Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Chemistry
On-line version ISSN 1996-840X
Print version ISSN 0379-4350
S.Afr.j.chem. (Online) vol.77 Durban 2023
http://dx.doi.org/10.17159/0379-4350/2023/v77a18
RESEARCH ARTICLE
Cu(II) catalyzed oxidation of L-phenylalanine by hexacyanoferrate(III) in cationic micellar medium
AbhishekSrivastavaI, *; Neetu SrivastavaII; Krishna SrivastavaIII
IDepartment of Chemistry, GLA University, Mathura, India
IIDepartment of Chemistry, D.D.U. Gorakhpur University, Gorakhpur, India
IIIFaculty of Chemical Sciences, Shri Ramswaroop Memorial University, Barabanki, India
ABSTRACT
The kinetics of Cu(II) accelerated oxidation of L-phenylalanine (Pheala) oxidation in cetyltrimethylammonium bromide (CTAB) by hexacyanoferrate(III) was investigated by registering the decline in absorbance at 420 nm. Employing the pseudo-first-order condition, the reaction's advancement has been examined as an indicator of[CTAB], [Cu(II)], [OH-], [Pheala], [Fe(CN)63-], temperature, and ionic strength. The results show that [CTAB] is the critical parameter with a discernible influence on reaction rate. Pheala interacts with [Fe(CN)6]3- in a 1:2 ratio, and this reaction exhibits first-order dependency with regard to [Fe(CN)63-]. In the investigated concentration ranges of Cu(II), [OH-], and [Pheala], the reaction demonstrates fractional-first-order kinetics. A positive salt effect is indicated by the linear rise in reaction rate with additional electrolytes. CTAB catalyzes the process substantially, and once at its peak, the rate remains basically constant as [CTAB] grows. Reduced repulsion between surfactant molecules' positive charge heads brought on by the negative-charged [Fe(CN)6]3-, OH-, and [Cu(OH)4]2- molecules may be responsible for the witnessed drop in CTAB CMC.
Keywords: copper(II), critical micellar concentration, hexacyanoferrate(III), kinetics and mechanism, L-phenylalanine
INTRODUCTION
Amino acids are crucial for metabolism as well as serving as the building blocks for the creation of proteins. Numerous fields, including biochemistry, fortification of feed and food, metabolism, microbiology, medicines, and nutrition use amino acids. Oxidation of amino acids plays an integral role in metabolism and medicine as organisms employ the oxidation of amino acids to control the quantity of protein in the body. Several scientists have already investigated the kinetic and mechanistic aspects of amino acid oxidation.1-5 The mechanism, however, varies amongst the various reaction systems. The oxidation of amino acids is extremely exciting since different oxidants result in diverse oxidation products.1-5 The mechanism via which various amino acids are oxidized must therefore be studied in detail.
A potent oxidizing agent, hexacyanoferrate(III) [HCF(III)] can oxidize a variety of organic and inorganic substrates in acidic as well as alkaline mediums.6-9 HCF(III) reactions in acid mediums are limited by its complexation with its reduced form HCF(II), which interferes with spectrophotometric monitoring. HCF(III) is very useful because of its stable reduction product as [Fe(CN)6]4-, high stability, water solubility, and moderate reduction potential.7 The nature of the substrate and the reaction media are crucial factors in the HCF(III) oxidation process, which, according to literature, proceeds via an outer-sphere electron transfer mechanistic pathway.9 Although several readily oxidizable substrates have been successfully oxidized using HCF(III), reactions involving amino acids with this oxidant are very slow and necessitate the addition of a catalyst. Pt(II), Ir(III), Ru(VI), Os(VIII), Ru(III), and Pd(III) were discovered to be the catalysts for these oxidation reactions.1-5,10 When coupled with HCF(III), Cu(II), one of the aforementioned metal ions, is especially potent at oxidizing amino acids. Several research articles describe the Cu(II) catalyzed oxidation of amino acids, antibiotics, alcohols, monosaccharides, and amines by HCF(III) in an aqueous environment.11-15 The generation of various intermediate complexes makes the catalytic mechanism highly complex.
The basic importance and applicability of the oxidation-reduction/ ligand exchange processes of complexes containing transition metals in analytical, organometallic, and synthetic chemistry encouraged a large number of scientists to explore their kinetics.16-19 Kinetic analyses on the oxidation of Co(II) or Fe(II) complexes and metal-catalyzed cyanide substitution from [Ru/Fe(CN)6]3- by nitrogen heterocyclic ligands have been reported by a number of authors.20-22 The procedures listed above have also been used to evaluate catalysts at the trace-level and drugs and other substances that have a strong affinity for catalysts.23-25
Due to their surface-active characteristics, surfactants are frequently employed in modern industries.26-28 Surfactants' amphiphilic structure, which includes both a tail (hydrophobic) and head (hydrophilic), is what gives them their surface activity.29 At modest concentrations, the water-based solution ofthe surfactant behaves as an electrolyte. Micellization takes place in an aquatic environment because one substrate contains both hydrophilic and hydrophobic components. The typical surfactant concentration at which micellization starts spontaneously is defined as the critical micelle concentration (CMC).30 Due to the repulsive and attractive interactions that exist between surfactant molecules, the molecules will self-associate beyond CMC and constitute micelles of different shapes and sizes. At 298 K, the CMC value of the cationic surfactant cetyltrimethylammonium bromide (CTAB) ranges from 0.8 X 10-3 mol dm-3 to 1.0 x 10-3 mol dm-3.31-33 In comparison to pure solvents, micelle-bound reactants experience a totally distinct reaction environment. The extent of substrate interaction with the micelle aggregates in a micellar medium determines the reaction rate.
