Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Animal Science
versão On-line ISSN 2221-4062
versão impressa ISSN 0375-1589
S. Afr. j. anim. sci. vol.53 no.3 Pretoria 2023
http://dx.doi.org/10.4314/sajas.v53i3.03
Microbiological quality assessment of milk and its fermented derivatives produced in the Sfax region, Tunisia
R. JarbouiI, II, III, #; H. GhamguiIII; S. SmaouiIV; E. AmmarI, V
ILaboratory of Environment Sciences and Sustainable Development, University of Sfax, Preparatory Institute of Engineering Studies of Sfax, Road Menzel Chaker km 0.5; 3018 Sfax, Tunisia
IIDepartment of Biology, Colleges of Sciences, Jouf University, Saudi Arabia
IIIFaculty of Sciences of Sfax, University of Sfax, Tunisia
IVHygienic Laboratory of Sfax, Ministry of Health, Road El Ain km 0.5, 3000 Sfax, Tunisia
VNational Engineering School of Sfax, BP 1173, 3038 Sfax, Tunisia
ABSTRACT
The aim of this study was to evaluate the microbial quality and safety of milk and its derivatives in the Sfax area, Tunisia. Two hundred and forty samples of ultra-high temperature sterilized milk (UHT) and pasteurized milk, and its derivatives (yoghurt, fermented milk, cheese, and butter), collected from industrial sale sites and traditional small-scale dairy processing units, were microbiologically analysed and the results compared to the European commission (EC, 2004) maximum limits. All the UHT sterilized milks analysed were free of microbial contamination and packaging-stable. However, pasteurized milk by thermal treatment at 63 °C for 30 min showed that 41 and 13% of samples were contaminated with total microbial count (TMC) and Escherichia coli. In the analysed samples, total and faecal coliform bacteria (TC and FC) counts exceeded the EU limits in 17 and 9% of industrial yoghurts; 85 and 54% of traditional yoghurts, and 57 and 29% of traditional butters, respectively. Furthermore, all studied fermented milks (Lben and Raieb) exceeded the EU coliform limits. Considering the traditional soft cheese made with pasteurized milk, 61% of the analysed samples respected EU limits in TC except one sample contaminated with both of E. coli and Staphylococcus aureus. Salmonella spp. and Listeria monocytogenes detection showed that only one pasteurized milk sample was contaminated by L. monocytogenes, without any Salmonella detection. Traditional producers must implement good manufacturing practices and hazard analysis critical control points (HACCP) to ensure integral safety and quality of dairy products.
Keywords: fermented milk products, pasteurized milk, microbiological quality, UHT milk
Introduction
Milk has a complex biochemical composition and its high water activity and nutritional value contribute to its function as an excellent growth medium for many microorganisms (Chawla, 2018; Franceschi et al., 2021). Consequently, it may vehicle and transmit foodborne pathogens to humans (Anonymous, 2004; Ahmed et al., 2014). Some bacteria are useful and necessary for the transformation of milk to other products; lactic acid bacteria and moulds are needed for cheese ripening and as yeasts to transform sugars to alcohol (Ben Hassine et al., 2015; Franceschi et al., 2021). Other bacteria may interfere, such as mesophilic bacteria and psychrophilic microorganism species, which are mainly the members of the genus, Pseudomonas, and include other germs infecting milk during its collection and/or storage (Ben Hassine et al., 2015). However, other contaminating bacteria are pathogenic agents such as Bacillus cereus (Keshtkar et al., 2016), Staphyíococcus aureus (Debbabi et al., 2018; Karzis et al., 2018), Escherichia coíi, Salmonella enterica (Lama et al., 2018), S. enteritidis (Yu et al., 2021), S. typhimurium (Tadielo et al., 2022), and Listeria monocytogenes (Deshapriya et al., 2006).
The most common thermal treatment processes widely used to extend milk shelf life are pasteurization and ultra-high temperature sterilized milk (UHT). Two pasteurization processes are used, the high temperature, short time (HTST) achieved by milk heat-treatment at 70-75 °C for 15-40 s, and longtime low temperature (LTLT), called the Holder method, by heating milk at 63 °C for 30 min. After pasteurization, milk is stored and distributed at +4 °C. The UHT involves the rapid heating of milk to at least 135 °C, for a few seconds. It is then packaged, stored, and distributed under room temperature, giving a shelf life of six months (Codex Aíimentarius, 2004; Zhang et al., 2022). The heat treatment improves the shelf life of milk by destroying pathogenic as well as spoilage microorganisms and preserves the safety and authentic good flavour of milk for a long time.
