cause irritation of the mucous membranes. Inhaled particles may be retained in the lungs
![]() Metallic niobium has a low order of toxicity due to poor absorption from stomach and intestines
Metallic niobium has a low order of toxicity due to poor absorption from stomach and intestines
![]() May cause irritation to the skin
May cause irritation to the skin
![]() May cause transient, mechanical irritation to the eyes. Chronic eye exposure may cause conjunctivitis
May cause transient, mechanical irritation to the eyes. Chronic eye exposure may cause conjunctivitis
![]() Niobium crosses the placental barrier in animals. Niobium, when inhaled, is retained mainly in the lungs, and secondarily in bones. It interferes with calcium as an activator of enzyme systems56.
Niobium crosses the placental barrier in animals. Niobium, when inhaled, is retained mainly in the lungs, and secondarily in bones. It interferes with calcium as an activator of enzyme systems56.
The TWA and STEL values have not been set but there is an occupational exposure limit55 of 15 mg/m3.
Prevention of potential risks
There are four general principles that should guide the setting of procedures to prevent or at least minimize the fire/explosion and health risks (adapted from57-59). These are discussed in the following sections.
Prevent the suspension of powder particles in the air
![]() Ideally, work should be conducted in an oxygen-free atmosphere (i.e. glove box etc.)
Ideally, work should be conducted in an oxygen-free atmosphere (i.e. glove box etc.)
![]() When this is not possible, handling should be done with great care and within the working zone of a suitable dust-extraction system to prevent any fugitive dust from settling within the facility
When this is not possible, handling should be done with great care and within the working zone of a suitable dust-extraction system to prevent any fugitive dust from settling within the facility
![]() Special care must be taken when cleaning equipment, bench tops, etc. and particularly if an accidental spill occurs. No compressed air hoses should be used
Special care must be taken when cleaning equipment, bench tops, etc. and particularly if an accidental spill occurs. No compressed air hoses should be used
![]() The dust extraction or venting system must be designed to convey powders at concentrations below their MEC.
The dust extraction or venting system must be designed to convey powders at concentrations below their MEC.
Eliminate all sources of ignition in powder-handling areas
![]() No smoking or smoking materials, including lighters or matches, should be allowed in areas where powder or dust is present
No smoking or smoking materials, including lighters or matches, should be allowed in areas where powder or dust is present
![]() No flame, spark-producing or propellant-actuated tools or activities should be carried out in an area where a powder is present
No flame, spark-producing or propellant-actuated tools or activities should be carried out in an area where a powder is present
![]() Locate electric motors and as much electrical equipment as possible outside the processing areas
Locate electric motors and as much electrical equipment as possible outside the processing areas
![]() Ensure that there are no hot surfaces (e.g. heating pads, hotplates etc.) in the powder-handling area
Ensure that there are no hot surfaces (e.g. heating pads, hotplates etc.) in the powder-handling area
![]() Conductive and non-sparking tools must be used for handling and cleaning
Conductive and non-sparking tools must be used for handling and cleaning
![]() Bonding and grounding of machinery must be done to prevent static electricity buildup
Bonding and grounding of machinery must be done to prevent static electricity buildup
![]() All moveable equipment, such as drums, containers, and scoops must be bonded and grounded during powder transfer by the use of clips and flexible ground leads
All moveable equipment, such as drums, containers, and scoops must be bonded and grounded during powder transfer by the use of clips and flexible ground leads
![]() Personnel should also be grounded when working with powders, and the flooring in a facility should be suitably surfaced to minimize charge buildup
Personnel should also be grounded when working with powders, and the flooring in a facility should be suitably surfaced to minimize charge buildup
![]() During transfer, powder must not be poured or slid on nonconductive surfaces
During transfer, powder must not be poured or slid on nonconductive surfaces
![]() Work clothing should be made of smooth, hard-finished, closely woven fire resistant/fire retardant fabrics which tend not to accumulate static electric charges.
Work clothing should be made of smooth, hard-finished, closely woven fire resistant/fire retardant fabrics which tend not to accumulate static electric charges.
The static charge buildup from even small objects can be sufficient to ignite an explosion of fine powders. Table IX shows the typical static charge energies that can be generated for various items.
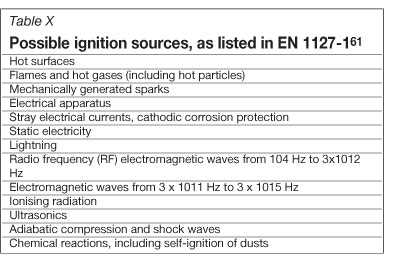
The European Standard EN 1127-1 lists the common ignition sources that have resulted in explosions or fires (Table X)61.