The amino acids oxidation by HCF(III) in an aqueous alkaline media under Cu(II) catalysis has been reported in numerous studies.11-15 However, very few such studies have been performed in surfactant medium.34-35 Therefore, in the current study, an effort is made to comprehend how cation ionic surfactant affects the Cu(II) promoted oxidation rate of Pheala by HCF(III).
MATERIALS AND METHODS
Reagent used
All chemicals utilized were of analytical grade. The stock solution of each reagent was directly prepared by weighing its accurate amount and further dissolving it in double-distilled water. To avoid the potential photo-decomposition of K3[Fe(CN)6].3H2O (Merck India, 99% pure), an amber-colored bottle was used to preserve its stock solution. L-phenylalanine (99% pure), sodium dodecyl sulfate (SDS) (99% pure), and Cetyltrimethylammonium bromide (99% pure) were purchased from Himedia, India, and utilized directly. The solution of Cu(NO3)2 (Sigma-Aldrich USA, 99% pure) was prepared by dissolving its calculated amount in distilled water. NaClO4 (Fisher Scientific, India, 99% pure) was utilized to control the reaction mixture's ionic strength, while Molechem's NaOH (99% pure) was employed for adjusting the reaction medium's pH.
Instrumentation
Using a DD LAB (model LAB.PHM.66800620) auto digital pH meter (DD Bioinfotech, New Delhi, India), verified with a predefined buffer solution, the pH of the reaction mixture was monitored. A double-beam UV-Visible spectrophotometer made by Electronics India, Haryana, India, model 2375, was deployed for the reaction's repeated spectrum scan and for measuring absorbance at 420 nm associated with the deterioration of HCF(III). Calibration of spectrophotometer was done by acidic potassium dichromate solution. IR spectra of the final product 3-methylbutanal was recorded on a Thermo Nicolet NEXUS 470 FTIR spectrophotometer (Spectralab Scientific Inc., Canada).
Kinetic procedure
The absorption values were not modified since, with the exception of the oxidizing agent, none of the reacting solutions exhibit significant absorption at the pertinent wavelength. Following 30 minutes of thermal equilibration at 298 K, all of the reactive solutions were quickly mixed in the sequence: Pheala, Cu(II), NaOH, CTAB, NaClO4, and [Fe(CN)6]3-. Immediately following a thorough shaking, the reaction mixture was poured into the spectrophotometric cell. An ingeniously constructed system of circulating water arrangement kept the cell compartment at a constant temperature. The absorbance decrease caused by [Fe(CN)6]3- was documented. The gradient of the line linking log(A„ - At) and time has been employed to calculate the rate constant (k') of the addressed reaction. Using calculated k' values, the impact of [OH], [CTAB], [Pheala], ionic strength, and [Fe(CN)63-] on the oxidation rate has been addressed. Calculated amounts of HCF(III) were allowed to stand with a tenfold surplus of Pheala in the presence of 0.20 mol dm-3 OH- and a fixed amount of Cu(II) at 298 K in a closed vessel until the reaction was completed. By determining the non-reacted [Fe(CN)6]3- spectrophotometrically at 420 nm, the reaction's stoichiometry is established. Based on the results, Pheala and [Fe(CN)6]3- interact at a mole ratio of 1:2, as demonstrated by the equation below.
RESULTS AND DISCUSSION
The kinetics of Cu(II) accelerated oxidation of Pheala by HCF(III) in CTAB micellar medium were investigated by monitoring the decline in absorbance at 420 nm. The reduction of HCF(III) to HCF(II) is accountable for the apparent drop in absorbance. Neither does the reaction take place in an acidic environment, and in a mild alkaline medium, it moves along extremely slowly at lower temperatures.
Therefore, the kinetic study, both in micellar and aqueous medium was performed in an alkaline medium (NaOH 0.20 mol dm-3) at 298 K temperature. The visible region's 420 nm absorption band is caused by [Fe(CN)6]3-.
Thin-layer chromatography identifies the oxidation product of Pheala as phenylacetaldehyde by the production of its 2,4-dinitrophenylhydrazone derivative. A chromatogram displaying only one peak for the prepared derivative indicates that only one product was formed. Previous research on amino acid oxidation via different oxidizing agents has also supported the production of phenylacetaldehyde.3-5
A polymerization test has been performed to assess the free radicals' existence during the oxidation process. Reaction mixtures are inertly stored for six h with known concentrations of acrylonitrile scavenger. Dilution with methanol produces white precipitates, demonstrating free radical involvement in this process. These trials failed without Pheala under similar settings, indicating the involvement of Pheala in free radical formation.
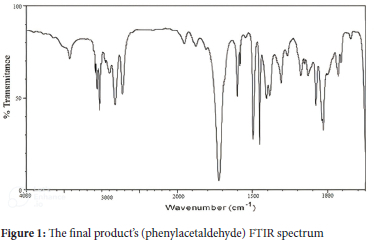
The end product, phenylacetaldehyde, has a distinctive FTIR spectrum with absorption bands at ~2900-3100 cm-1, 2730 cm-1, and 2831 cm-1, and 1734 cm-1, which correlate to C-H str., C-H str. (aldehyde proton), and C=O str. respectively (Figure 1). Phenyl acetaldehydes' production is further supported by the elemental analysis data for H, 6.73; O, 13.36; and C, 79.91.
