Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Chemistry
versão On-line ISSN 1996-840X
versão impressa ISSN 0379-4350
S.Afr.j.chem. (Online) vol.78 Durban 2024
http://dx.doi.org/10.17159/379-4350/2024/v78a01
RESEARCH ARTICLE
Colour removal from wastewaters by an electrooxidation method
Sermin Günaslan; Baybars Ali Fil; Cansu Elgün; Sevim Alya Cihan
Balikesir University, Engineering Faculty, Department of Environmental Engineering, Balikesir, Turkey
ABSTRACT
This study examined the potential of electrooxidation techniques for treating Rhodamine B dyestuff from aqueous solutions made from synthetic wastewater. The Rhodamine B dyestuff was electro-oxidized by using five anodes coated with "Ti/IrO2/RuO2" as anode material and five stainless steel cathodes as cathode material. The initial concentration of Rhodamine B, the type of support electrolyte, the current density, and the initial pH of the wastewater were evaluated. KCl, NaCl, NaNO3 and Na2SO4 were used as supporting electrolytes. Colour removal performance was optimum when the initial pH was approximately 5, the current density was 1.78 mA cm-2, and the supporting electrolyte was NaCl at a concentration of 10 mM. Under ideal circumstances, colour removal was shown to be 99.72% effective.
Keywords: electrooxidation, stainless steel cathode, supporting electrolyte type, Ti/IrO2/RuO2 anode,
INTRODUCTION
The world's fast rising population and increasing requirements, combined with developing technology and industrialization, have elevated environmental pollution to a dangerous level. Without a doubt, the treatment of water, which is one of our most essential necessities, is critical. The chemicals found in effluent from industrial activity have a harmful impact on living organisms. This makes water purification essential. Coloured wastewaters from the textile industry containing organic materials cause issues in receiving habitats that are both aesthetically displeasing and biologically problematic. In addition, rapid industrialization causes more dangerous contaminants to spread to the receiving environment due to changing product diversity and composition. As a result, hazardous chemicals in dye-containing wastewater must be eliminated before release.1
Global economic development is positively impacted by the textile industry. China is the largest exporter of textiles of all kinds, followed by the EU, India, and the USA.2 The categorization of the textile sector is based on the types of fabrics they produce, such as cellulosic fabrics derived from plants (such as cotton, rayon, and linen), protein fabrics derived from animals (such as wool, silk, and mohair), and synthetic fabrics derived from man-made materials (such as nylon, polyester, and acrylic). In textile factories, both dry and wet techniques are used to produce fibre. The wet process produces effluent that is highly polluted and consumes a significant amount of potable water. The procedures used in this process include sourcing, sizing, de-sizing, bleaching, mercerizing, dyeing, printing, and finishing.3, 4
A wide range of concentrations of dyes in textile effluents have been recorded. According to another study, there are 10 to 50 mg L-1 of dye in textile effluent.5 However, reactive dyes are apparently released in concentrations of 60 mg L-1,6 and between 100 and 200 mg L-1 in cotton mills.7 Vandevivere et al. mentioned dye effluents with a concentration of 600 to 800 mg L-1.8 According to Koprivanac et al., a reactive dye effluent had a concentration of 7000 mg L-1.9 When compared with other references, this concentration is incredibly high, and it may be that they quoted the wastewater discharge from a particular textile factory. According to Abid et al., 14 Ramadhan textile companies in Iraq observed dye outflow concentrations ranging from 20 to 50 mg L-1.10 Sivakumar reported that the final clarifier from a textile plant in India had an Acid Orange 10 outflow concentration of 45 mg L-1.11 The dye concentrations released by dye houses, according to Ghaly et al., varied from 10 to 250 mg L-1.2 The American Dye Manufactures Institute (ADMI) stated that dye concentrations in coloured effluents ranged from 1000 to 1500 ADMI units.12
Dyes are hazardous substances that can cause mutagenesis and cancer in humans. As a result, their removal from wastewater is critical for both human and environmental health. A considerable number of studies on the elimination of these chemical substances have been conducted, particularly in recent years, and numerous treatment approaches are being investigated and developed.13 Methods such as electro-Fenton,14 electrooxidation,15-17 electrocoagulation,18 Fenton,19,20 photo-Fenton,21 adsorption,22 ozonation,23 and reverse osmosis10 have been used in the treatment of such wastewater.