Limit the size of the fire or explosion
![]() There should be regular cleaning of the laboratory and equipment to prevent any buildup of dust
There should be regular cleaning of the laboratory and equipment to prevent any buildup of dust
![]() Work with the minimum amount of powder that is needed
Work with the minimum amount of powder that is needed
![]() Ensure powder containers are closed immediately after use
Ensure powder containers are closed immediately after use
![]() Store all containers (sealed) in a room separate from the handling areas. This room must comply with the necessary requirements for fire-proof storage. Longterm storage should be in argon-filled steel drums with tightly fitting clamp-on sealable lids. The metal powder should not be stored in areas containing flammable liquids or other combustible materials, due to the differences in fire-fighting methods
Store all containers (sealed) in a room separate from the handling areas. This room must comply with the necessary requirements for fire-proof storage. Longterm storage should be in argon-filled steel drums with tightly fitting clamp-on sealable lids. The metal powder should not be stored in areas containing flammable liquids or other combustible materials, due to the differences in fire-fighting methods
![]() Have fireproof containers readily available for disposal of any spilled or burning powders
Have fireproof containers readily available for disposal of any spilled or burning powders
![]() There should be no false ceilings or difficult-to-access sections (e.g. cabling conduits etc.) in the working or storage areas where dust could collect unnoticed
There should be no false ceilings or difficult-to-access sections (e.g. cabling conduits etc.) in the working or storage areas where dust could collect unnoticed
![]() The dust extraction system must also be cleaned and maintained on a regular basis
The dust extraction system must also be cleaned and maintained on a regular basis
![]() The clothing worn by workers should not have exposed cuffs, turn-ups, or pockets or other features where dust might accumulate.
The clothing worn by workers should not have exposed cuffs, turn-ups, or pockets or other features where dust might accumulate.
The US National Fire Protection Agency (NFPA) specifies immediate cleaning when a dust layer exceeds 0.4 mm (1/64 inch) over at least 5 per cent of the facility area (this includes the floor, overhead structures, equipment, counters etc.) 62. While this may be difficult to implement, it certainly emphasizes the seriousness of the potential risk.
Protect personnel from exposure
There must be provision of suitable clothing and equipment to protect personnel while working in a powder handling and processing area. These should include:
![]() respirators (with correct filters)
respirators (with correct filters)
![]() chemically resistant and fire-retardant laboratory coats/overalls
chemically resistant and fire-retardant laboratory coats/overalls
![]() gloves (both for fire protection but also to prevent skin contact)
gloves (both for fire protection but also to prevent skin contact)
![]() safety glasses/face shields with hair/head protection. These should remain in the working area to prevent carry-over of dust to other areas (offices etc.). Suitable storage and changing facilities would be required.
safety glasses/face shields with hair/head protection. These should remain in the working area to prevent carry-over of dust to other areas (offices etc.). Suitable storage and changing facilities would be required.
A programme of regular inspection, cleaning, replacement of filters, laundry of clothing etc. should be instituted.
Before leaving the facility, personnel should be required to wash their hands and faces and any other exposed areas to remove any powder residues.
Fire fighting procedures
Detailed procedures that are suitable for a particular facility should be sought from professional firefighters. However the following guidelines provide a starting point:
![]() It is extremely important that, should a fire start, there is a rapid response to deal with it as this can quickly escalate in intensity.
It is extremely important that, should a fire start, there is a rapid response to deal with it as this can quickly escalate in intensity.
![]() The burning powder should if possible be deposited into a suitable fire-proof container (appropriate nonsparking shovels etc must be available)
The burning powder should if possible be deposited into a suitable fire-proof container (appropriate nonsparking shovels etc must be available)
![]() If this is not feasible, then the correct extinguishant must be used (see Table XI). These must be kept close at hand and personnel should be trained in their use
If this is not feasible, then the correct extinguishant must be used (see Table XI). These must be kept close at hand and personnel should be trained in their use
![]() Once the fire has been dealt with to the best of their ability, personnel should leave the area after turning off equipment and closing any doors
Once the fire has been dealt with to the best of their ability, personnel should leave the area after turning off equipment and closing any doors
![]() Trained firefighters should then ensure that the fire is adequately dealt with and the area is safe for cleanup operations to take place
Trained firefighters should then ensure that the fire is adequately dealt with and the area is safe for cleanup operations to take place
![]() Care must be taken to thoroughly and safely clean the facility and personnel must wear protective equipment. The debris must also be collected and stored in a suitable container for appropriate disposal.
