Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
African Entomology
On-line version ISSN 2224-8854
Print version ISSN 1021-3589
AE vol.31 Pretoria 2023
http://dx.doi.org/10.17159/2254-8854/2023/a13597
RESEARCH ARTICLE
Do thermal requirements of Dichrorampha odorata, a shoot-boring moth for the biological control of Chromolaena odorata, explain its failure to establish in South Africa?
Slindile B NqayiI, II; Costas ZachariadesII, III; Julie CoetzeeIV; Martin HillI; Frank ChidawanyikaV, VI; Osariyekemwen O UyiVII; Andrew J McConnachieVIII
ICentre for Biological Control, Department of Zoology and Entomology, Rhodes University, Makhanda, South Africa
IIPlant Health and Protection, Agricultural Research Council, Cedara, South Africa
IIISchool of Life Sciences, University of KwaZulu-Natal, Pietermaritzburg, South Africa
IVCentre for Biological Control, Botany Department, Rhodes University, Makhanda, South Africa
VDepartment of Zoology and Entomology, University of the Free State, Bloemfontein, South Africa
VIInternational Centre for Insect Physiology and Ecology (ICIPE), Nairobi, Kenya
VIIDepartment of Animal and Environmental Biology, University of Benin, Benin City, Nigeria
VIIIWeed Research Unit, New South Wales Department of Primary Industries, Biosecurity and Food Safety, Orange, Australia
ABSTRACT
Chromolaena odorata (L.) RM King and H Rob. (Asteraceae) has been subject to a biological control programme in South Africa for over three decades. A shoot-tip boring moth, Dichrorampha odorata Brown and Zachariades (Lepidoptera: Tortricidae), originating from Jamaica, was released as a biological control agent in 2013 but despite the release of substantial numbers of the insect, it has not established a permanent field population. Because climate incompatibility is a major constraint for classical biological control of invasive plants, and based on the differences in climate between Jamaica and South Africa and field observations at release sites, aspects of the thermal physiology of D. odorata were investigated to elucidate reasons for its failure to establish. Developmental time decreased with increasing temperatures ranging from 20 °C to 30 °C, with incomplete development for immature stages at 18 °C and 32 °C. The developmental threshold, t, was calculated as 8.45 °C with 872.4 degree-days required to complete development (K). A maximum of 6.5 generations per year was projected for D. odorata in South Africa, with the heavily infested eastern region of the country being the most eco-climatically suitable for establishment. The lower lethal temperature (LLT50) of larvae and adults was -4.5 and 1.8 °C, respectively. The upper lethal temperature (ULT50) for larvae was 39.6 °C whilst that of adults was 41.0 °C. Larvae thus had better cold tolerance compared to adults whereas adults had better heat tolerance compared to larvae. The critical thermal (CT) limits for adults were 3.4 ± 0.07 to 43.7 ± 0.12 °C. Acclimation at 20 °C for 7 days resulted in increased cold and heat tolerance with a CTmin. and CTmax of 1.9 ± 0.06 and 44.4 ± 0.07 °C respectively, compared to the relative control, acclimated at 25 °C. Acclimation at 30 °C improved neither cold (CTmin: 5.9 ± 0.08 °C) nor heat tolerance (CTmax: 42.9 ± 0.10 °C). These results suggest that thermal requirements fall within field temperatures and are thus not the main constraining factor leading to poor establishment of D. odorata in South Africa.
Keywords: biological invasions; climate; CLIMEX; developmental threshold; geographic range
INTRODUCTION
Classical weed biological control relies on the establishment and persistence of a natural enemy (chiefly insects, mites and fungal pathogens) introduced from the region of origin of the weed into the invaded range (Goolsby et al. 2013). Successful establishment of an insect as a biological control agent depends on a number of possible factors. Such factors include correct genetic matching of the target plant and biocontrol agent, and/or the ability of a population of the agent to persist from the introduction of a limited number of individuals (where stochastic events, dispersal and predation of the agent play a role). The biological control agent must have an ability to cope with the novel environment into which it is introduced (where climatic similarity to the native range, in concert with the agent's climatic adaptability, as well as the presence of novel predators, are important) (McFadyen 1998; van Driesche et al. 2009; Harms et al. 2020). Environmental temperature is one of the most important abiotic factors affecting the performance of insects (Terblanche et al. 2005; May and Coetzee 2013; Hough-Goldstein et al. 2016), including the establishment of insects as biocontrol agents in their region of release (Angilletta et al. 2002; Terblanche et al. 2005; Harms et al. 2021); other abiotic factors affecting the success of establishment of biocontrol agents include rainfall and humidity (Byrne et al. 2002; van Lenteren et al. 2006; Cowie et al. 2016; Muskett et al. 2020).
Well-established standard laboratory protocols enable researchers to link ambient temperature with development rate, and determine the optimal developmental temperature for insect species (Campbell et al. 1974; Wagner et al. 1991). These data can then be used to generate a degree-day model which can be mapped onto the country of introduction to predict the number of generations the insect will be able to complete in a year (Byrne et al. 2003; May and Coetzee 2013; Ramanand et al. 2017; Muskett et al. 2020). The more the generations, the higher the rate of population growth and likelihood of establishment (Muskett et al. 2020). The degree-day model can also be used to compare the number of predicted generations in the region of origin with that in the region of introduction (Muskett et al. 2020).
Similarly, the role of extreme temperatures on mortality and mobility of an insect species can be determined through standardised laboratory techniques. These involve the determination of both critical thermal limits (CT), where insects temporarily lose their locomotory ability, and lethal temperature limits (LT50), which predict the temperatures at which the insect will die (Uyi et al. 2017; Chidawanyika et al. 2020). Depending on the frequency and magnitude (compared to the CT and LT50 values) of extreme-temperature events in the field, they can have a significant impact on the likelihood of establishment of the insect (Chown and Terblanche 2007; Muskett et al. 2020). Finally, it has been shown that insects often display plasticity in their thermal tolerances, depending on factors such as photoperiod, acclimation, day temperature and rate of temperature change (Terblanche et al. 2007). For example, a change in thermal tolerances can be achieved through exposure to a temperature regime that is different from the norm for a few days in the laboratory prior to release (Chidawanyika et al. 2017).
Chromolaena odorata (L.) RM King and H Rob. (Asteraceae), commonly known as chromolaena or triffid weed, is native to the Americas, with a large range stretching from the southern United States of America to northern Argentina (Zachariades et al. 2009). It is also an invasive weed, with widespread populations in the humid tropics and subtropics of Africa, Asia and Oceania, including South Africa (Zachariades et al. 2009). However, the C. odorata population invading southern Africa is of a different biotype ('southern African biotype' - SAB) and genotype to that invading other parts of the Old World, and has an origin in the Greater Antilles, in particular Jamaica or Cuba (Zachariades et al. 2009; Paterson and Zachariades 2013; Shao et al. 2018). The weed was first reported as naturalised in KwaZulu-Natal (KZN) province, South Africa in the 1940s (Zachariades et al. 2011). Since then, the weed's distribution has expanded to include the warmer, higher-rainfall parts of Mpumalanga, Limpopo and Eastern Cape provinces (Fig 1a), as well as the neighbouring countries of Eswatini and Mozambique (Goodall and Erasmus 1996).