However, milk and its products could be contaminated by several pathogens that cause milk spoilage during and/or after heat treatment. The growth of spore-forming bacteria after heat treatment and microbiological quality of raw milk is a critical factor influencing milk shelf life and its products. Raw milk with a high microbial count could cause a serious coagulation problem (Melini et al., 2017). Indeed, the long-time refrigeration of raw milk before heating treatment can increase the growth of psychrotrophic microorganisms that produce thermostable enzymes such as proteinases and lipases (Fitouhi et al., 2018).
The first milk microbial contamination source is the infected or sick lactating animal. Secondly, microbial pollution occurs along the milk value chain including dairymen, milk handlers, unsanitary utensils and/or milking equipment, and water supplies used in sanitary activities. Other milk microbial contamination sources happen during its handling, transportation, and storage (Anonymous, 2004; Debbabi et al., 2018; Franceschi et al., 2021).
The common method used to extend milk shelf life in Tunisia is Holder pasteurization, which is especially practical in traditional small-scale processing units. The main factors influencing the quality of products in these traditional units include the lack of knowledge of good hygiene practices by unqualified workers and poor preservation conditions. Industrial processing plants use UHT thermal treatment at 140 °C for 4 s (Fitouhi et al., 2018) and Holder pasteurization for some milk products, based on HACCP guidelines to manage food safety and the quality of products (Samet-Bali et al., 2016; Debbabi et al., 2018; Fitouhi et al., 2018).
Fermented milk products are a very popular food in the daily diet of humans, which plays an important nutritional role in modern style-life (Ahmed et al., 2014). In Tunisia, a great diversity of dairy products is available, including raw and pasteurized milk, as well as fermented milk such as yoghurt, Lben, Raieb, cheese, cream, and butter (Debbabi et al., 2018; Fitouhi et al., 2018). These perishable foods could be contaminated by antibiotics, pesticides (Klibi et al., 2018), detergents, disinfectants, and brominated compounds (Ben Hassine et al., 2015).
Several studies have investigated the microbial quality of milk and its products through the world; in Egypt (Ahmed et al., 2014; Abbas et al., 2016), in Saudi Arabia (Al-Otaibi, 2009), in Sri Lanka (Deshapriya et al., 2006), in Iran (Koushki et al., 2015); in South Africa (Karzis et al., 2018), in Korea (Ryu et al., 2021), in India (Mallappa et al., 2021), and in Tunisia (Dabbabi et al., 2018; Fitouhi et al., 2018; Klibi et al., 2018). However, in Sfax region, Tunisia, a limited number of investigated samples in few studies have considered the microbial contamination of UHT (tetra-pack packaging) and pasteurized milk, as well as its fermented products (Samet-Bali et al., 2016). The present study aimed to investigate the microbial quality assessment of cow's milk and its derivatives collected from industrial processing plants and traditional small-scale units in Sfax city (Tunisia), and to evaluate the hygiene and safety states of these agro-food products.
Materials and methods
Sampling was undertaken between January 2018 and April 2018 to assess the bacteriological quality of milk products. Two hundred and forty samples of UHT and pasteurized milk and their fermented products (yoghurt, Raieb, Lben, cheese, and butter) were collected from different industrial and traditional sales sites located in Sfax city (34° 44' N, 10° 45' E) in Tunisia (Table 1). Raieb and the Lben are the two main traditional dairy products consumed by Tunisian people. The first one is a milk product prepared by spontaneous fermentation at room temperature (25 ± 2 °C); its coagulation requires up to 18 h. After acidification and coagulation, the product is called "Raieb". Lben is prepared by spontaneous acidification of untreated raw milk until coagulation, followed by churning to recover the traditional butter. The preparation of the Lben involves warm water addition during the churning to enhance fatty phase separation by coalescence.
The samples were collected aseptically in sterile bags, kept in ice-cool box, and then immediately transported within 6 h to the Laboratory of Hygiene of Sfax, belonging to the Ministry of Health in Tunisia, where they were microbiologically analysed. The microbial concentration values were interpreted according to the European Commission (EC) limits for UHT sterilized milk, pasteurized milk, and its products (EC, 2004).