Care must be taken to thoroughly and safely clean the facility and personnel must wear protective equipment. The debris must also be collected and stored in a suitable container for appropriate disposal.
Conclusions
This paper has attempted to give an overview of the potential hazards that can arise when working with metal powders in general, but also with special reference to zirconium, tantalum, hafnium, and niobium.
There are well-established methods to characterize powders for their explosive potential and to provide limits for safe operation. These can be used to help establish and maintain a safe working environment.
The steps to characterize the potential risk as well as to prevent an explosion are summarized in Figure 20.
The impact of powders on health is difficult to quantify and it would be best to err on the side of caution by preventing exposure, as far as possible. This can be effectively achieved by the provision of suitable protective equipment as well as having properly designed venting systems installed.
Educating personnel on the risks and safe working procedures is also vitally important to avoid the occurrence of an explosive dust accident.
Table XII lists some pertinent standards relating to the prevention of metal powder fires and explosions.
References
1. Investigation Report, Combustible Dust Hazard Study, November 2006, accessed at: wwww.csb.gov/assets/document/Dust_Final_Report_ Website_11-17-06.pdf. [ Links ]
2. Combustible Dust Policy Institute. http://dustexplosions.blogspot.com/2011/01/legislators-more-regulation-is-comdust.html [ Links ]
3. US Chemical Safety Board website, www.csb.gov/investigations /investi-gations.aspx?Type=1&F_All=y. [ Links ]
4. Hought, J. www.baghouse.com/2011/03/09/one-dust-explosion-per-week-in-uk-and-2000-per-year-in-europe-says-new-study/ [ Links ]
5. Peckham, M. <http://techland.time.com/2011/05/24/combustible-dust-plant-explosion-may-cost-apple-500000-ipads/#ixzz1PKNoxPQD [ Links ]
6. US Chemical Safety Board report number 2004-01-I-IN: Aluminium Dust Explosion, www.csb.gov/assets/document/Hayes_Report.pdf. [ Links ]
7. Ostiguy, C., Soucy, B., Lapointe, G., Woods, C., Ménard, L., and Trottier, M. Health Effects of Nanoparticles, IRSST Report R-589, Second Edition, www.irsst.qc.ca/media/documents/PubIRSST/R-589.pdf [ Links ]
8. Industrial Fire Prevention web-site, http://industrialfireprevention. blogspot.com/2010/04/fire-triangle-and-fire-tetrahedron.html. [ Links ]
9. Cholin, J.M. http://www.oshainfo.gatech.edu/DustExplosions.pdf [ Links ]
10. Cashdollar, K.L. Journal of Loss Prevention in the Process Industries, vol. 13, 2000. pp. 183-199. [ Links ]
11. Dreizin, E. Progress in Energy and Combustion Science, vol. 35, 2009. pp. 141-167. [ Links ]
12. Eckhoff, R.K. Dust Explosions in the Process Industries, Third Edition, Gulf Professional Publishing, ISBN 0-7506-7602-7. [ Links ]
13. The Office of Health, Safety and Security, http://homer.ornl.gov/nuclearsafety/ns/techstds/standard/hdbk1081/hbk1081d.html [ Links ]
14. Hertzberg, M., Zlochower, I.A., and Cashdollar, K.L. Metal Dust Combustion: Explosion Limits, Pressures and Temperatures, Twenty Fourth Symposium (International) on Combustion, The Combustion Institute, 1992. pp. 1827-1835. [ Links ]
15. ASTM International, ASTM E2651 - 10 Standard Guide for Powder Particle Size Analysis, http://www.astm.org/Standard/index.shtml. [ Links ]
16. ASTM International, ASTM E1515 - 07 Standard Test Method for Minimum Explosible Concentration of Combustible Dusts, http://www.astm.org/Standard/index.shtml. [ Links ]