In order to handle wastewaters with a high organic content and colour, the dye industry uses a variety of treatment procedures. One of the most crucial of these processes is electrooxidation. Indirect or direct oxidation forms the basis for the electrooxidation of organic substances through electrodes such as graphite, coated titanium, platinum and boron doped diamond. An electrochemical oxidation procedure can either directly or indirectly oxidize organic pollutants on the anode surface.24
In the direct anodic process, the pollutants are initially adsorbed to the anode surface, and then electron transfer takes place from the anode surface. The catalytic activity of the anode, which is aided by the diffusion rate of the organic compound to the anode's active sites and the applied current intensity, determines the rate at which organic pollutants are directly oxidized. Oxidizing agents such as hypochlorite, chlorine, ozone, and hydrogen peroxide may be created anodically during indirect electrooxidation of organic materials.25
In the electrooxidation process, the anode electrode actively participates. As a result, one of the characteristics useful in this process is the anode's catalytic activity. Additionally, factors like current, pH, temperature, and the rate at which organic molecules and other oxidants diffuse, are crucial. The chloride ions in the wastewater may convert to chlorine if the anode has a high enough potential, or secondary processes such as the direct oxidation of organic molecules may also take place.
As a result, the removal of coloured pollutants that produce unwanted problems in the receiving environment, by an electrooxidation method was investigated in this study. Variables like the supporting electrolyte concentration, supporting electrolyte type, initial dye concentration, current density, and initial pH value of the wastewater, were assessed.
EXPERIMENTAL
The chemical formula and analytical purity of Rhodamine B, a cationic dye employed in the investigation, was C28H31ON203 and 99.0% (±1.0), respectively. The molecular structure of Rhodamine B is shown in Figure 1, and its estimated molecular mass is around 479.02 g mol-1. The artificial wastewater utilized in the tests was created artificially from clean water and diluted appropriately before use. The Rhodamine B concentration in the sample solution was determined spectrophotometrically (DR LANGE CADAS 30-S UV-Vis spectrophotometer) at a wavelength of 554 nm. Calibration curves were plotted between absorbance and concentration of the dye solution. Samples were taken at different contact times to determine the time required to reach the desired removal efficiency. Each experimental point was an average of three independent adsorption tests.
The study looked into the electrooxidation approach for treating synthetically manufactured wastewater containing Rhodamine B. The anodes were Ti/IrO2/RuO2 (mixed metal oxide coated on titanium) sieve type anodes, and the cathode material was steel plates. Five anodes and five cathodes were connected in parallel. Figure 2 depicts the experimental setup.

Equations (1) to (3) were used to calculate the removal efficiency, energy consumption, and current density respectively. Removal Efficiency:

where %n is the percentage removal efficiency, Ct is the dye concentration at any time t (mg L-1), C0 is the initial dye concentration (mg L-1), EC is the energy consumption (kW h m-3), V is the system voltage (V), V is the wastewater volume (m3), I is the current intensity (A), t is the time (h), J is the current density (mA cm-2), and A is the wet electrode surface area (cm2).