The detection of food-borne pathogens in the sampled milk and its fermented products was qualitatively determined after enrichment. An amount of 25 g of each sample was enriched at a 1:10 ratio in buffered peptone water (BPW; VWR International) for 18 h at 37 °C. One millilitre of the stock solution or of its decimal dilutions was placed in a plate, to which ~15 mL of Plate Count Agar (PCA) medium (BioMé-rieux, Marcy l'Etoile, France) were added. These plates were incubated at 30 + 1 °C for 72 + 3 h. The plates with the number of colonies between 15 and 300 were counted (ISO, 2007). The detection of β-glucuronidase-positive E. coli was carried out in industrial and traditional pasteurized milk and in traditional soft cheese according to ISO (2019) on tryptone bile x-glucuronide (TBX) medium (BioMérieux, Marcy l'Etoile, France). The result was obtained directly by counting the characteristic colonies after incubation at 44 + 1 °C for 24 + 1 h. Furthermore, the enumeration and isolation of total faecal coliforms were performed on Violet Red Bile Lactose (VRBL) medium (BioMérieux, Marcy l'Etoile, France) according to ISO (2007) after incubation at 30 °C and 44 °C for 18 to 24 h for total and faecal coliforms, respectively. The plates with a number of colonies between 10 and 150 were counted (ISO, 2007). The number (N) of colony-forming units per ml or per gram of sample (cfu/mL; cfu/g) was calculated considering the number of colonies obtained in the Petri dishes, according to the standard NF/ISO (2017):
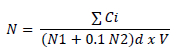
Σ Ci: Sum of characteristic colonies counted on all retained Petri dishes;
N1: Number of Petri dishes retained at the first dilution;
N2: Number of Petri dishes retained at the second dilution;
d: Dilution rate corresponding to the first dilution;
V: Inoculated volume (mL);
N: Number of microorganisms (cfu/mL).
Isolation and enumeration of S. aureus
From the stock suspension, 0.1 mL was spread on Baird Parker (BP) medium plates (Merck KGaA, Darmstadt, Germany). The Petri dishes were incubated at 37 + 1 °C for 24 + 1 h. Two colony types were detected: typical, black-coloured, bright, converse colonies surrounded by a clear area and often an opalescent ring and atypical black-coloured colonies with no transparent edges. These two types of colonies underwent confirmatory tests (ISO, 2000). Five typical and atypical colonies were then taken from the Petri dish. For the same colony, a streak on Chapman medium (Merck KGaA, Germany) and another 2 cm steak on DNA agar (Merck KGaA, Germany) were performed. The Petri dishes were incubated at 37 + 1 °C for 24 + 1 h. In Chapman medium, S. aureus is positive when surrounded by a bright yellow zone.
In DNA agar, the polymerized DNA are precipitated in the presence of 1N HCl and the medium becomes opaque. If the bacteria produce sufficient deoxyribonucleases, a clear halo resulting from DNA hydrolysis appears around the colonies. Coagulase is an enzyme that occurs during the exponential growth phase and allows the clotting of rabbit plasma oxalate citrate. Weckman & Catlin (1957) showed a good correlation between deoxyribonuclease production and coagulase activity in S. aureus strains. A colony was inoculated in 0.5 mL of brain-heart infusion (BHI) broth medium (Merck KGaA, Germany) and incubated for 18-24 h at 37 + 1 °C. After incubation, 0.1 mL of the culture was mixed in a sterile haemolysis tube with 0.3 mL of rabbit plasma. The tube was incubated at 37 + 1 °C for 24 + 1 h. Staphyíococcus aureus strains induced plasma coagulation most often after 30 min to 3 h of incubation (ISO, 2000).
ISO 6579-2017 (2017) is the horizontal reference method used for the detection of Salmonella spp. For Salmonella detection, 25 mL of each type of milk and its products were enriched in 225 mL of buffered peptone water (Alpha Bioscience, Maryland, USA), then incubated at 37 + 1 °C for 18 + 1 h. Then, 0.1 mL of the pre-enrichment was inoculated in 10 mL of Rappaport-Vassiliadis soya (RVS) broth (Merck KGaA, Germany) and incubated at 42 + 1 °C for 24 + 1 h. Suspected Salmonella colonies were sub-cultured on xylose lysine deoxycholate agar (XLD) medium (Merck KGaA, Germany, 24 h, 37 °C). Biochemical tests were subsequently performed on one or more characteristic colonies to confirm the identification of Salmonella. The API 10S gallery (BioMérieux, France) was used for the biochemical identification.