We have not obtained and change in the absorbance maximum of Fe(CN)63- after mixing Fe(CN)63- and Cu2+ (both in aqueous and micellar medium). The result confirms that Fe(CN)63- will not exhibit any type of interaction with Cu2+ under the studied reaction conditions (Figure S1 and S2).
Impact of variation of [OH-] on oxidation rate
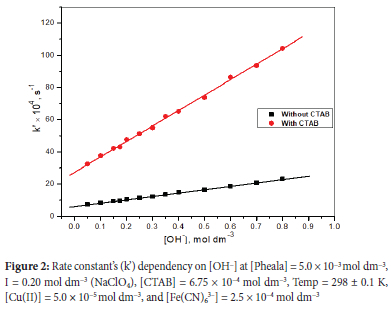
Previous research on organic moieties' oxidation by [Fe(CN)6]3- has demonstrated the importance of [OH-] in determining the reaction rate. By calculating the rate constant at various [OH-] concentrations, the oxidation rate was initially explored in the [OH-] range of 0.05 mol dm-3 to 0.8 mol dm-3.
The graph of k' versus [OH-], illustrated in Figure 2, shows a linear relationship with positive intercepts. In the investigated range of OH- concentrations, the reaction rate is slower at lower [OH-] and accelerates significantly with [OH-]. The occurrence of a lesser responsive protonated state of Pheala and [Fe(CN)6]3- is thought to be the cause of the lowered rate at lesser pH. At greater [OH-], [Fe(CN)6]3-, like Pheala, appears primarily in its deprotonated state.36-37 The occurrence of merely a deprotonated version of the reducing and oxidizing agent accounts for the increment in reaction rate observed in a strongly alkaline solution. The reaction moves roughly 4.5 times faster in CTAB micellar media compared to aqueous conditions (Figure 2), which seems consistent with previous findings on the oxidation of organic substrates catalyzed/mediated by micelles.38-39
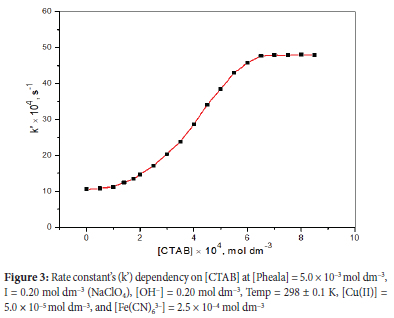
Impact of variation of [CTAB] on oxidation rate
To investigate how [CTAB] impacts the oxidation rate, every other parameter was kept constant while [CTAB] was adjusted from 0.5 x 10-4 mol dm-3 to 8.5 x 10-4 mol dm-3. The [CTAB] against k graph (Figure 3) demonstrates the increases in oxidation rate with [CTAB] up to 6.5 x 10-4 mol dm-3 (around CTAB's CMC) and then remains nearly constant in the examined [CTAB] range. At 6.5 x 10-4 mol dm-3 [CTAB], the maximum oxidation rate was observed; this result is somewhat lower compared to the CMC for previously reported aqueous CTAB. CTAB's calculated CMC in the studied reaction state is 6.12 x 10-4 mol dm-3 which is slightly less compared to the published data in an aquatic environment.31-33 This is determined by the junction of the two straight lines in the graph of k' versus [CTAB] (Figure S3).
The acceleration or suppression of the rate of reaction in a micellar media is caused by the disbursement of reactants in micellar and aqueous pseudophases and the variation in their reaction rates.40-43 Electrostatic and/or hydrophobic interactions between reactants and surfactant aggregates, as well as structural changes in water molecules, influence reaction rate. At concentrations lower than CMC, the surfactant in monomeric form serves as a catalyst, accelerating the reaction rate. Catalytic micelles are formed by monomeric surfactant and substrate molecule aggregation, which speeds up the process. Premicellar regions also impact surfactant medium reaction rates.44-45 The substrate is less reactive in micellar aggregates compared to premicellar complexes.46 Premicellar complexes disintegrate and micelles form once the reaction rate peaks at CMC, sustaining the reaction rate.
The CTAB medium's reaction rate is accelerated by the surfactant's capacity to form nanoscale micelles that gather all reacting entities closer to promote a smoother reaction. Cationic surfactant CTAB creates a micelle with an outer layer that is positively charged. Therefore, cationic species shouldn't get to the substrate and micellar surface in the stern layer.47 Because ofthe significantly positive charged micellar exterior, the negatively charged [Cu(OH)4]2-, [Fe(CN)6]3-, and OH- are able to come closer to the reactant molecules (in the stern layer) despite being repelled electrostatically.48 CTAB thus facilitates and accelerates the Cu(II) catalyzed reaction involving [Fe(CN)6]3-and Pheala (Figure 4).