RESULTS AND DISCUSSION
Effect of supporting electrolyte type on colour removal
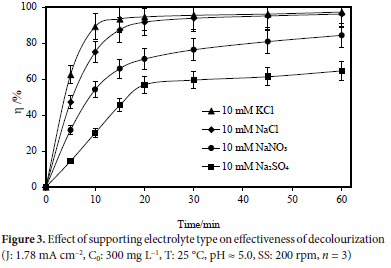
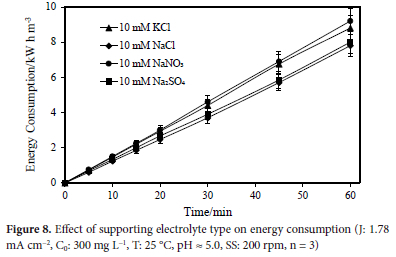
The effect on color removal by electrooxidation of the following supporting electrolytes: NaCl, KCl, Na2SO4, and NaNO3, at a concentration of 10 mM utilizing a Ti/IrO2/RuO2 anode was investigated under the following conditions: 1.78 mA cm-2 current density, 300 mg L-1 initial dye concentration, 25 °C temperature, 5.0 natural pH, 60-minute reaction period, and 200 rpm stirring speed. Figure 3 displays the experimental findings. NaCl, KCl, NaNO3 and Na2SO4 all had colour removal efficiencies of 96.23, 97.31, 84.38, and 64.59%, respectively. It has been determined that choosing the right supporting electrolyte is crucial for eradicating colour pollution. With the help of current density and pH level, chlorine in wastewater has been converted into 0Cl" and H0Cl, which are powerful chlorine oxidants.26
Effect of supporting electrolyte concentration on colour removal
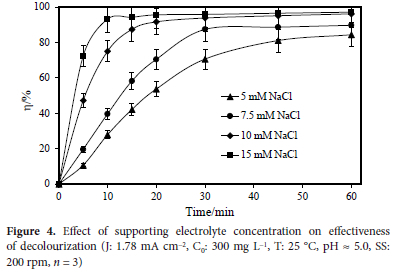
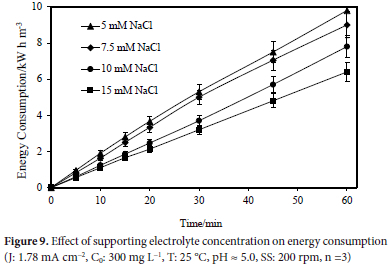
The effect of the concentration of the supporting electrolyte on the colour removal effectiveness was examined in this part of the investigation. The concentration of the supporting electrolyte is one of the most crucial factors affecting indirect electrooxidation.27 The effect of the supporting electrolyte concentration was studied at room temperature, 300 mg L-1 initial dye concentration, natural pH value, and 1.78 mA cm-2 current density for 60 minutes. Figure 4 depicts the results obtained. As the results show, increasing the concentration of the supporting electrolyte improved the purifying efficiency.
Effect of initial dye concentration on colour removal
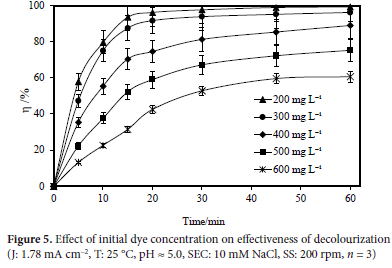
The effect of the initial dye concentration on electrooxidation of Rhodamine B with a Ti/IrO2/RuO2 anode at initial Rhodamine B concentrations of 200, 300, 400, 500, and 600 mg L-1 was examined. Other experimental settings were a current density of 1.78 mA cm-2, a pH of 5.0, a temperature of 25 °C, a supporting electrolyte concentration of 10 mM NaCl, a mixing speed of 200 rpm, and a reaction period of 60 minutes. For the initial dye concentrations of 200, 300, 400, 500, and 600 mg L-1, removal efficiencies of 99.25, 96.23, 89.02, 75.28, and 60.68% were attained, respectively. Figure 5 depicts the acquired results. As the colour concentration increased, the total colour removal increased as well. Although high purification efficiencies were reached at low colour concentrations (approximately 99.25% for 200 mg L-1), at higher colour concentrations (about 96.23% for 300 mg L-1), the total amount of dye removed increased.28
Effect of initial solution pH on colour removal
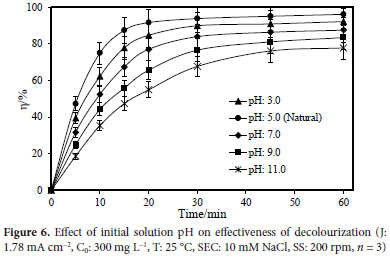
The change in the initial pH value of the solution is an important characteristic to consider in terms of the creation of intermediate agents by the electrode material utilized. Under acidic conditions, it is anticipated that a hypochlorous acid solution predominates in the effluent. Because hypochlorous acid has a high oxidation potential, colour loss at low pH levels is caused by this. At a pH of 7, the reaction rate slows down. Free chlorine can be cathodically reacted into chlorine and perchlorate at pH levels that are neutral. Hypochlorite and hypochlorous acid, which have a high oxidative potential in water, would therefore be present in lower concentrations.29 Other variables were held constant in order to study the effect of the starting pH value of the solution that is intended to be purified by means of the electrooxidation approach. The initial pH of the solution was set to a precise value. The study examined how the pH affected the ability of the batch electrooxidation to remove colour from the synthetic wastewater. The supporting electrolyte was maintained constant at 10 mM NaCl.