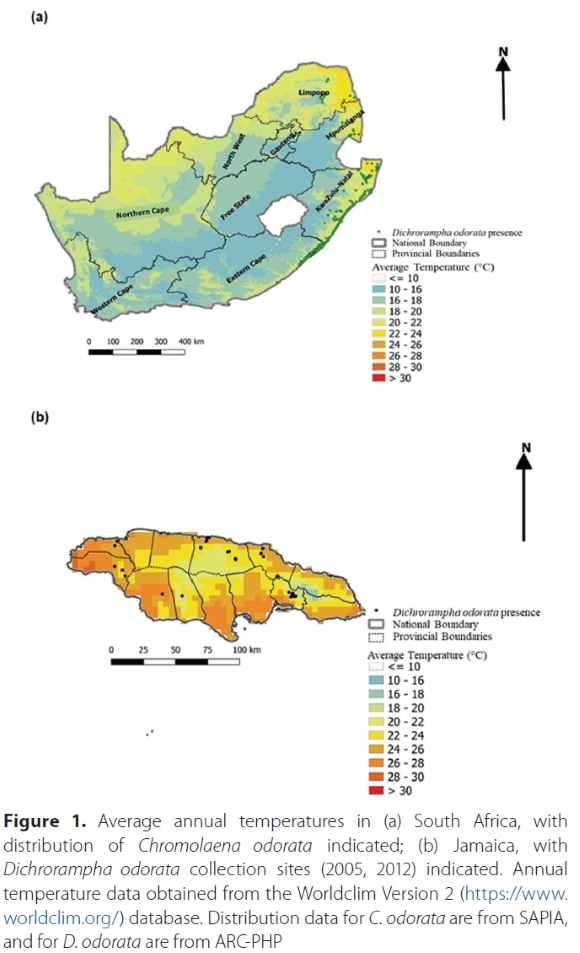
Since the start of the biocontrol programme in 1988, eight species of insects have been released as biocontrol agents against C. odorata in South Africa, of which two (a leaf-feeding moth, Pareuchaetes insulata (Walker) (Lepidoptera: Erebidae) and a leaf-mining fly, Calycomyza eupatorivora Spencer (Diptera: Agromyzidae), have established (Zachariades et al. 2021). A number of other candidate biocontrol agents have been investigated but not released (Zachariades 2021). The relatively low success rate of this programme with regards to releases and establishments can be ascribed, inter alia, to both incompatibility between genotypes of C. odorata from which natural enemies were collected and the SAB (for areas outside the Greater Antilles) (Zachariades et al. 2011), and poor climatic compatibility (for much of the native range of C. odorata, including the Greater Antilles) (Robertson et al. 2008). The climate of Jamaica, even at the altitudinal limit of C. odorata (850 m - ARC-PHP unpubl. data), differs substantially from that of the areas of southern Africa invaded by C. odorata. Köppen-Geiger climate classification of 130 predominantly Cfa (warm temperate climate, fully humid) and Cwa (warm temperate climate with dry winter) in areas in which C. odorata is present; at a latitude of around 18°, Jamaica falls within the humid tropics (Köppen-Geiger climate classification of Af and Am in areas in which C. odorata is present), and experiences higher rainfall and humidity, shorter dry seasons, and higher temperatures (Figure 1b). Moreover, projected differences in global models suggest that the SAB C. odorata has different climatic requirements compared to Jamaica and the rest of the world (Kriticos et al. 2005; Robertson et al. 2008). Minimum temperatures in particular differ markedly from those during the South African winter (Table 1; Nqayi 2019). Climatic mismatches in the native and invasive ranges of weeds are quite common, particularly regarding species with more tropical native ranges that invade temperate regions; and this can lead to problems in establishment of biocontrol agents (Harms et al. 2021). The SAB C. odorata was shown to be at the edge of its climatic tolerance in the colder and drier parts of its southern African range (te Beest et al. 2013). Nevertheless, the climatic differences between Jamaica and South Africa did not prevent the establishment of C. eupatorivora, collected in Jamaica, on C. odorata in South Africa.
Chromolaena odorata outcompetes surrounding vegetation partly through its rapid vertical growth rate during the rainy season (Zachariades et al. 2009). A tip-destroying biocontrol agent thus was deemed to be important in reducing this competitiveness. Several insect species within this niche were considered (Zachariades et al. 2011), including a stem-tip boring moth discovered in Jamaica and Cuba, Dichrorampha odorata Brown and Zachariades (Lepidoptera: Tortricidae) (Strathie and Zachariades 2004). A culture of this moth was collected in Jamaica in 2005, proved easy to rear under quarantine conditions, and was both host specific and damaging (Dube et al. 2017, 2019).
Dichrorampha odorata adults lay flimsy, scale-like eggs, stuck singly on the upper surface of young, fully-expanded C. odorata leaves (Dube et al. 2017, 2019; Nqayi 2019). Once the larvae hatch, they navigate to a nearby shoot tip and bore into it, killing the meristematic tissue. The larva causes the terminal 2-3 cm of the stem tip to swell slightly as it bores down (Dube et al. 2017). The mature larva exits the stem tip and pupates inside a leaf roll on a nearby leaf (Brown and Zachariades, 2007; Dube et al. 2017). The first releases of D. odorata in South Africa were made in 2013, and several thousand individual moths were subsequently released (mainly as pupae placed in a container hung on a tree branch) in KZN, Mpumalanga and Limpopo provinces, but the insect has not established (Zachariades et al. 2019). Because it is unlikely that there is incompatibility between the insect and the genotype of its host plant in southern Africa, and factors such as release effort were accounted for in Zachariades et al. (2019). Therefore we surmised that lack of establishment was most likely due to incompatibility in ecological climatic conditions of native region and introduced region. This study was thus undertaken to determine aspects of the thermal biology of a biocontrol agent, the shoot-tip boring moth (D. odorata), in South Africa. Specifically, we determined its lower and upper thermal limits, measured as critical thermal minima (CTmin) and maxima (CTmax), and lower and upper lethal temperatures (LLT50 and ULT50). We also investigated the developmental threshold of the shoot-tip boring moth and used these data to establish a degree-day model. This was used to predict the potential number of generations that D. odorata was capable of completing across C. odorata-invaded regions in South Africa. Although humidity and precipitation are also potential limiting climatic factors, these were not considered in this current study.
MATERIALS and METHODS
Study site and plants
The study was conducted in the quarantine facility of the Agricultural Research Council - Plant Health and Protection (ARC-PHP) laboratory, at Cedara in KZN province, South Africa. In order to rear host plants, fresh cuttings of C. odorata were collected from the field in Pietermaritzburg, KZN (-29.57719°, 30.33728°) and treated with SeradixTM (No. 1) rooting hormone before planting in a seedling tray with vermiculite as medium. The seedling tray was then placed in a heated mist-bed with fine sprinkler irrigation every 20 minutes under glasshouse conditions where temperature ranged between 22 °C and 27 °C.
The cuttings were kept under these conditions for approximately 2 weeks to allow for root development before transplanting them into plastic pots (25 cm diameter) with river sand and Gromor Potting Medium™ (Cato Ridge, South Africa) at 1:1 ratio. The potted plants were then grown in a greenhouse tunnel and hand-watered once daily. Two weeks after being potted out, 5 ml of Multicote 8TM (Haifa Group RSA (Pty) Ltd) fertiliser was added to each pot. Terminal shoot tips of the plants were nipped off to stimulate growth of dormant lateral buds. The plants were introduced to insects when plants were at least 25 cm tall, with the broad leaves favoured by the moths for laying their eggs.
Study organisms: insects and rearing conditions
All insects were sourced from an existing culture that was established in 2005 in the ARC-PHP quarantine facility. The moths were maintained in mesh-covered steel-framed cages (50 × 50 × 82 cm) in an insectary under controlled conditions (22-27 °C and 35-80% RH).