17. ASTM International, ASTM E2019 - 03(2007) Standard Test Method for Minimum Ignition Energy of a Dust Cloud in Air, http://www.astm.org/Standard/index.shtml. [ Links ]
18. ASTM International, ASTM E2021 - 09 Standard Test Method for Hot-Surface Ignition Temperature of Dust Layers, http://www.astm.org/Standard/index.shtml. [ Links ]
19. ASTM International, ASTM E1491 - 06 Standard Test Method for Minimum Auto-ignition Temperature of Dust Clouds, http://www.astm.org/Standard/index.shtml. [ Links ]
20. ASTM International, ASTM E1226 - 10 Standard Test Method for Explosibility of Dust Clouds, http://www.astm.org/Standard/index.shtml. [ Links ]
21. Dufaud, O., Traore', M., Perrin, L., Chazelet, S., and Thomas, D. Experimental investigation and modelling of aluminium dusts explosions in the 20 L sphere, Journal of Loss Prevention in the Process Industries, vol. 23, 2010. pp. 226-236. [ Links ]
22. Bouillard, J., Vignes, A., Dufaud, O., Perrin, L., and Thomas, D. Ignition and explosion risks of nanopowders, Journal of Hazardous Materials, vol. 181, 2010. pp. 873-880. [ Links ]
23. US National Fire Protection Association, NFPA 484: Standard for Combustible Metals, 2009, www.nfpa.org. [ Links ]
24. Cashdollar, K.L. and Zlochower, I.A. Explosion Temperatures of Metals and Other Elemental Dust Clouds, Proceedings of the Sixth International Symposium on Hazards, Prevention, and Mitigation of Industrial Explosions (Halifax, NS, Canada, Aug. 27 - Sept. 1, 2006). pp. 98-113 http://www.cdc.gov/niosh/mining/pubs/pdfs/etoma.pdf. [ Links ]
25. Chemetall, Precautionary Handling Advicefor Zirconium, Zirconium Hydride, Titanium, Titanium Hydride and Zr/Ni- Alloys in Powder Form, April 2010, http://www.specialmetals.chemetall.com/pdf/A0ECD7C3FC93C0CAC125771 F0025346F_0.pdf. [ Links ]
26. Lí, Q., Lin, B., Li, W., Zhai, C., and Zhu, C. Explosion characteristics of nano-aluminum powder-air mixtures in 20 L spherical vessels, Powder Technology vol. 212, iss. 2, 10 October 2011. pp. 303-309. [ Links ]
27. Randeberg, E. and Eckhoff, R.K. Measurement of minimum ignition energies of dust clouds in the <1 mJ region, Journal of Hazardous Materials, vol. 140, 2007. pp. 237-244. [ Links ]
28. OSHA, http://www.wfmma.org/wood-industry-resources/nep_combustibledust. pdf. [ Links ]
29. Crohmiq. http://crohmiq.com/mie-fibc-minimum-ignition-energy-antistatic-big-bags.html. [ Links ]
30. OSHS. http://www.osh.dol.govt.nz/order/catalogue/archive/staticelec-tricity.pdf [ Links ]
31. Matsuda, T. and Yamaguma, M. Tantalum dust deflagration in a bagfilter dust-collecting device, Journal of Hazardous Materials, vol. 77, 2000. pp. 33-42. [ Links ]
32. Wu, H-C., Chang, R-C., and Hsiao, H-C. Research of minimum ignition energy for nano Titanium powder and nano Iron powder, Journal of Loss Prevention in the Process Industries, vol. 22, iss. 1, January 2009. pp. 21-24. [ Links ]
33. Chemetall. www.specialmetals.chemetall.com/pdf/Zirconium_metal_ powde _AB_dry.pdf [ Links ]
34. ESPI Metals. http://www.espi-metals.com/msds's/Zirconium.htm [ Links ]
35. Centres for Disease Control and Prevention. http://www.cdc.gov/niosh/npg/npgd0585.html [ Links ]
36. Atlantic Equipment Engineers. http://micronmetals.thomasnet.com/Asset/HF-101-MSDS.doc [ Links ]
37. ESPI Metals. https://www.utdallas.edu/research/cleanroom/safety/msd/documents/hafnium.pdf. [ Links ]
38. Tico Titanium. http://www.ticotitanium.com/wp-content/themes/tico/pdfs/MSDS_Niobium_ Base_ Alloys _Solids.pdf. [ Links ]
39. Canadian Centre for Occupational Health and Safety. http://www.ccohs.ca/oshanswers/chemicals/chem_profiles/aluminum_powder/working_alu.html [ Links ]