In the experiments, the Rhodamine B dye was electro-oxidized with initial wastewater pH values set at 3.0, the wastewater natural pH, 7.0, 9.0, and 11.0; with the other variables kept constant as follows: 25 °C temperature; 10 mM NaCl concentration; 200 rpm mixing speed; 1.78 mA cm-2 constant current density; 300 mg L-1 initial dye concentration; and 60-minute reaction time. Figure 6 depicts the effect of initial wastewater pH on the colour removal efficacy. The maximum removal was observed at acidic pH values, as shown in Figure 6. Treatment efficiencies of 92.26, 96.23, 87.57, 83.49 and 77.85% were obtained for pH 3, 5, 7, 9 and 11, respectively.
Effect of current density on colour removal
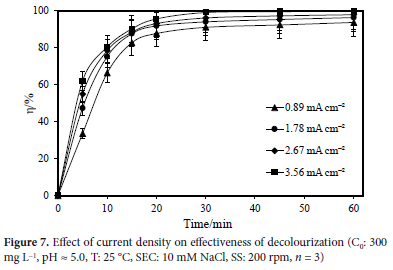
Either a constant potential difference or a constant current intensity is required for electrochemical reactions to occur. Constant current intensity was used in this experiment. A direct current power was used to apply the desired constant current intensity to the electrochemical reactor's electrodes, resulting in the desired level of oxidation. The rate of direct or indirect electrooxidation inside the system could be adjusted depending on the applied current intensity. While this parameter was being studied, variables like the initial pollutant concentration, mixing rate, initial pH of the solution, temperature, and the type and concentration of the supporting electrolyte were all kept constant.
Figure 7 illustrates how the dye concentration decreased over time in synthetic wastewaters with an initial dye concentration of 300 mg L-1 by using current densities of 0.89, 1.78, 2.67, and 3.56 mA cm-2, for natural wastewater pH (5.0), 60-minute of reaction duration, 25 °C temperature, and 10 mM NaCl concentration, and a stirring speed of 200 rpm. An essential element of the electrochemical process is current density. It is an incontrovertible fact that as the current intensity grows, so does the efficacy of colour removal. After 60 minutes of electrolysis, colour removal efficiencies for 0.89, 1.78, 2.67, and 3.56 mA cm-2 current densities were determined to be 93.59, 96.23, 97.77, and 99.72%, respectively. This can be explained by the greater creation rate of oxidants at high current densities, such as chlorine/hypochlorite and hydroxyl radicals.30,31
Effect of all parameters on energy consumption values
Results were attained at 1.78 mA cm-2 current density, 300 mg L-1 initial dye concentration, 25 °C temperature, 5.0 natural pH, 60-minutes of reaction time, and 200 rpm stirring speed. At the end of the reaction, consumption of energy values can be listed 10 mM NaCl, 10 mM KCl, 10 mM NaNO3 and 10 mM Na2SO4 as; 7.8, 8.8, 9.2, and 8.0 kW h m-3, respectively. In Figure 8, the effect of supporting electrolyte type on energy consumption is shown graphically.