Thermal biology studies
Critical thermal limits and thermal acclimation
A programmable water bath (Haake C25P, Thermo Electro Corporation, Karlsruhe, Germany) connected to a series of double-jacketed holding chambers as standard equipment ('organ pipes') was used to determine critical thermal limits (CTmin and CTmax) following the methods outlined in Terblanche et al. (2008). Ten newly enclosed D. odorata adults were individually placed into the organ pipes, which were plugged with cotton wool to prevent the insects from escaping before the onset of each trial. The cotton wool was moistened with water to prevent dehydration of the adult moths. A thermocouple connected to a digital thermometer (Fluke Corporation, Australia Ltd., Sydney) was inserted into the control chamber to record the actual temperature experienced by adults in the chambers. The water bath was pre-set at 25 °C for 15 minutes, to allow adults to equilibrate with chamber temperatures. Next, temperatures were decreased (CTmin) or increased (CTmax) at a ramping rate of 0.25 °C per minute until all individuals within each trial had lost locomotory function (determined as the lack of ability to self-right upon mild prodding with a camel-hair brush) (Lachenicht et al. 2010). This rate was chosen as it is an ecologically relevant rate resembling temperature changes in the natural environment (e.g. Nyamukondiwa and Terblanche 2010; Chidawanyika et al. 2017). The temperatures at which each individual lost locomotory function were recorded. The experiment was replicated three times, using newly eclosed D. odorata insects. The CTmin and CTmax and their respective standard errors were calculated from these data.
To determine the effects of thermal acclimation on critical thermal limits, D. odorata pupae, harvested from the culture, were placed inside three growth chambers (LabCon, South Africa) set at 20 °C, 25 °C (control) and 30 °C for 7 days. After this acclimation period, the CTmin and CTmax of each group were assessed, as above. For each acclimation treatment, 10 adults were assessed, and each trial was replicated three times. Differences in critical thermal limits between each group were analysed using one-way ANOVAs in StatisticaTM version 13.2 (Tibco Software Inc.).
Lethal temperatures
Lower (LLT50) and upper lethal temperatures (ULT50) of adults and 6th instar larvae were assessed using a standard "plunge" protocol (Chidawanyika et al. 2017) using a programmable water bath (Haake C25P, Thermo Electro Corporation, Karlsruhe, Germany). Newly eclosed D. odorata adults and mature larvae (6th instar) were placed into plastic vials (25 insects per temperature treatment) in groups of 5 per vial, and the vial was sealed with moistened cotton wool to retain sufficient humidity. Vials (5 vials with 5 insects per vial) were placed inside a sealed Ziploc bag before being immersed in the water bath. The water bath was initially set at 25 °C for 15 minutes to ensure body temperature was in equilibrium with ambient temperature. The insects were then exposed to one of several set, constant temperature treatments for 2 h (larval high-temperature treatments = 30, 35, 38, 40, 42, 44 °C; larval low-temperature treatments = 10, 5, 0, -3, -6, -9 °C; adult high-temperature treatments = 33, 35, 38, 40, 42, 45 °C; adult low-temperature treatments = 10, 5, 2, 0 °C). A digital thermometer (Fluke Corporation, Australia Ltd., Sydney) connected to a type-K thermocouple was used to monitor the temperature inside the water bath to ensure that the desired temperature was achieved and maintained during the treatment. To avoid ice formation at sub-zero temperatures, 70% alcohol was used in the water bath. The same numbers of insects per temperature were kept at room temperature (25 °C) as controls.
During recovery following exposure to each temperature, the adults or larvae were placed in Petri dishes with filter paper together with shoot tips of C. odorata in a bouquet, to allow the surviving larvae to feed ad libitum and adults to perch. The Petri dishes were then placed in a growth chamber (Labcon, South Africa) set at constant 25 °C for 24 h and monitored for the survival of the insects. 'Survival' was considered as normal behaviour, i.e. walking or flying for adults and boring into shoot tips in the case of larvae. The effect of temperature on larval and adult survival was analysed using a Generalised Linear Model (GLM) and the LT50 (the temperature causing 50% of tested individuals to die in a given period (Li et al. 2011)) was calculated using SPSS (version 20.0; SPSS IBM, Chicago, USA) statistical software.
Developmental thresholds
Development time and survival of D. odorata were studied at seven constant temperatures: 15, 18, 20, 25, 27, 30 and 32 °C. All trials were performed in constant environment growth chambers (LabCon, South Africa) set at one of the above temperature treatments, with photoperiod set at 12L:12D. iButton dataloggers (model DS 1923, Maxim Integrated Products, San José, USA, 0.5 °C accuracy) were used to log microclimates (temperature and relative humidity) inside each temperature chamber, every hour, to ensure experimental temperatures were maintained.
Egg development
In order to obtain eggs for the development studies, six cages of 0.9 × 0.4 × 0.4 m, comprising a steel frame and fine gauze panelling, were placed in a quarantine glasshouse maintained at 20-30 °C. One C. odorata plant (~25 cm tall) was placed inside a cage. Two newly eclosed adults, consisting of one putative male (visually smaller in body size) and one putative female (visually larger in body size), were placed inside the cage with the plant for mating and oviposition. After 24 h of exposing adults to the plant to allow for oviposition, the adults were removed and placed back in the culture. The number of eggs laid (visible as white dots on the upper surface of leaves) was recorded for each plant and these were then placed into one of the seven growth chambers (with each growth chamber accommodating 8-12 plants) at the set experimental temperature. Plants in chambers were irrigated using BlumatTM (Tropf-Blumat, Germany) clay cones. Eggs were monitored daily and hatching date was recorded. At the lower temperatures (15, 18 and 20 °C), there was high egg mortality initially, as a result of lower humidity due to the cooling system in the chambers (RH = 26.9 ± 0.26% at low temperatures and 58.0 ± 1.46% at higher temperatures). Plants with eggs in these chambers were subsequently covered with porous plastic bags (280 mm × 400 mm × 30 μm (Range Plastic cc)) to decrease airflow around the plant and prevent eggs from dehydrating. Duration of egg development (number of days to hatching) was calculated for each egg at each temperature treatment.
Larval and pupal development
After hatching, neonate larvae migrated to the closest shoot tips and tunnelled inside C. odorata shoot tips. Damaged C. odorata shoots were dissected 10 days later to determine how many larvae were present in each shoot. Fresh shoot tips collected from stock plants were used to create a bouquet with 3-4 shoot tips, by cutting the terminal 70-80 mm of a shoot tip off the plant using secateurs and individually wrapping the base of each shoot tip with a piece of damp tissue paper and tin foil. Each bouquet was placed into a glass Petri dish (9 cm in diameter × 1 cm high) lined with filter paper moistened with a 2% sodium hypochlorite solution to prevent pathogen growth. Each larva that had been dissected from the shoot tips was placed onto its own separate C. odorata shoot-tip bouquet using a fine paint brush, to prevent competition. Larvae dissected from shoot tips readily bored into new tips on which they were placed. Petri dishes were placed inside plastic containers (36 cm × 26 cm × 8 cm) lined with moistened tissue paper and sealed with a lid to prevent leaf material from drying out from the airflow inside each chamber. Each plastic container with nine Petri dishes was placed in its respective growth chamber and larval development at each temperature was monitored daily. The bouquets were replaced once a week to provide fresh plant material for larvae - larvae were dissected out of the old shoot tips and placed onto the new shoot tips. The larval development period (number of days from hatching to pupation) was calculated, as was the pupal period (number of days from pupation to adult eclosion) and duration of adult survival at each temperature. One-way ANOVA was used to compare development time and survival between temperatures. A linear relationship between temperature and developmental rate was established for D. odorata using the reduced major axis regression method (Ikemoto and Takai 2000). This method graphs the product of developmental time and temperature (DT) against developmental time (D), follows a straight line equation (y = a + bx, where y = DT, a = K, b = t, and x = D) and does not require an estimation of standard error because its line parameters are the direct parameters, K (rate of development) and t (developmental threshold) (Ikemoto and Takai, 2000). StatisticaTM version 13.2 (Tibco Software Inc.) and SPSS Statistical software were used to analyse the data.