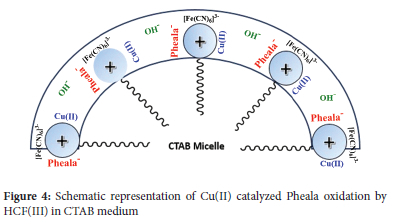
The oxidation rate in the current reaction system advances with [CTAB] even with [CTAB] is less than its CMC. The creation of a pre-micellar complex as a result of the agglomeration of the surfactant's monomeric form with the molecules that are reacting is what causes the oxidation rate to increase; this aggregation promotes the reaction and increases its rate. The peaks in the rate-surfactant pattern are caused by two conflicting effects. Reactants started binding in the Stern layer promptly as the micelle formation process starts, and they are then transferred into a small volume of the micellar pseudophase. Because of the concentration effect, the acceleration results. The dilution of the interacting reactants in the micellar pseudophase offsets the concentration effect as the concentration of surfactant increases. In contrast to larger concentrations of surfactant, where these two opposing effects are reconciled and the rate-surfactant profile is consistent, lower surfactant concentrations are characterized by the former influence dominating the latter. CTAB's CMC is observed to be somewhat lower in the examined reaction condition than in the aqueous environment. Electrostatically, the negatively charged OH-, [Cu(OH)4]2-, and [Fe(CN)6]3- molecules will be drawn to the positively charged CTAB molecule. Reduced repulsion between surfactant molecules' positive charge heads brought on by the negative-charged [Fe(CN)6]3-, OH-, and [Cu(OH)4]2- molecules may be responsible for the witnessed drop in CTAB CMC.49
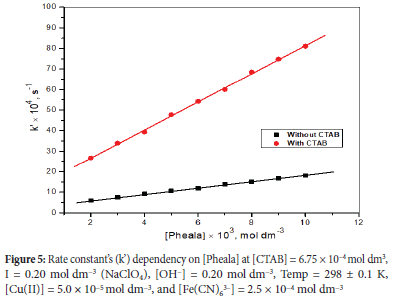
Impact of variation of [Pheala] on oxidation rate
Under the optimal [OH-], the influence of [Pheala] on the rate of reaction was examined at 298 K in the 2.0 x 10-3 mol dm-3 to 10.0 x 10-3 mol dm-3 range. The k' versus [Pheala] graph (Figure 5) produced a straight line with positive intercepts. We found fractional-first-order kinetics in both aqueous and micellar environments for the amino acid. CTAB micellar media oxidizes Pheala faster compared to an aqueous medium.
Impact of variation of [Fe(CN)63-] on oxidation rate
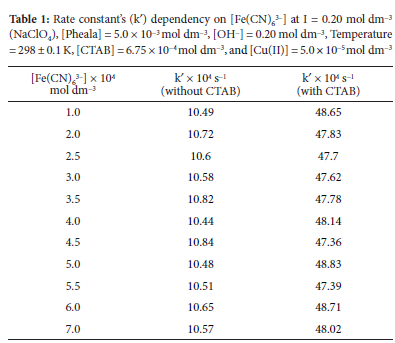
Under the optimum conditions of [Pheala] and [OH-], while fixing the rest reaction variables, the rate constant was calculated with reference to [Fe(CN)6]3- in the concentration band of 1.0 x 10-4 mol dm-3 to 7.0 x 10-4 mol dm-3. In accordance with the calculated rate constant (k') for each [Fe(CN)63-], the [Fe(CN)63-] range under consideration exhibits first-order kinetics (Table 1).
Impact of variation of [Electrolyte] on oxidation rate
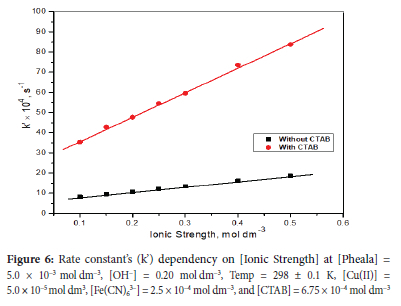
Ionic strength's effect on oxidation rate will be examined by altering the concentration of neutral electrolyte (NaClO4) in the 0.1 mol dm-3 to 0.5 mol dm-3 range. The other variables of the reaction were maintained at [Cu(II)] = 5.0 x10-5 mol dm-3, [Pheala] = 5.0 x 10-3 mol dm-3, Temp. = 298 ± 0.1 K, [Fe(CN)63-] = 2.5 x 10-4 mol dm-3, and [OH-] = 0.20 mol dm-3. A positive salt effect could be seen on the graph plotted between k' versus ionic strength (I) (Figure 6).
Impact of variation of temperature on oxidation rate
Under the optimum reaction conditions, as anticipated, the increases in oxidation rate were observed when the temperature increased (293 to 323 K). To ensure a minimum probability of product degradation, the temperature range was selected. The reaction proceeds uniformly at a modest pace at 298 K, hence this temperature was preferred for the kinetic investigation. The Arrhenius and Erying equation was exploited to calculate the Ea (energy of activation), AH# (enthalpy of activation), AS* (entropy of activation), and AG# (free energy of activation) values which are 19.52 kJ mol-1, 17.02 kJ mol-1, -244.10 J K-1 mol-1, and 89.74 kJ mol-1, in surfactant medium and 27.67 kJ mol-1, 25.19 kJ mol-1, -211.59 J K-1 mol-1, and 88.24 kJ mol-1in an aqueous environment, respectively. The lower value of Ea and AH* in CTAB micellar medium compared to aqueous environment also supports the enhancement of reaction rate by CTAB micelles. Large negative entropy of activation in both medium also supports the formation of activated complex.