The effect of the supporting electrolyte concentration on the energy consumption values in studies conducted at 5, 7.5, 10 and 15 mM concentrations of NaCl at room temperature, with an initial dye concentration of 300 mg L-1, a natural pH value, and a current density of 1.78 mA cm-2 for 60 minutes was examined. It was concluded that the energy consumption was 9.80 kW h m-3 in the presence of 5 mM NaCl, 9.00 kW h m-3 in the presence of 7.5 mM NaCl, 7.80 kW h m-3 in the presence of 10 mM NaCl, and 6.40 kW h m-3 in the presence of 15 mM NaCl. Figure 9 shows the effect of the supporting electrolyte concentration on energy consumption.
The effect of the initial Rhodamine B dye concentration on the electrooxidation at values of 200, 300, 400, 500, and 600 mg L-1, and utilizing a Ti/IrO2/RuO2 anode, was examined. Additionally, the temperature was 25 °C, the pH value was the natural value (5.0), the supporting electrolyte concentration was 10 mM NaCl, the stirring speed was 200 rpm, and the reaction period was 60 minutes. Energy consumption values of 6.80 kW h m-3 for 200 mg L-1, 7.80 kW h m-3
for 300 mg L-1, 8.60 kW h m-3 for 400 mg L-1, 9.80 kW h m-3 for 500 mg L-1, and 10.80 kW h m-3 for 600 mg L-1, were calculated. The effect of initial dye concentration on energy consumption is given in Figure 10.
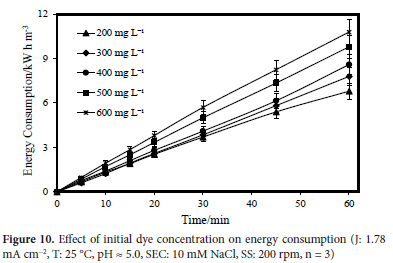
Experiments examining the effect of Rhodamine B dye on energy consumption when the initial wastewater pH values were 3, the natural wastewater pH (pH ~5.0), 7, 9 and 11 were carried out. The other parameters were kept constant at the following values: a concentration of 10 mM NaCl, a temperature of 25 °C, a stirring speed of 200 rpm, an initial dye concentration of 300 mg L-1, a constant current density of 1.78 mA cm-2, and a reaction time of 60 minutes. Energy consumption values of 6.80, 7.80, 8.60, 9.80, and 10.80 kW h m-3 for pH 3, 5, 7, 9 and 11, respectively, were observed. In Figure 11, the effect of the initial wastewater pH value on energy consumption is illustrated graphically.
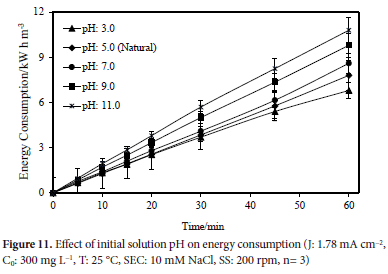
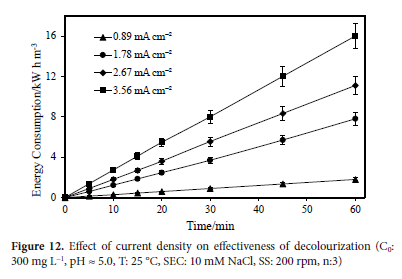
In another experiment the conditions were maintained at the natural wastewater pH value (5.0), 10 mM NaCl concentration, 60 min reaction time, and 25°C temperature, and 200 rpm stirring speed. The synthetic wastewater in the study contained 300 mg L-1 of dye, and its concentration decreased over time by applying different current densities, such as 0.89, 1.78, 2.67, and 3.56 mA cm-2. After 60 minutes of electrolysis, the energy consumption values calculated for 0.89, 1.78, 2.67 and 3.56 mA cm-2 current densities, were determined to be 1.80, 7.80, 11.10, and 16.00 kW h m-3 respectively. The effect of applied current density on energy consumption is shown in Figure 12.
Comparison with other studies
Some studies concerned with different dyestuff removals were compared with the current study and are presented in Table 1.
CONCLUSIONS
In this study, the investigation of the elimination of colour pollution caused by Rhodamine B dye utilizing Ti/IrO2/RuO2 sieve type anodes led to the following fundamental conclusions.