Degree-day calculations
The CLIMEX model database (CLIMEX v. 4, Hearne Scientific Software; Kriticos et al. 2015) was used to obtain daily maximum and minimum temperature records for locations throughout Africa, North America and the Caribbean. The parameters K and t were used to calculate the accumulated degree days (°D) for each location according to the equation below, where Tmax and Tminrepresent the maximum and minimum temperatures experienced, and t represents the lower developmental threshold for D. odorata:
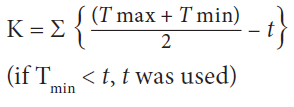
The available degree days were then calculated for each of the locations in Africa, North America and the Caribbean. This enabled the calculation of the number of generations that D. odorata is likely to complete in localities throughout Africa, North America and the Caribbean (where the South African C. odorata is thought to have originated). These data were mapped, using ArcGIS v. 10.2 (ArcMap function), to illustrate the likely suitability of these localities for the establishment and persistence of the moth.
Climate data
To better understand the potential climatic limitations on the establishment of D. odorata, microclimate data (temperature, humidity and rainfall) were recorded using iButton dataloggers (model DS 1923, Maxim Integrated Products, San José, USA, 0.5 °C accuracy) at four release sites where D. odorata was field-released in KZN province. The Worldclim database (Fick and Hijmans 2017) was used to obtain average annual temperatures in the invasive range of C. odorata in South Africa and the native range in Jamaica (Figure 1). We obtained the weather data for Jamaica from a reputable weather station which records and stores weather data in Jamaica and is used as the weather database source to determine average, minimum and maximum annual temperatures from one of the areas in which D. odorata was originally collected (Table 1).
RESULTS
Critical thermal limits and thermal acclimation
The mean CTmin and CTmax of D. odorata adults were 4.4 ± 0.22 °C and 43.7 ± 0.12 °C (mean ± SE, n = 30), respectively. Acclimation affected the CTmin and CTmax of D. odorata adults in all temperature treatments (20 °C, 25 °C, and 30 °C) (CTmin 1.95 ± 0.06 °C, 3.36 ± 0.07 °C and 5.92 ± 0.08 °C and CTmax 44.41 ± 0.07 °C, 43.67 ± 0.12 °C and 42.92 ± 0.09 °C), with CTmin being more responsive to cold acclimation (F2,87 = 818.87, p < 0.001).
Lethal temperatures
The temperatures that D. odorata was exposed to, and the life stage of the moth, significantly affected the survival of the insects both at lower and upper test temperatures (Table 2). As expected, exposure to increasingly lower or higher temperatures resulted in increased mortality (Figures 2 and 3). There was 100% mortality of larvae recorded at 9 °C and 44 °C, and at 0 °C and 45 °C for adults (Figures 2 and 3). The LLT50 of larvae and adults were determined to be -4.50 °C and 1.83 °C, respectively, and the ULT50 were 39.64 °C and 41.02 °C, respectively.
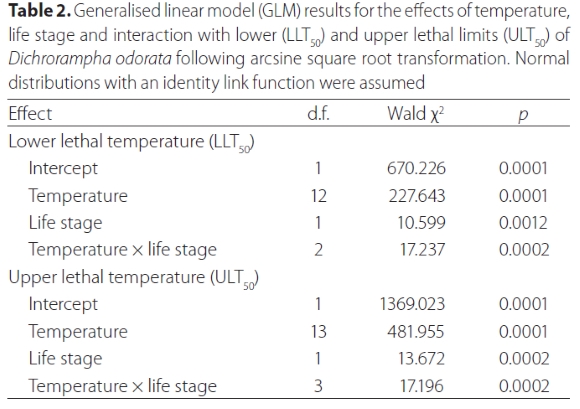
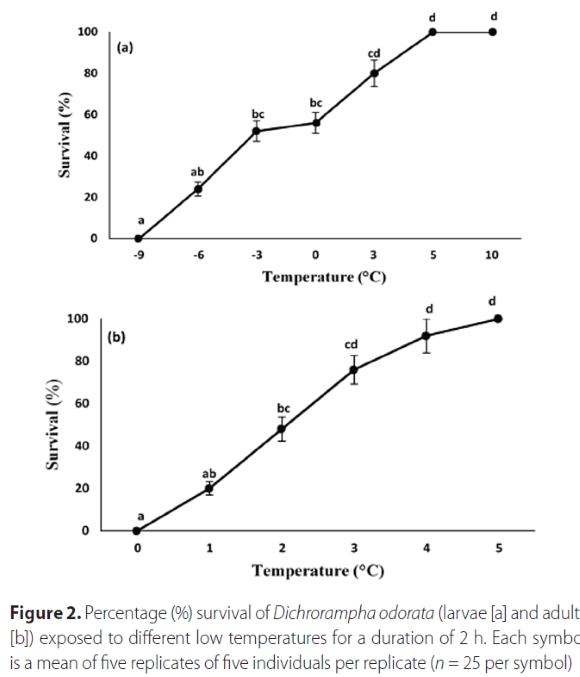
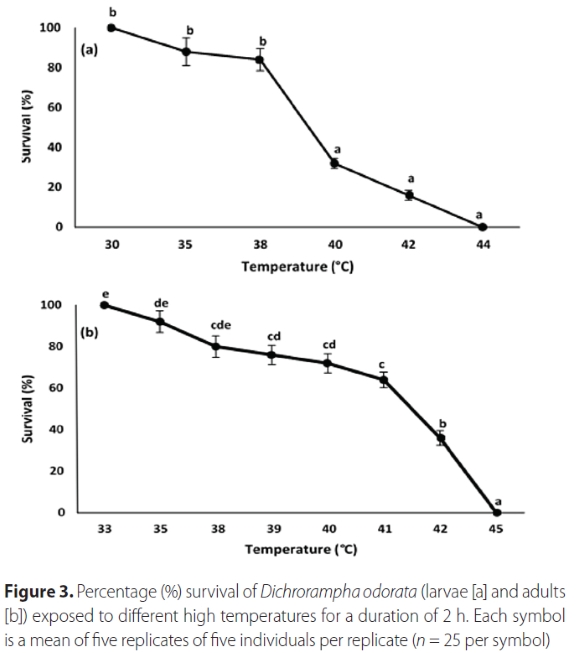
Lower developmental threshold (t) and rate of development (K)
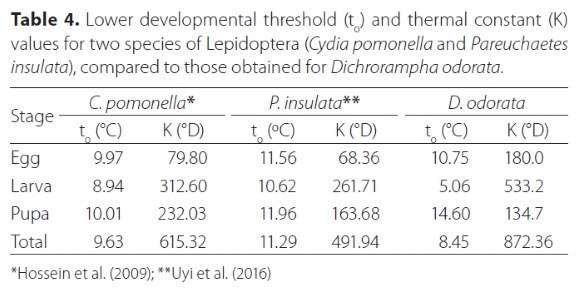
Dichrorampha odorata did not complete development at constant rearing temperatures of 15 °C (where no eggs hatched), 18 °C (where one egg hatched but the resultant larva died after 2 days), or 32 °C (where 21 eggs hatched but larvae died before pupation, after developing for 16.0 ± 1.8 days). Complete development occurred at the constant rearing temperatures of 20, 25, 27 and 30 °C (Table 3). Developmental time increased as temperatures decreased. Adult progeny from each test temperature were maintained at these temperatures; adults from the 20 °C trial lived longer (8.6 ± 0.97 days) than those from higher temperatures (F1,62 = 9.83; p = 0.001) (Table 3). Using the reduced major axis regression equation, the lower temperature threshold (to) for all stages combined was determined as 8.45 °C and the thermal constant (K) as 872.4 °D (y = 8.45x + 872.36; R2 = 0.98; p = 0.005) (Table 4), indicating that at the lower developmental threshold temperature of 8.45°C, the moth takes 872.4 days to complete development from egg to adult.