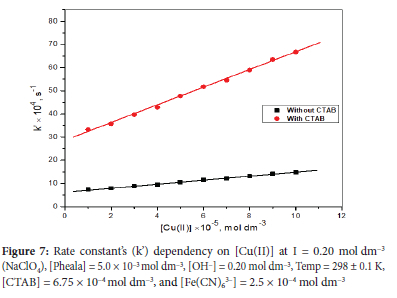
Impact of variation of [Cu(II)] on oxidation rate
As Cu(II) catalyzed oxidation of Pheala could eventually be employed to measure Cu(II) at trace levels, it is imperative to investigate the impact of [Cu(II)] on reaction rate. The valve of k' was evaluated after the reactants were mixed under optimal reaction conditions with variable [Cu(II)]. In the examined concentration range, Cu(II) exhibits fractional-first-order dependence as shown by a linear relationship between [Cu(II)] and k' (Figure 7).
Impact of anionic surfactant "sodium dodecyl sulfate (SDS)" on oxidation rate
Surfactants, depending on their charges, may enhance the rate in addition to homogenizing organic substrates in aqueous media. In a micellar CTAB (cationic surfactant) medium, the rate of Cu(II) catalyzed L-Leu oxidation is almost 4.5 times faster than in aqueous conditions, with the maximum rate near the CMC of CTAB. By taking the concentration ofSDS in its CMC range (8.2 x 10-3 -8.5 x10-3 mol dm-3), the effect of anionic surfactant on the oxidation of L-Leu has been examined under optimal reaction conditions. The calculated value of ko reported in Table 2, demonstrates that the reaction rate in the CTAB medium is extraordinarily high whereas it is slowed down in the SDS medium even in the presence of Cu(II).

Mechanism
Cu(II) has been established as a potent catalyst in a variety of oxidation processes. Taking into account the present kinetic analysis and past literature, we outlined the most likely mechanistic pathway for the oxidation of Pheala by hexacyanoferrate(III), catalyzed by Cu(II) via Equations 2-6 (Scheme 1).50
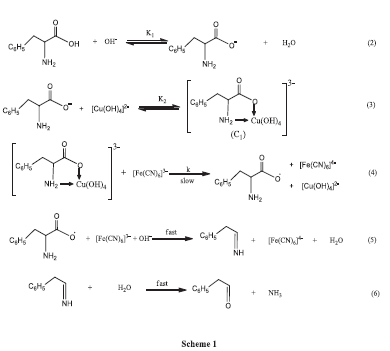
According to the findings, hexacyanoferrate(III)-catalyzed amino acid oxidation involves multiple steps and advances via the formation of a soluble Pheala-Cu intermediate complex (C1) involving Pheala and Cu(II). In the next step (slowest step) the complex C1 interacts with [Fe(CN)6]3- to produce L-phenylalanine free radical, [Fe(CN)6]4-with rejuvenation of catalyst. The resulting free radical quickly interacts with [Fe(CN)6]3- in the presence of OH-, and then hydrolysis results in the final oxidation product, phenylacetaldehyde.
The most sluggish step in the provided scheme, Equation 4, is regarded as the step that determines the rate.

Using Eq. 3 we get
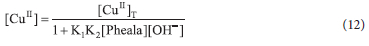
Using Equations 11 and 12 we get;
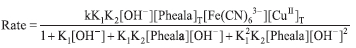
Due to the low concentration of Pheala, the term can be neglected; Equation 12 transforms to;
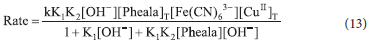
Under pseudo-first-order conditions, the rate of catalyzed reaction can be represented by Eq. 13. Where k' represents the rate constant of the catalyzed reaction.
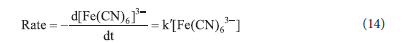
From Eq. 13 and 14, we get
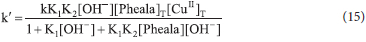
The first order dependence of reaction rate on [Fe(CN)63-] is in accordance with the rate law. The fractional-first-order reliance of oxidation rate on both [Pheala] and [catalyst] further supports the generation of the Pheala-Cu complex in the equilibrium step. The positive salt effect also supports the emergence of an intermediate complex involving identically charged species which is further confirmed by the large negative entropy of activation value. The first order dependence on [Fe(CN)63-] and fractional-first-order dependence on [Pheala] and [catalyst] suggests the involvement of intermediate complex and [Fe(CN)6]3- in the rate determining step.
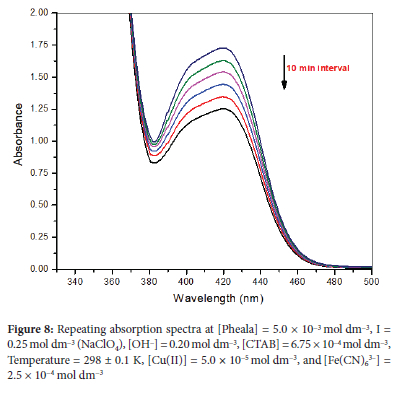
The oxidation of Pheala by [Fe(CN)6]3- promoted by Cu(II) in a basic environment, as seen in the repeating spectral scan recorded with the inclusion of CTAB exhibits a constant drop in absorbance at 420 nm (Figure 8). Pheala consumes the hexacyanoferrate(III) during the reaction, causing all peaks attributed to it to get shorter over time as [Fe(CN)6]3- is eventually converted to [Fe(CN)6]4-.
CONCLUSION
This investigation adds to our understanding of the Cu(II) accelerated oxidation ofamino acids by [Fe(CN)6]3- in CTAB micellar environment. In the studied range of [Cu(II)], [OH-], and [Pheala], the reaction displays fractional-first-order kinetics. The reaction exhibits first-order dependency with regard to [Fe(CN)63-]. A positive salt effect is seen by the reaction rate's incremental trend with electrolyte content. CTAB micellar media has a reaction rate almost 4.5 times swifter than an aqueous environment. Reduced repulsion between surfactant molecules' positive charge heads brought on by the negative-charged [Fe(CN)6]3-, OH-, and [Cu(OH)4]2- molecules may be responsible for the witnessed drop in CTAB CMC. The comprehensive kinetic examination can be used to successfully quantify Cu(II) at the micro-level in the CTAB micellar media.