1. Studies analyzing the type of supporting electrolyte have found that the chlorine-containing substances such as KCl and NaCl are the most efficient supporting electrolytes. The effectiveness of colour removal was 96.26 and 97.31%, respectively.
2. The supporting electrolyte concentration depends on the type of supporting electrolyte used; here NaCl values of 5, 7.5, 10, and 15 mM were investigated. About 20% more colour was removed when the supporting electrolyte concentration was raised from 5 mM to 10 mM. When the supporting electrolyte concentration was raised to 15 mM, the colour removal efficiency did not significantly alter, thus, 10 mM was shown to be the ideal supporting electrolyte content.
3. Studies that examined the effect of the initial dye concentration were conducted at 200 - 600 mg L-1. As can be observed from the results of increasing dye concentration, the computed value of the removal efficiency declined with concentration while the amount of dye removed grew.
4. In the initial pH value experiments, the maximum removal effectiveness was found to be 96.23% at pH:5.00, which is the natural pH of wastewater.
5. The removal efficiency was shown to be considerably impacted by the current density. For the highest current density measurement of 3.56 mA cm-2, it was determined to be 99.72%.
Based on all of these findings, it was determined that the electrooxidation approach can be used successfully to remove colour from wastewater containing dyes.
ACKNOWLEDGEMENTS
The authors are grateful for the financial support of the Balikesir University Scientific Research Project Department (Project No: 2023/026).
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare that are relevant to the content of this article.
ORCID IDs
Sermin Günaslan: https://orcid.org/0000-0003-2395-2808
Baybars Ali Fil: https://orcid.org/0000-0003-3085-224x
Cansu Elgün: https://orcid.org/0000-0001-7197-8661
Sevim Alya Cihan: https://orcid.org/0000-0003-2646-7285
REFERENCES
1. Buthelezi SP, Olaniran AO, Pillay B. Textile dye removal from wastewater effluents using bioflocculants produced by indigenous bacterial isolates. Molecules. 2012;17(12):14260-14274. https://doi.org/10.3390/molecules171214260 [ Links ]
2. Ghaly A, Ananthashankar R, Alhattab M, Ramakrishnan VV. Production, characterization and treatment of textile effluents: A critical review. Chem Eng Process. 2014; 5. [ Links ]
3. Liu RR, Tian Q, Yang B, Chen JH. Hybrid anaerobic baffled reactor for treatment of desizing wastewater. Int J Environ Sci Technol. 2010;7(1):111-118. https://doi.org/10.1007/BF03326122 [ Links ]
4. Babu BR, Parande AK, Raghu S, Kumar TP. Cotton textile processing: Waste generation and effluent treatment. J Cotton Sci. 2007;11(3):141-143. [ Links ]
5. Laing IG. The impact of effluent regulations on the dyeing industry. Rev Prog Color Relat Top. 1991;21(1):56-71. https://doi.org/10.1111/j.1478-4408.1991.tb00081.x [ Links ]
6. Shelley T. Dye pollution clean-up by synthetic mineral. Int Dyer. 1994;79:26-31. [ Links ]
7. Gâhr F, Hermanutz F, Oppermann W. Ozonation - An important technique to comply with new German laws for textile wastewater treatment. Water Sci Technol. 1994;30(3):255-263. https://doi.org/10.2166/wst.1994.0115 [ Links ]
8. Vandevivere PC, Bianchi R, Verstraete W. Review: Treatment and reuse of wastewater from the textile wet-processing industry: Review of emerging technologies. J Chem Technol Biotechnol. 1998;72(4):289-302. https://doi.org/10.1002/(SICI)1097-4660(199808)72:43.0.CO;2-# [ Links ]