The percentage survival (combined immature stages) at 30 °C was significantly lower than that at any of the three other temperatures (F3,129 = 7.787, p < 0.001). There was no significant difference in survival of combined immature stages between 20, 25 and 27 °C. The highest overall survival (66.7%) was recorded at 27 °C, followed by 20, 25 and 30 °C (63.0, 61.1, 19.4% overall survival respectively). At this optimal temperature of 27 °C, total development time was 48 days (Table 3).
Degree-day model
The map generated by the degree-day model using CLIMEX weather station data suggested that D. odorata is capable of completing a maximum of 6.3 generations per year in South Africa, especially along the east coast where C. odorata is most prevalent (Figure 4), while in other parts of Africa, it is capable of completing a maximum of nine generations per year. In Jamaica, from where it was collected within its native range, D. odorata can complete a maximum of between 6.3 and 7.4 generations per year (Figure 5).of Jamaica indicated by arrow, and enlarged at top right.
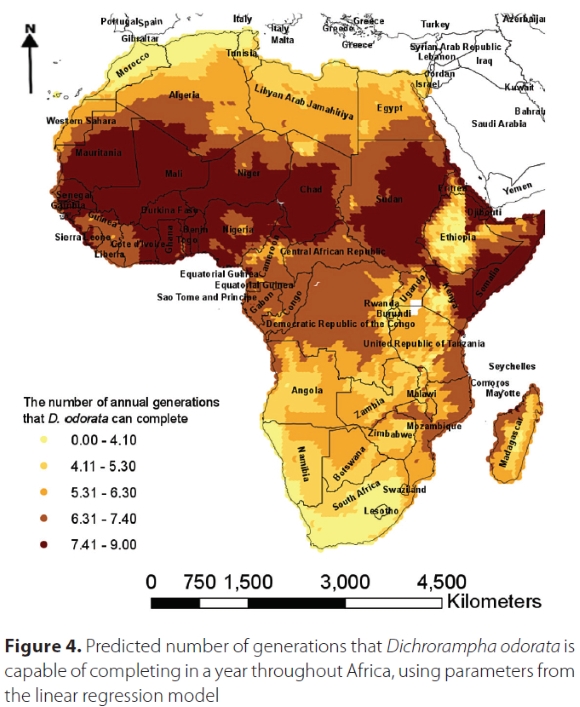
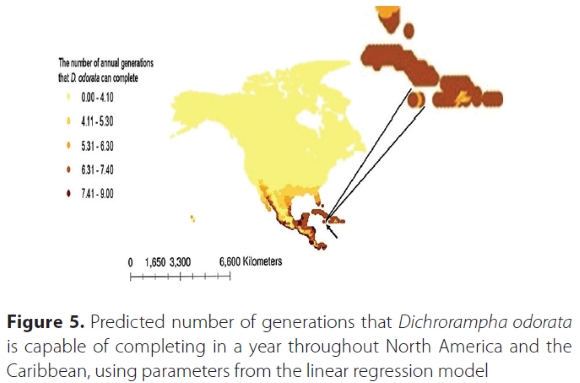
Climate data
Data collected at the four release sites (all situated along the coast of KZN province, South Africa), which were regarded as most climatically favourable in terms of temperature, humidity and rainfall, indicated that temperatures fell within the critical and lethal temperature limits of D. odorata recorded in this study. However, minimum temperature data recorded from a low-altitude (Kingston) weather station in Jamaica were considerably higher than those at the most climatically suitable sites in South Africa during winter (Table 1). Across the four South African release sites (all along the KZN coastline), daily mean minimum and maximum temperatures (averaged across 11 months) were 16.6 °C and 25.9 °C respectively. The equivalent temperatures from the weather station in Jamaica were 21.6 °C and 31.7 °C. This means that on average, minimum and maximum daily temperatures in South Africa were 5.0 °C cooler and 5.8 °C cooler, respectively, than those in Jamaica. Furthermore, the minimum daily temperature for the coldest month, averaged across the four D. odorata release sites in South Africa (11.6 °C), was 8.5 °C lower than that in Jamaica (20.1 °C). The maximum daily temperature for the warmest month in South Africa (29.0 °C) was 5.0 °C lower than that in Jamaica (34.0 °C).
DISCUSSION
Environmental temperature mediates development, activity, survival, population dynamics and ultimately geographic range of ectotherms including biocontrol agents (Angilletta et al. 2002; Terblanche et al. 2005; van Lenteren et al. 2006). In this study, adult critical thermal limits of D. odorata ranged between 4.4 and 43.7 °C. These temperatures fell within the mean thermal range of the targeted sites in South Africa, suggesting that both heat and cold conditions are suitable for D. odorata activity. Furthermore, the D. odorata adults assayed in this study showed capacity for adjustment of their thermal tolerance as both CTmin and CTmax improved to 1.95 and 44.4 °C, respectively, following acclimation, thereby underlying the suitability of the targeted habitats in South Africa for adult D. odorata. The temperatures that caused 100% adult mortality following acute exposure to static temperatures were 9 °C and 44 °C, beyond the mean annual temperatures.
Microhabitat temperatures in our study sites also fell within the thermal tolerance range of D. odorata. Overall, the lowest recorded temperature across the three release sites in South Africa was 12 °C whilst the highest was 34.5 °C. Given that 100% mortality following cold exposure in adults was realised at -9 °C, it is highly unlikely that the mild winter temperatures in the targeted areas may be lethal or limit adult activity. Moreover, because the host plant, C. odorata, is frost-intolerant (Goodall and Erasmus 1996), it does not grow in areas which reach close to freezing point, and none of the sites at which D. odorata has been released reach temperatures at which the LLT50 would be experienced by either the larvae or adults. There is a slim possibility that some sites may reach ULT50 temperatures, but this is probably a rare event (Muskett et al. 2020). The CTs of D. odorata adults indicate they should remain active across the range of temperatures experienced in the field.
The larvae of D. odorata were more tolerant to cold than adults, while adults were slightly more tolerant to heat than larvae. Other studies have reported that thermal tolerance in insects varies significantly between life stages (Marais et al. 2009; Uyi et al. 2017). In general, less- or non-mobile life stages such as larvae are more tolerant to thermal extremes as compensation for poor behavioural responses, such as flight from potentially deleterious environments (Mutamiswa et al. 2019). It is interesting to note that in this study this assertion was only supported under low temperature test conditions whilst the converse was true for high temperature test conditions.
Apart from survival and critical limits for activity, thermal parameters such as lower developmental threshold and number of degree-days are important indices for determining potential establishment and geographic distribution of biocontrol agents (Manrique et al. 2008; May and Coetzee 2013). In our study, developmental rate (time taken for the insect to go through all of its developmental stages) of D. odorata increased with temperature, but was halted beyond 30 °C. Microhabitat data revealed several days where temperatures exceeded 30 °C, which can compromise development and ultimately population establishment. It is therefore plausible that maximum temperatures in the field may be limiting establishment through impaired development. Moreover, typical heat stress in insects, even at acute time-scales, can lead to irreversible protein damage (Chown and Nicholson 2004).