40. Huang, Y., Risha, G.A., Yang, V., and Yetter, R.A. Effect of particle size on combustion of aluminum particle dust in air, Combustion and Flame, vol. 156, iss. 1, January 2009. pp. 5-13. [ Links ]
41. Abbasi, T. and Abbasi, S.A. Dust explosions-Cases, causes, consequences, and control, Journal of Hazardous Materials, vol. 140, 2007. pp. 7-44. [ Links ]
42. Matsuda, T., Yashima, M., Nifuku, M., and Enomoto, H. Some aspects in testing and assessment of metal dust explosions, Journal of Loss Prevention in the Process Industries, vol. 14, 2001. pp. 449-453. [ Links ]
43. Eastern Research Group, Inc., July 2011, http://www.osha.gov/dsg/combustibledust /expert_forum_summary_report.pdf. [ Links ]
44. Cadwallader, L.C. Dust Combustion Safety Issuesfor Fusion Applications, Idaho National Engineering and Environmental Laboratory, Bechtel BWXT, Idaho, LLC, May 2003. http://www.inl.gov/technicalpublications/Documents/2699859.pdf [ Links ]
45. OSHA. www.osha.gov/SLTC/hedthguidelines/hafmum/recogmtion.html [ Links ]
46. Dastidar, A.G., Amyotte, P.R., Going, J., and Chatrathi, K. Flamtnabüity limits of dusts: Minimum inerting concentrations, Process Safety Progress, vol. 18, no. 1, 1999. pp. 56-63. [ Links ]
47. McClellan, G., Rodriguez, J., and Millage, K. http://www.dtic.mil/ndia/2007cbis /wednesday/mcclellanWed1130.pdf [ Links ]
48. Buzea, C., Pacheo, I., and Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity, Biointerphases, vol. 2, iss. 4, 2007. pp. MR17-MR172. http://arxiv.org/ftp/arxiv/papers/0801/0801.3280.pdf. [ Links ]
49. Centres for Disease Control and Prevention. http://www.cdc.gov/niosh/npg/npgd0677.html [ Links ]
50. http://en.wikipedia.org/wiki/Zirconium. [ Links ]
51. Centres for Disease Control and Prevention. http://www.cdc.gov/niosh/idlh/7440677.html [ Links ]
52. Kerwien, S.C. Toxicity of tungsten, molybdenum, and tantalum and the environmental and occupational laws associated with their manufacture, use, and disposal. http://www.dtic.mil/cgi-bin/GetTRDoc?AD=ADA310298 & Location=U2&doc =GetTRDoc.pdf. [ Links ]
53. Cabot Corporation. http://wwww.cabot-corp.com/wcm/msds/en-gb/TA/TANTALUM-EUR-EN.pdf. [ Links ]
54. Centres for Disease Control and Prevention. http://www.cdc.gov/niosh/npg/npgd0309.html. [ Links ]
55. ESPI Metals. http://www.espi-metals.com/msds's/niobium.htm. [ Links ]
56. Lenntech. http://www.lenntech.com/periodic/elements/nb.htm#ixzz1REOn7nj1. [ Links ]
57. The Aluminum Association. Recommendations for storage and handling of aluminium powders and paste, 4th edition, 2006. [ Links ]
58.POULSEN, E. wvww.titanium.org/files/ItemFileA4631.pdf [ Links ]
59. Poulsen, E. Safety-Related Problems in the Titanium Industry in the Last 50 Years, JOM, vol. 52, no. 5, 2000. pp. 13-17. [ Links ]
60. Eckhoff, R.K. and Randeberg, E. Journal of Loss Prevention in the Process Industries, vol. 20, iss. 4-6, July-November 2007. pp. 396-401. [ Links ]
61. European Standard. EN 1127-1 'Explosive atmospheres - Explosion prevention and protection - Part 1: Basic concepts and methodology', 2007. [ Links ]
62. US National Fire Protection Association. NFPA 654: Standard for the Prevention of Fire and Dust Explosions from the Manufacturing, Processing, and Handling of Combustible Particulate Solids, www.nfpa.org. [ Links ]
63. National Fire Protection Agency, http://www.nfpa.org/ [ Links ]
64. Australian/New Zealand Standards, http://www.standards.org.au/ [ Links ]
65. South African National Standards, https://www.sabs.co.za/ [ Links ]
©The Southern African Institute of Mining and Metallurgy, 2012. SA ISSN2225-6253. This paper was first presented at the ZrTa2011 New Metals Development Network Conference, 12-14 October 2011, Mount Grace Country House & Spa, Magaliesburg.