9. Koprivanac N, Bosanac G, Grabaric Z, Papic S. Treatment of wastewaters from dye industry. Environ Technol. 1993;14(4):385-390. https://doi.org/10.1080/09593339309385304 [ Links ]
10. Abid MF, Zablouk MA, Abid-Alameer AM. Experimental study of dye removal from industrial wastewater by membrane technologies of reverse osmosis and nanofiltration. Iran J Environ Health Sci Eng. 2012;9:17. https://doi.org/10.1186/1735-2746-9-17 [ Links ]
11. Sivakumar D. Role of Lemna minor Lin. in treating the textile industry wastewater. Int J Text Eng Process. 2014;8(3):208-212. [ Links ]
12. O'Neill C, Hawkes FR, Hawkes DL, Lourenço ND, Pinheiro HM, Delée W Colour in textile effluents - sources, measurement, discharge consents and simulation: a review. J Chem Technol Biotechnol. 1999;74(11):1009-1018. https://doi.org/10.1002/(SICI)1097-4660(199911)74:113.0.CO;2-N. [ Links ]
13. Akbas H. Investigation of color removal by electrocoagulation method. Tekirdag: Graduate School of Natural and Applied Sciences Department of Environmental Engineering, Namik Kemal University; 2014. [ Links ]
14. Wang C-T, Hu J-L, Chou W-L, Kuo Y-M. Removal of color from real dyeing wastewater by Electro-Fenton technology using a three-dimensional graphite cathode. J Hazard Mater. 2008;152(2):601-606. https://doi.org/10.1016/j.jhazmat.2007.07.023 [ Links ]
15. Parsa JB, Shojaat R. Removal of organic dye pollutants from wastewater by electrochemical oxidation. Phys Chem Liquids. 2007;45(4):479-485. https://doi.org/10.1080/00319100601089752 [ Links ]
16. Degermenci G. Removal of reactive azo dye using platinum-coated titanium electrodes with the electro-oxidation process. Desalination Water Treat. 2021;218:436-443. https://doi.org/10.5004/dwt.2021.26981 [ Links ]
17. Fil BA. The effect of initial dyestuff pollution and temperature on the investigation of color removal from synthetic wastewater by electrooxidation method. Turkish J Eng Res Edu. 2022;1(2):80-85. [ Links ]
18. Yilmaz AE. Determination of the optimum conditions in the removal of color from synthetic textile wastewater using electrocoagulation method. Fresenius Environ Bull. 2012;21(5):1052-1060. [ Links ]
19. Meric S, Lofrano G, Belgiorno V. Treatment of reactive dyes and textile finishing wastewater using Fenton's oxidation for reuse. Int J Environ Pollut. 2005;23(3):248-258. https://doi.org/10.1504/IJEP.2005.006865 [ Links ]
20. Degermenci N, Degermenci G, Ulu H. Decolorization of reactive azo dye from aqueous solutions with Fenton oxidation process: effect of system parameters and kinetic study. Desalination Water Treat. 2019;169:363-371. https://doi.org/10.5004/dwt.2019.24672 [ Links ]
21. Degermenci GD. Decolorization of reactive azo dye by fenton and photo-fenton processes in aqueous solution: the influence of operating conditions, kinetics study, and performance comparison. Bull Chem Soc Ethiop. 2022;37(1):197-210. https://doi.org/10.4314/bcse.v37i1.16 [ Links ]
22. Fil B, Özmetin C, Korkmaz M. Cationic dye (methylene blue) removal from aqueous solution by montmorillonite. Bull Korean Chem Soc. 2012;33(10):3184-3190. https://doi.org/10.5012/bkcs.2012.33.10.3184 [ Links ]
23. Sevimli MF, Sarikaya HZ. 0zone treatment of textile effluents and dyes: effect of applied ozone dose, pH and dye concentration. J Chem Technol Biotechnol. 2002;77(7):842-850. https://doi.org/10.1002/jctb.644. [ Links ]
24. Fil BA. Treatment of Pistachio Processing Wastewater by Electrooxidation. Erzurum: Graduate School of Natural and Applied Sciences, Ataaturk University; 2014. [Ph.D. [ Links ]]].