In this study, only one egg hatched at 18 °C, and none at 15 °C. Surprisingly, these lower temperatures allowed larval development to pupation, albeit at a slow rate. This therefore suggests that the thermal sensitivity of D. odorata varies with ontogeny, and that poor egg hatch and subsequent slow development at lower temperatures may impede establishment in the targeted sites. However, the eggs were exposed to the lower experimental temperatures (15 °C and 18 °C) for their entire development, which is not typical in the field where temperatures fluctuate. Muskett et al. (2020) similarly recorded poor egg hatch and immature survival at constant temperatures below 20 °C for Catorhintha schaffneri Barilovsky and Garcia (Hemiptera: Coreidae), a biocontrol agent for Pereskia aculeata Miller (Cactaceae) in a similar invaded range as C. odorata, but this did not prevent the insect's establishment in South Africa.
Weather station data from areas invaded by C. odorata, as well as microclimate data from 'optimal' release sites, indicate that both adults and larvae (especially 20 day-old-larvae) should survive and maintain mobility at short time-scales. However, although average annual temperatures at some of the D. odorata collection sites in Jamaica are similar to those at some of the release sites in South Africa. The mean daily minimum temperatures of the coldest months at four coastal release sites (where minimum temperatures are highest) are more than 5 °C lower than those of Shortwood, Jamaica, the island on which D. odorata was originally collected. Even at higher-altitude C. odorata sites in Jamaica (Gordon Town at 345 m and Mavis Bank at 884 m), minimum temperatures were consistently several degrees higher on average than winter minimums in South Africa (Nqayi 2019). These sustained cold temperatures could have serious implications for persistence in the field. The neonate larvae may have higher thermal tolerance limits than older larvae. Given that the adults of this moth lay eggs on the leaves, neonates may be exposed to unfavourably low temperatures for a longer duration in the field as they must move to find suitable shoots for feeding. The neonates also spend time boring into the shoots of their host plant. The fact that the adults are nocturnal and must find mates at night when temperatures are low is also of ecological concern. Typically, the consequences of exposing insects to unfavourably low temperatures over a long time-scale may result in slow development, reduced mobility, exposure to natural enemies, reduced mating potential, and/or increased mortality (Lachenicht et al. 2010; Uyi et al. 2017). Uyi et al. (2017) found that the minimum temperatures in Florida, USA, where Pareuchaetes insulata (Walker) (Lepidoptera: Erebidae) was collected, remained substantially higher than winter temperatures in South Africa, and that the locomotive ability of the nocturnally feeding larvae of this species was impaired at temperatures below 11 °C. Although this did not prevent the establishment of P. insulata, the authors attributed the variable performance of the moth in South Africa to these factors. It is surmised that the leaf-mining fly C. eupatorivora, also collected from C. odorata across Jamaica, may have established, inter alia, because it has diurnally active adults whose activities are less affected by low night-time temperatures than those of D. odorata.
Other moth species with similar developmental thresholds to that of D. odorata are able to survive in similar or cooler habitats. Hossein et al. (2009), working on Cydia pomonella L. (Lepidoptera: Tortricidae), a widespread pest of deciduous fruit in temperate regions, recorded a slightly higher (9.97 °C) developmental temperature threshold than that of D. odorata (8.45 °C) in our study. It is interesting to note, however, that the thermal constant (K-value) for D. odorata was higher than those of C. pomonella and P. insulata (Table 4). Perhaps this long development period at the lower development temperature threshold (18 times longer than at the optimal developmental temperature of 27 °C) helps explain the poor establishment record of D. odorata in South Africa.
The CLIMEX degree-day model for D. odorata in areas where C. odorata is invasive in South Africa indicates that the moth should be able complete a maximum of 6.5 generations per year, which is only slightly lower than in Jamaica where the insect was collected. However, factors other than temperature may play a role in the lack of establishment of D. odorata in South Africa. The eggs are extremely thin and fragile, and die if the leaf on which they have been deposited is removed from the parent plant, even at a late stage of the egg's development. This may indicate that D. odorata eggs are very susceptible to changes in the microclimate of the leaf surface (Pincebourde and Woods 2012) which may be induced by decreases in atmospheric humidity. In the heated mass-rearing glasshouse at ARC-PHP Cedara, a high proportion of eggs has been noted to die during winter, when RH is low (SB Nqayi, pers. obs.).
In summary, this study indicated that the aspects of the thermal biology ofthe shoot-boring moth D. odorata investigated do not explain its lack of establishment on C. odorata in South Africa, especially along the east coast. However, this lack of establishment could also be due to one or more of several factors not yet investigated: (1) the vulnerability of juvenile stages to cold and other factors such as humidity; (2) possible reduced feeding by older nocturnal larvae during night-time low temperatures; (3) reduced mating and reproductive activities of adult moths during night-time low temperatures in winter months; (4) susceptibility of adults to low humidity; for cold and (5) an increased impact of predation and/or parasitism on survival due to slower development rates at cooler temperatures. Future studies should focus on exploring factors that were not studied in the current study. Selection tolerance during rearing, especially among the juvenile stages, may also be prioritised and investigated to improve field establishment; collection of fresh field material from high-altitude and if possible drier areas of Jamaica and Cuba may also improve establishment prospects (Harms et al. 2021). Similar cases, where weeds invade more temperate habitats than the climatic limits of their native range, are quite common, and may affect the establishment and performance of biocontrol agents released against them in these areas (Harms et al. 2021). However, this also depends on the biology of the species of biocontrol agent; should establishment of D. odorata fail in South Africa, other biocontrol agents with a more robust biology are available. For example, the stem-galler Polymorphomyia basilica Snow (Diptera: Tephritidae), also collected in Jamaica, was recently approved for release following host-range testing (Dube et al. 2020); and given that adults are diurnally active and long-lived, it is hoped that establishment will occur. Finally, D. odorata remains an option for more tropical, humid parts of the invasive range of C. odorata, should the need for additional biocontrol agents in these areas arise.
ACKNOWLEDGEMENTS
The authors would like to thank the Department of Forestry, Fisheries and the Environment: Natural Resource Management Programmes for funding research on the biocontrol of Chromolaena odorata at ARC-PHP, and Rhodes University for financial support of the first author. Thanks to the ARC-PHP staff at Cedara: Sthembiso Dlomo, Nompilo Mhlongo, Derrick Nkala and Samora Mqolombeni, for technical assistance, and insect and plant culture maintenance. We thank Ms Pippa Muskett for assistance with mapping. The Meteorological Service of Jamaica is thanked for provision of data. Lastly, we thank anonymous reviewers for their constructive comments which helped to improve the manuscript.
ORCID IDS
Slindile B Nqayi - https://orcid.org/0000-0002-1645-2859
Costas Zachariades - https://orcid.org/20000-0002-5855-9558
Julie Coetzee - https://orcid.org/0000-0002-0364-3349
Martin Hill - https://orcid.org/0000-0003-0579-5298
Frank Chidawanyika - https://orcid.org/0000-0002-4601-768X
Osariyekemwen O Uyi - https://orcid.org/0000-0001-5268-4676
Andrew J McConnachie - https://orcid.org/0000-0002-2947-4408
REFERENCES
Angilletta JRMJ Jr, Niewiarowski PH, Navas CA. 2002. The evolution of thermal physiology in ectotherms. Journal of Thermal Biology 27(4): 249-268. https://doi.org/10.1016/S0306-4565(01)00094-8 [ Links ]
Brown WJ, Zachariades C. 2007. A new species of Dichrorampha (Lepidoptera: Tortricidae: Grapholitini) from Jamaica: a potential biocontrol agent against Chromolaena odorata (Asteraceae). Proceedings of the Entomological Society of Washington 109: 938-947. [ Links ]
Byrne MJ, Currin S, Hill MP. 2002. The influence of climate on the establishment and success of the biocontrol agent Gratiana spadicea, released on Solanum sisymbriifolium in South Africa. Biological Control 24(2): 128-134. https://doi.org/10.1016/S1049-9644(02)00021-X [ Links ]
Byrne MJ, Coetzee J, Mcconnachie AJ, Parasram W, Hill MP. 2003. Predicting climate compatibility of biological control agents in their region of introduction. In: Cullen Briese JM, Kriticos DT, Lonsdale DJ, Morin WM, Scott JK. (eds). Proceedings of the XI International Symposium on Biological Control of Weeds, CSIRO Entomology, Canberra, Australia.