SUPPLEMENTARY MATERIAL
Supplementary information for this article is provided in the online supplement.
ORCID IDS
Abhishek Srivastava: https://orcid.org/0000-0003-2463-1213
Neetu Srivastava: https://orcid.org/0000-0002-0690-9990
Krishna Srivastava: https://orcid.org/0000-0002-8276-8886
REFERENCES
1. Sharanabasamma K, Angadi MA, Tuwar SM, Kinetics and mechanism of ruthenium(III) catalyzed oxidation of l-proline by hexacyanoferrate(iii) in aqueous alkali. Open Catal J. 2011;4:1-8. https://doi.org/10.2174/1876214X01104010001 [ Links ]
2. Singh HS, Singh B, Gupta A, Singh AK, Kinetics and mechanism of the oxidation of alpha-amino acids by osmium tetroxide in aqueous alkaline medium by spectrometric stopped flow technique. Oxid Commun. 1999;22:146-153. [ Links ]
3. Goel A, Sharma R, A kinetic and mechanistic study on the oxidation of arginine and lysine by hexacyanoferrate (III) catalysed by iridium (III) in aqueous alkaline medium. J Chem Eng Mater Sci. 2012;3:1-6. https://doi.org/10.5897/JCEMS11.049 [ Links ]
4. Nowdari A, Adari KK, Gollapalli NR, Parvataneni V, Kinetics and mechanism of oxidation of l-cystine by hexacyanoferrate(III) in alkaline medium. E-J Chem. 2009;6:93-98. https://doi.org/10.1155/2009/615750 [ Links ]
5. Goel A, Sharma S, Mechanistic study of the oxidation of L-phenylalanine by hexacyanoferrate(III) catalyzed by iridium(III) in aqueous alkaline medium. Trans Met Chem. 2010;35:549-554. https://doi.org/10.1007/s11243-010-9362-1 [ Links ]
6. Gupta A, Pandey A, Catalysed oxidation of amino acid in aqueous alkaline medium. Ind J Sci Res. 2017;13:66-72. [ Links ]
7. Sharma P, Sailani R, Meena A, Khandelwal CL, A kinetic and mechanistic study of the osmium(VIII)-catalysed oxidation of crotyl alcohol by hexacyanoferrate(III) in aqueous Alkaline medium. J Chem Res. 2020;44:295-300. https://doi.org/10.1177/1747519819900622 [ Links ]
8. Pandey E, Grover N, Kambo N, Uphadyay SK, Aggregation in oxidation of aspartic and glutamic acids by chloramine-T in presence of surfactants: A kinetic study. Ind J Chem. 2004;43A:1183-1192. [ Links ]
9. Naik RM, Srivastava A, Verma AK, The kinetics and mechanism of ruthenium(III)-catalyzed oxidation of tris(2-amino ethyl)amine by hexacyanoferrate(iii) in aqueous alkaline medium. Turk J Chem. 2008;32:495-503. https://doi.org/10.3906/che-0612-7 [ Links ]
10. Bagalkoti J, Nandibewoor ST, Investigation of kinetics and mechanism of oxidation of acetoacetanilide in alkaline medium using hexacyanoferrate(III). Monatsh Chem. 2019;150:469-1478. https://doi.org/10.1007/s00706-019-02482-8 [ Links ]
11. Asghar BH, Altas HM, Fawzi A, Copper (II) catalysis for oxidation of L-tryptophan by hexacyanoferrate(III) in alkaline medium : a kinetic and mechanistic approach. J Saudi Chem Soc. 2017;21:887-898. https://doi.org/10.1016/j.jscs.2015.12.003 [ Links ]
12. Tazwar G, Devra V, Kinetic study of uncatalyzed and Cu(II) catalyzed oxidation of ofoxacin in aqueous alkaline medium and their mechanistic aspects. SN Appl Sci 2019;1:729. https://doi.org/10.1007/s42452-019-0764-1 [ Links ]
13. Duke FR, Bulgrin VC, Copper catalysls in the hexacyanoferrate(llI) oxidation of mercaptoacetate. J Phys Chem. 1975;79:2323-2324. https://doi.org/10.1021/j100588a019 [ Links ]
14. Olusanya SM, Odebunm EO, A comparative study of kinetics and mechanism of oxidation of maltose and lactose by copper (ii) ion and hexacyanoferrate(III) ion in alkaline solution. Pac J Sci Technol. 2011;12:328-333. [ Links ]
15. Mádlo K, Hasned A, Vepfek-Siska J, Catalytic effect of copper(II) ions on reactions with potassium hexacyanoferrate(III). Collect Czech Chem Commun. 1975;41:7-13. https://doi.org/10.1135/cccc19760007 [ Links ]
16. Iioka T, Takahashi S, Yoshida Y, Matsumura Y, Hiraoka S, SatoH, A kinetics study of ligand substitution reaction on dinuclear platinum complexes: Stochastic versus deterministic approach. J Comput Chem. 2019;40:279-285. https://doi.org/10.1002/jcc.25588 [ Links ]
17. Naik RM, Srivastava A, Tiwari AK, Yaday SBS, Verma AK, Kinetic and mechanistic studies of oxidation of amine-N-polycarboxylates complexes of cobalt(II) by periodate ions in aqueous medium. J Iran Chem Soc. 2007;4:63-71. https://doi.org/10.1007/BF03245804 [ Links ]