25. Kul S, B oncukcuoglu R, Yilmaz AE, Fil BA. Treatment of olive mill wastewater with electro-oxidation method. J Electrochem Soc. 2015;162(8):G41-G47. https://doi.org/10.1149/2.0451508jes [ Links ]
26. Fil BA, Boncukcuoglu R, Yilmaz AE, Bayar S. Electro-oxidation of pistachio processing industry wastewater using graphite anode. Clean (Weinh). 2014;42(9):1232-1238. https://doi.org/10.1002/clen.201300560 [ Links ]
27. Malpass GRP, Miwa DW, Mortari DA, Machado SAS, Motheo AJ. Decolorisation of real textile waste using electrochemical techniques: effect of the chloride concentration. Water Res. 2007;41(13):2969-2977. https://doi.org/10.1016/j.watres.2007.02.054 [ Links ]
28. Asghar HMA, Ahmad T, Hussain SN, Sattar H. Electrochemical 0xidation of Methylene Blue in Aqueous Solution. Int J Eng Res Appl. 2015;6(5): 352355. https://doi.org/10.7763/IJCEA.2015.V6.508. [ Links ]
29. Gotsi M, Kalogerakis N, Psillakis E, Samaras P, Mantzavinos D. Electrochemical oxidation of olive oil mill wastewaters. Water Res. 2005;39(17):4177-4187. https://doi.org/10.1016/j.watres.2005.07.037 [ Links ]
30. Ma HZ, Zhuo QF, Wang B. Electro-catalytic degradation of methylene blue wastewater assisted by Fe203-modified kaolin. Chem Eng J. 2009;155(1-2):248-253. https://doi.org/10.1016/jxej.2009.07.049. [ Links ]
31. Gu L, Wang B, Ma HZ, Kong WP. Catalytic oxidation of anionic surfactants by electrochemical oxidation with Cu0-Co203-P043- modified kaolin. J Hazard Mater. 2006;137(2):842-848. https://doi.org/10.1016/j.jhazmat.2006.03.012 [ Links ]
32. Cotillas S, Llanos J, Canizares P, Clematis D, Cerisola G, Rodrigo MA, Panizza M. Removal of Procion Red MX-5B dye from wastewater by conductive-diamond electrochemical oxidation. Electrochim Acta. 2018;263:1-7. https://doi.org/10.1016/j.electacta.2018.01.052 [ Links ]
33. Senthilkumar S, Basha CA, Perumalsamy M, Prabhu HJ. Electrochemical oxidation and aerobic biodegradation with isolated bacterial strains for dye wastewater: combined and integrated approach. Electrochim Acta. 2012;77:171-178. https://doi.org/10.1016/j.electacta.2012.05.084 [ Links ]
34. Bogdanowicz R, Fabianska A, Golunski L, Sobaszek M, Gnyba M, Ryl J, Darowicki K, 0ssowski T, Janssens SD, Haenen K, et al. Influence of the boron doping level on the electrochemical oxidation of the azo dyes at Si/ BDD thin film electrodes. Diamond Related Materials. 2013;39:82-88. https://doi.org/10.1016/j.diamond.2013.08.004 [ Links ]
35. An H, Cui H, Zhang W, Zhai J, Qian Y, Xie X, Li Q. Fabrication and electrochemical treatment application of a microstructured Ti02-NTs/Sb-Sn02/Pb02 anode in the degradation of C.I. Reactive Blue 194 (RB 194). Chem Eng J. 2012;209:86-93. https://doi.org/10.1016/jxej.2012.07.089 [ Links ]
36. Maljaei A, Arami M, Mahmoodi NM. Decolorization and aromatic ring degradation of colored textile wastewater using indirect electrochemical oxidation method. Desalination. 2009;249(3):1074-1078. https://doi.org/10.1016/j.desal.2009.05.016 [ Links ]
37. Farizoglu B, Fil BA, Uzuner S, Biçakci S, Er E, Kara EN. Reactive black 5 removal with electro-oxidation method using Ti/Ir02/Ru02 anode and stainless steel cathode. Int J Electrochem Sci. 2018;13(4):3288-3296. https://doi.org/10.20964/2018.04.58 [ Links ]
Received 26 April 2022
Revised 09 June 2023
Accepted 01 August 2023
* To whom correspondence should be addressed Email: baybarsalifil2@gmail.com