Campbell A, Frazer BD, Gilbert N, Gutierrez AP, Mackauer M. 1974. Temperature requirements of some aphids and their parasites. Journal of Applied Ecology 11(2): 431-438. https://doi.org/10.2307/2402197 [ Links ]
Chidawanyika F, Nyamukondiwa C, Strathie L, Fischer K. 2017. Effects of thermal regimes, starvation and age on heat tolerance of the parthenium beetle Zygogramma bicolorata (Coleoptera: Chrysomelidae) following dynamic and static protocols. PLoS One 12(1): e0169371. https://doi.org/10.1371/journal.pone.0169371 [ Links ]
Chidawanyika F, Chikowore G, Mutamiswa R. 2020. Thermal tolerance of the biological control agent Neolema abbreviata and its potential geographic distribution together with its host Tradescantia fluminensis in South Africa. Biological Control 149: 104315. https://doi.org/10.1016/j.biocontrol.2020.104315 [ Links ]
Chown SL, Nicolson SW. 2004. Insect Physiological Ecology: Mechanisms and Patterns. New York, USA: Oxford Press. https://doi.org/10.1093/acprof:oso/9780198515494.001.0001. [ Links ]
Chown SL, Terblanche JS. 2007. Physiological diversity in insects: Ecological and evolutionary contexts. Advances in Insect Physiology 33: 50-152. https://doi.org/10.1016/S0065-2806(06)33002-0 [ Links ]
Cowie BW, Venturi G, Witkowski ET, Byrne MJ. 2016. Does climate constrain the spread of Anthonomus santacruzi, a biological control agent of Solanum mauritianum, in South Africa? Biological Control 101: 1-7. https://doi.org/10.1016/j.biocontrol.2016.06.005 [ Links ]
Dube N, Zachariades C, Munyai TC, Uyi OO. 2017. Laboratory studies on the biology and host range of Dichrorampha odorata (Lepidoptera: Tortricidae), a biological control agent for Chromolaena odorata (Asteraceae). Biocontrol Science and Technology 27(2): 222-236. https://doi.org/10.1080/09583157.2016.1274879 [ Links ]
Dube N, Uyi O, Zachariades C, Munyai TC, Whitwell M. 2019. Impact of the shoot-boring moth Dichrorampha odorata (Lepidoptera: Tortricidae) on growth and reproductive potential of Chromolaena odorata (Asteraceae) in the laboratory. Biocontrol Science and Technology 29(4): 350-364. https://doi.org/10.1080/09583157.2018.1562038 [ Links ]
Dube N, Zachariades C, Uyi O, Munyai TC. 2020. Life history traits and host suitability of a gallforming fly, Polymorphomyia basilica (Diptera: Tephritidae), for the biological control of Chromolaena odorata (Asteraceae) in South Africa. Arthropod-Plant Interactions 14(2): 237-250. https://doi.org/10.1007/s11829-019-09731-x [ Links ]
Fick SE, Hijmans RJ. 2017. WorldClim 2: new 1 km spatial resolution climate surfaces for global land areas. International Journal of Climatology 37(12): 4302-4315. https://doi.org/10.1002/joc.5086 [ Links ]
Goodall JM, Erasmus DJ. 1996. Review of the status and integrated control of the invasive alien weed, Chromolaena odorata, in South Africa. Agriculture, Ecosystems and Environment. 56(3): 151-164. https://doi.org/10.1016/0167-8809(95)00647-8 [ Links ]
Goolsby JA, Racelis AE, Goolsby JB, Kirk AA, Cristofaro M, Grusak MA, De Leon AP. 2013. Evaluation of biogeographical factors in the native range to improve the success of biological control agents in the introduced range. Biocontrol Science and Technology 23(10): 1213-1230. https://doi.org/10.1080/09583157.2013.822848 [ Links ]
Harms NE, Cronin JT, Diaz R, Winston RL. 2020. A review of the causes and consequences of geographical variability in weed biological control successes. Biological Control 151: 104398. https://doi.org/10.1016/j.biocontrol.2020.104398 [ Links ]
Harms NE, Knight IA, Pratt PD, Reddy AM, Mukherjee A, Gong P, Coetzee J, Raghu S, Diaz R. 2021. Climate mismatch between introduced biological control agents and their invasive host plants: improving biological control of tropical weeds in temperate regions. Insects. 12(6): 549. https://doi.org/10.3390/insects12060549 [ Links ]
Hossein RA, Yaghoub F, Gholamreza R, Mohammadreza R. 2009. Temperature-dependent development and temperature thresholds of codling moth (Lepidoptera: Tortricidae) in Iran. Entomological Society of America 38: 885-895. [ Links ]
Hough-Goldenstein J, Lake EL, Shropshire KJ, Moore RA, D'Amico V. 2016. Laboratory and field-based temperature-dependent development of a monophagous weevil: implications for integrated weed management. Biological Control 92: 120-127. https://doi.org/10.1016/j.biocontrol.2015.10.009. [ Links ]
Ikemoto T, Takai K. 2000. A new linearized formula for the law of total effective temperature and the evaluation of line-fitting methods with both variables subject to error. Environmental Entomology 29(4): 671-682. https://doi.org/10.1603/0046-225X-29.4.671 [ Links ]
Kriticos DJ, Yonow T, Mcfadyen RE. 2005. The potential distribution of Chromolaena odorata (Siam weed) in relation to climate. Weed Research 45(4): 246-254. https://doi.org/10.1111/j.1365-3180.2005.00458.x [ Links ]
Kriticos DJ, Maywald GF, Yonow T, Zurcher EJ, Herrmann NI, Sutherst RW. 2015. CLIMEX Version 4: Exploring the Effects of Climate on Plants, Animals and Diseases. Canberra, ACT, Australia: CSIRO. [ Links ]
Lachenicht W, Clusella-Trullas S, Boardman L, Le Roux C, Terblanche JS. 2010. Effects of acclimation temperature on thermal tolerance, locomotion performance and respiratory metabolism in Acheta domesticus L. (Orthoptera: gryllidae). Journal of Insect Physiology. 56(7): 822-830. https://doi.org/10.1016/j.jinsphys.2010.02.010. [ Links ]
Manrique V, Cuda JP, Overholt WA, Diaz R. 2008. Temperature-dependent development and potential distribution of Episimus utilis (Lepidoptera: Tortricidae), a candidate biological control agent of Brazilian peppertree in Florida. Environmental Entomology 37: 862-870. https://doi.org/10.1093/ee/3Z4.862. [ Links ]
Marais E, Terblanche JS, Chown SL. 2009. Life stage-related differences in hardening and acclimation of thermal tolerance traits in the kelpfly, Paractora dreuxi (Diptera, Helcomyzidae). Journal of Insect Physiology 55(4): 336-343. https://doi.org/10.1016/j.jinsphys.2008.11.016 [ Links ]
May B, Coetzee J. 2013. Comparisons of the thermal physiology of water hyacinth biological control agents: predicting establishment and distribution pre- and post-release. Entomologia Experimentalis et Applicata 147(3): 241-250. https://doi.org/10.1111/eea.12062 [ Links ]
McFadyen REC. 1998. Biological control of weeds. Annual Review of Entomology 43(1): 369-393. https://doi.org/10.1146/annurev.ento.43.1.369 [ Links ]