18. Naik RM, Srivastava A, Verma AK, Yadav SBS, Singh R, Prasad S, The kinetics and mechanism of oxidation of triethylene tetraamine hexaacetate. Bioinorg React Mech. 2007;6:185-192. https://doi.org/10.1515/irm.2007.6.3.185 [ Links ]
19. Omondi RO, Stephen O, Ojwach SO, Jaganyi D, Review of comparative studies of cytotoxic activities of Pt(II), Pd(II), Ru(II)/(III) and Au(III) complexes, their kinetics of ligand substitution reactions and DNA/ BSA interactions. Inorganica Chim Acta. 2020;512:119883. https://doi.org/10.1016/j.ica.2020.119883 [ Links ]
20. Srivastava A, Naik RM, Rai J, Asthana A, The Ag(I) - Promoted Substitution of Cyanide from Hexacyanoferrate(II) with Pyrazine: A Kinetic and Mechanistic Study. Russ J Phys Chem A. 2021;95:2545-2552. https://doi.org/10.1134/S0036024421130227 [ Links ]
21. Singh A, Singh A, Kinetics and mechanism of mercury catalysed ligand exchange reaction between hexacyanoferrate(II) and phenanthroline in aqueous medium. Prog React Kinet Mech. 2013;38:105-118. https://doi.org/10.3184/146867813X13600909461700 [ Links ]
22. Naik RM, Tewari RK, Singh PK, Tiwari AK, Prasad S, The mercury(ii) catalyzed ligand exchange between hexacyanoferrate(ii) and pyrazine in aqueous medium. Trans Met Chem. 2005;30:968-977. https://doi.org/10.1007/s11243-005-6266-6 [ Links ]
23. Prasad S, Naik RM, Srivastava A, Application of ruthenium (III) catalyzed oxidation of Tris(2-amino ethyl) amine in trace determination of ruthenium in environmental water samples. Spectrochimca Acta A. 2008;70:958-965. https://doi.org/10.1016/j.saa.2007.10.011 [ Links ]
24. Srivastava A, Sharma V, Prajapati A, Srivastava N, Naik RM, Spectrophotometric determination of ruthenium utilizing its catalytic activity on oxidation of hexacyano ferrate(II) by periodate ion in water samples. Chem Chem Technol. 2019;13:275-279. https://doi.org/10.23939/chcht13.03.275 [ Links ]
25. Srivastava A, Sharma V, Singh VK, Srivastava K, A Simple and sensitive inhibitory kinetic method for the carbocisteine determination. J Mex Chem Soc. 2022;66:57-69. https://doi.org/10.29356/jmcs.v66i1.1654 [ Links ]
26. Das B, Kumar B, Begum W, Comprehensive review on applications of surfactants in vaccine formulation, therapeutic and cosmetic pharmacy and prevention of pulmonary failure due to COVID-19. Chem Africa 2022;5:459-480. https://doi.org/10.1007/s42250-022-00345-0 [ Links ]
27. Zahed MA, Matinvafa MA, Azari A, Biosurfactant, a green and effective solution for bioremediation of petroleum hydrocarbons in the aquatic environment. Discov Water 2022;5:2. https://doi.org/10.1007/s43832-022-00013-x [ Links ]
28. Mohanambigai DC, Jenif D, Applications of surfactants in current situations - A comprehensive review. SPAST Abstracts 2021;1:1. [ Links ]
29. Karimi MA, Mozaheb MA, Hatefi-Mehrjardi A, A new simple method for determining the critical micelle concentration of surfactants using surface plasmon resonance of silver nanoparticles. J Anal Sci Technol. 2015;6:1-8. https://doi.org/10.1186/s40543-015-0077-y [ Links ]
30. Shah S, Chatterjee SK, Bhattarai A, The effect of methanol on the micellar properties of dodecyltrimethylammonium bromide (dtab) in aqueous medium at different temperatures. J Surfactants Deterg. 2016;19:201-207. https://doi.org/10.1007/s11743-015-1755-x [ Links ]
31. Tiwari S, Mall C, Solanki PP, CMC studies of CTAB, SLS & tween 80 by spectral and conductivity methodology to explore its potential in photogalvanic cell. Surf Interfaces 2020;18:100427. https://doi.org/10.1016/j.surfin.2019.100427 [ Links ]
32. Shah SK, Bhattarai A, Interfacial and micellization behavior of cetyltrimethylammonium bromide (ctab) in water and methanol-water mixture at 298.15 to 323.15 K. J Chem. 2020:4653092. https://doi.org/10.1155/2020/4653092 [ Links ]
33. Rauf A, Baloch MK, Khan A, Khan Z, Rauf S, Effect of concentration and molecular mass of peo on the micellization and thermodynamic behaviour of cetyltrimethylammonium bromide (ctab) in aqueous peo-ctab mixed system. J. Chil. Chem. Soc. 2016;61:3013-3017. https://doi.org/10.4067/S0717-97072016000300001 [ Links ]