Muskett PC, Paterson ID, Coetzee JA. 2020. Ground-truthing climate-matching predictions in a post-release evaluation. Biological Control 144: 104217. https://doi.org/10.1016/j.biocontrol.2020.104217 [ Links ]
Mutamiswa R, Machekano H, Chidawanyika F, Nyamukondiwa C. 2019. Life-stage related responses to combined effects of acclimation temperature and humidity on the thermal tolerance of Chilo partellus (Swinhoe) (Lepidoptera: crambidae). Journal of Thermal Biology 79: 85-94. https://doi.org/10.1016/j.jtherbio.2018.12.002 [ Links ]
Nqayi SB. 2019. Climatic suitability of Dichrorampha odorata Brown and Zachariades (Lepidoptera: Tortricidae), a shoot-boring moth for the biological control of Chromolaena odorata (L.) R.M. King and H. Robinson (Asteraceae) in South Africa [MSc thesis]. Rhodes University, Makhanda, South Africa. [ Links ]
Nyamukondiwa C, Terblanche JS. 2010. Within-generation variation of critical thermal limits in adult Mediterranean and Natal fruit flies Ceratitis capitata and Ceratitis rosa: thermal history affects short-term responses to temperature. Physiological Entomology 35(3): 255-264. https://doi.org/10.1111/j.1365-3032.2010.00736.x [ Links ]
Paterson ID, Zachariades C. 2013. ISSRs indicate that Chromolaena odorata invading southern Africa originates in Jamaica or Cuba. Biological Control 66(2): 132-139. https://doi.org/10.1016/j.biocontrol.2013.04.005 [ Links ]
Ramanand H, McConnachie AJ, Olckers T. 2017. Thermal tolerance of Liothrips tractabilis, a biological control agent of Campuloclinium macrocephalum recently established in South Africa. Entomologia Experimentalis et Applicata 162(2): 234-242. https://doi.org/10.1111/eea.12528 [ Links ]
Robertson MP, Kriticos DJ, Zachariades C. 2008. Climate matching techniques to narrow the search for biological control agents. Biological Control 46(3): 442-452. https://doi.org/10.1016/j.biocontrol.2008.04.002 [ Links ]
Shao X, Li Q, Lin L, He T. 2018. On the origin and genetic variability of the two invasive biotypes of Chromolaena odorata. Biological Invasions 20(8): 2033-2046. https://doi.org/10.1007/s10530-018-1677-4 [ Links ]
Strathie LW, Zachariades C. 2004. Insects for the biological control of Chromolaena odorata: surveys in the northern Caribbean and efforts undertaken in South Africa. In: Day, MD, McFadyen, R. E. (Eds). Proceedings of the Sixth International Workshop on Biological Control and Management of Chromolaena. ACIAR Technical Reports 55: 45-52.
Te Beest M, Elschot K, Olff H, Etienne RS. 2013. Invasion success in a marginal habitat: an experimental test of competitive ability and drought tolerance in Chromolaena odorata. PLoS One 8(8): e68274. https://doi.org/10.1371/journal.pone.0068274 [ Links ]
Terblanche JS, Sinclair BJ, Klok CJ, Mcfarlane ML, Chown SL. 2005. The effects of acclimation on thermal tolerance, desiccation resistance and metabolic rate in Chirodica chalcoptera (Coleoptera: Chrysomelidae). Journal of Insect Physiology 51(9): 1013-1023. https://doi.org/10.1016/j.jinsphys.2005.04.016 [ Links ]
Terblanche JS, Deere JA, Clusella-Trullas S, Janion C, Chown SL. 2007. Critical thermal limits depend on methodological context. Proceedings of the Royal Society B 274(1628): 2935-2943. https://doi.org/10.1098/rspb.2007.0985 [ Links ]
Terblanche JS, Clusella-Trullas S, Deere JA, Chown SL. 2008. Thermal tolerance in a south-east African population of tsetse fly Glossina pallidipes (Diptera: Glossinidae): implications for forecasting climate change impacts. Journal of Insect Physiology 54(1): 114-127. https://doi.org/10.1016/j.jinsphys.2007.08.007 [ Links ]
Uyi OO, Zachariades C, Hill MP, Mcconnachie AJ. 2016. Temperature-dependent performance and potential distribution of Pareuchaetes insulata, a biological control agent of Chromolaena odorata in South Africa. Biological Control 61: 815-825. https://doi.org/10.1007/s10526-016-9760-1 [ Links ]
Uyi OO, Zachariades C, Marais E, Hill MP. 2017. Reduced mobility but high survival: thermal tolerance and locomotor response of the specialist herbivore, Pareuchaetes insulata (Walker) (Lepidoptera: Erebidae), to low temperatures. Bulletin of Entomological Research 107(4): 448-457. https://doi.org/10.1017/S0007485316001103 [ Links ]
Van Driesche R, Hoddle M, Center T. 2009. Control of pests and weeds by natural enemies: an introduction to biological control. European Journal of Entomology 106(2): 323. https://doi.org/10.14411/eje.2009.038 [ Links ]
Van Lenteren JC, Bale J, Bigler F, Hokkanen HMT, Loomans AJ. 2006. Assessing risks of releasing exotic biological control agents of arthropod pests. Annual Review of Entomology 51(1): 609-634. https://doi.org/10.1146/annurev.ento.51.110104.151129 [ Links ]
Wagner TL, Olson RL, Willers JL. 1991. Modeling arthropod development time. Journal of Agricultural Entomology 8: 251-279. [ Links ]
Zachariades C, Day M, Muniappan R, Reddy GVP. 2009. Chromolaena odorata (L.) King and Robinson (Asteraceae). In: Muniappan R, Reddy GVP, Raman A. (Eds). Biological Control of Tropical Weeds Using Arthropods. Cambridge, U.K.: Cambridge University Press; p. 130-162. https://doi.org/10.1017/CBO9780511576348.008 [ Links ]
Zachariades C, Strathie LW, Retief E, Dube N. 2011. Progress towards the biological control of Chromolaena odorata (L.) R.M. King and H. Rob. (Asteraceae) in South Africa. African Entomology 19: 282-302. [ Links ]
Zachariades C, Dube N, Nqayi SB, Dlomo SI, Uyi OO. 2019. Attempts to establish Dichrorampha odorata on Chromolaena odorata in South Africa. In: H.L. Hinz et al. (Eds), Proceedings of the XV International Symposium on Biological Control of Weeds, Engelberg, Switzerland, pp. 272-274.
Zachariades C. 2021. A catalogue of natural enemies of invasive alien plants in South Africa: classical biological control agents considered, released and established, exotic natural enemies present in the field, and bioherbicides. African Entomology 29(3): 1077-1142. https://doi.org/10.4001/003.029.1077. [ Links ]
Zachariades C, Van Der Westhuizen L, Heystek H, Dube N, Mcconnachie AJ, Nqayi SB, Dlomo DI, Mpedi P, Kistensamy Y. 2021. Biological control of three Eupatorieae weeds in South Africa: 2011-2020. African Entomology 29(3): 742-767. https://doi.org/10.4001/003.029.0742 [ Links ]
 Correspondence:
Correspondence:
SB Nqayi
Email: sitholenqayi@gmail.com
Received: 30 March 2022
Accepted: 11 July 2022