34. Zourab SM, Ezzo EM, El-Aila HJ, Salem JKJ, Oxidation of amines by potassium ferricyanide in AOT surfactant systems. J Disper Sci Technol. 2003;24:67-77. https://doi.org/10.1081/DIS-120017945 [ Links ]
35. Srivastava A, Manjusha, Srivastava N, Naik RM, Kinetic Study of Ru(III) promoted oxidation of l-tryptophan in an anionic surfactant medium by hexacyanoferrate(III). J Mex Chem Soc 2023;67:46-59. https://doi.org/10.29356/jmcs.v67i1.1829 [ Links ]
36. Ramanaiah M, Sailaja BBV, Protonation equilibria of L-phenylalanine and maleic acid in cationic micellar media. Chem Speciat Bioav. 2014;26:119-125. https://doi.org/10.3184/095422914X13957810218711 [ Links ]
37. Domingo PL, Garc BA, Leal JM, Acid-base behaviour of the ferricyanide ion in perchloric acid media. Spectrophotometric and kinetic study. Can J Chem. 1990;68:228-235. https://doi.org/10.1139/v90-030 [ Links ]
38. Chowdhury S, Rakshit A, Acharjee A, Ghosh A, Mahali K, Saha B, Trivalent ruthenium and iridium salt: Excellent homogeneous catalysts for cyclic alcohol oxidation in micellar media. Tenside Surfact Det. 2020;57:298-309. https://doi.org/10.3139/113.110696 [ Links ]
39. Chowdhury S, Rakshit A, Acharjee A, Ghosh A, Mahali K, Saha B, Ru (III) catalysed oxidation of 2-propanol by Cr (VI) in micellar media. J Mol Liq. 2019;290:1247. https://doi.org/10.1016/j.molliq.2019.111247 [ Links ]
40. Dubey S, Sharma N, Khandelwal CL, Kinetics and mechanism of osmium(VIII)-catalyzed oxidation of hypophosphite by hexacyanoferrate(III) in aqueous alkali. Trans Met Chem. 2003;28:176-181. https://doi.org/10.1023/A:1022906921313 [ Links ]
41. Naik RM, Kumar B, Kinetics and Mechanism of Formation of [Fe(CN)5D-PA]3- by Ligand Substitution Reaction in the Presence of Surfactant Micelle in Acidic Media. J Dis Sci Technol. 2012;33:647-653. https://doi.org/10.1080/01932691.2011.579823 [ Links ]
42. Graciani MM, Rodriguez MA, Moyá ML, Study of the ligand substitution reaction Fe (CN)5 H2O3- + pyrazine in micellar solutions. Int J Chem Kinet. 1997;29:377-384. [ Links ]
43. Bunton CA, Nome F, Quina FH, Romsted LS, Ion binding and reactivity at charged aqueous interfaces. Acc Chem Res. 1991;24:357-364. https://doi.org/10.1021/ar00012a001 [ Links ]
44. Brinchi L, Profio PD, Germani R, Savelli G, Tugliani M, Bunton CA, Hydrolyses of dinitroalkoxyphenyl phosphates in aqueous cationic micelles acceleration by premicelles. Langmuir 2000;16:10101-10105. https://doi.org/10.1021/la000799s [ Links ]
45. López-Cornejo P, Mozo JD, Roldán E, Dominguez M, Sanchez F, Kinetic study of the reaction*[Ru(bpy)3]2+ + S2O82- in solutions of Brij-35 at premicellar and micellar concentrations. Chem Phys Lett. 2002;352:33-38. https://doi.org/10.1016/S0009-2614(01)01287-8 [ Links ]
46. Piszkiewicz P, Cooperativity in bimolecular micelle-catalysed reactions. inhibition of catalysis by high concentrations of detergent. J Am Chem Soc. 1977;99:7695-7697. https://doi.org/10.1021/ja00465a046 [ Links ]
47. Sen PK, Gani N, Pal B, Effects of microheterogeneous environments of sds, tx-100, and tween 20 on the electron transfer reaction between lleucine and AuCl4-/AuCl3(OH). Ind Eng Chem Res. 2013;52:2803-2813. https://doi.org/10.1021/ie302656d [ Links ]
48. Acharjee A, Rakshit A, Chowdhury S, Malik S, Barman MK, Ali MA, Saha B, Micellar catalysed and heteroaromatic base promoted rate enhancement of oxidation of an alicyclic alcohol in aqueous medium. J. Mol Liq 2019;277:360-371. https://doi.org/10.1016/j.molliq.2018.12.082 [ Links ]
49. Ghosh A, Das P, Saha D, Sar P, Ghosh SK, Saha B, Rate enhancement via sodium dodecyl sulfate (SDS) encapsulation of metal-mediated cerium(IV) oxidation of D-mannitol to D-mannose at room temperature and pressure: a kinetic and mechanistic approach. Res Chem Intermed. 2016;42:2619-2639. https://doi.org/10.1007/s11164-015-2171-6 [ Links ]
50. Jimenez R, Bueno E, Cano I, Corbacho E, Micellar effects on a ligand substitution reaction: Kinetics of the formation of [Fe(CN)5(µ-pz) Ru(NH3)5]-, from [Fe(CN)5H2O]3- and [Ru(NH3)5pz]2+, in the presence of anionic micelles. Int J Chem Kinet. 2004;26:627-633. https://doi.org/10.1002/kin.20038 [ Links ]
Received 18 April 2023
Revised 31 July 2023
Accepted 03 August 2023
* To whom correspondence should be addressed Email: aabhichem@gla.ac.in