Three steps were carried out for the detection of L. monocytogenes in milk products (ISO, 2017). Firstly, 25 g of the product were added to 225 mL of Fraser broth (Merck, France). The mixture was incubated at 30 + 1 °C for 25 h + 1 h. Secondly, a transplant was carried out on polymyxin acriflavine lithium chloride ceftazidime aesculin mannitol agar (known as PALCAM medium; Alpha Bioscience, Maryland, USA) from the enrichment primary culture and then incubated at 37 + 1 °C for 48 + 2 h. The lack of growth on the PALCAM medium proves the absence of Listeria in the first enrichment. When suspicious colonies were detected, 0.1 ml of the first enrichment culture was added to 10 mL of Fraser broth and then incubated at 37 + 1 °C for 48 + 2 h. Inoculation was made on PALCAM medium, and incubated at 37 + 1 °C for 24 to 48 h, where Listeria sp. were presented as an olive green colony, with a central depression surrounded by a black halo.
For the Listeria biochemical identification, five typical colonies were inoculated on tryptone soy yeast extract agar (TSYEA) medium (Alpha Bioscience, Maryland, USA) and then incubated at 37 + 1 °C for 24 + 1 h. Listeria appeared as small, glossy, and transparent colonies in translucent chains with regular edges. These colonies were subjected to biochemical identification based on Gram stain, catalase test, and mobility followed by an API 10 Listeria gallery identification (Bio-Mérieux, France).
All the analyses were conducted in triplicate. The results were expressed as mean ± standard deviation. Comparisons between the groups were performed using SPSS with one-way ANOVA tests, followed by a Student t-test; differences were considered significant at P <0.05.
Results and discussion
A total of 43 samples of pasteurized milk and 50 samples of sterile UHT milk were microbiologically analysed (Table 2). All the sterile UHT milk samples were free of microbial contamination, regardless of the sterilization process used. A stability test was performed on these samples following incubation at 25 and 30 °C for 21 days. The results showed no change in the packaging, odour, appearance, and pH (pH = 6.74 ± 0.04) of UHT milk. The results revealed an increment of total microbial count (TMC) in pasteurized milk, with 41 samples (98%) exhibiting high growth of TMC with a mean (± SD) of 2 x 108 cfu/mL (± 25%) and 13 samples (30%) showed E. coli growth with a mean (± SD) of 4.52 x 106 cfu/mL (± 10%). There is a difference between TMC and E. coli in all tested samples (P <0.05), but there was no difference (P >0.05) in microbial count in the pasteurized milk samples. These results exceeded the European Commission (EC) maximum limit of total microbial count (5 x105 cfu/mL) and of E. coli (103 cfu/mL), which confirmed the unsatisfactory safety of pasteurized milk samples destined for consumers. The UHT heating process (140 °C for 4 sec) should eliminate pathogenic microorganisms and increase the shelf life of unopened packages, which can then be stored for six months at ambient temperature (Machado et al., 2017). Fitouhi et al. (2018) mentioned that UHT sterilization of milk inactivated vegetative bacteria and most spore-forming bacteria. While testing 254 samples of pasteurized milk, Koushki et al. (2015) reported that the total microbial count and E. coli contamination exceeded the standard limits in 61.1 and 8.7% of the samples, respectively. Other studies have shown a substantial contamination of pasteurized milk by total mesophilic flora and E. coli that exceeds the standard limits, indicating an insufficient efficiency of the thermal treatment applied by dairy producers (Belli et al., 2013; Woldemariam & Asres, 2017; Osman et al., 2020).
Samet-Bali et al. (2016) reported that pasteurization treatment may destroy many microorganisms in milk, but its improper handling could re-contaminate the milk after thermal treatment. The high concentration of total microbial count in pasteurized milk could be related to a high microbial contamination of the raw milk used. Several studies reported that a higher microbial load of raw milk resulted in higher total microbial count of pasteurized milk (Salman & Hagar, 2013; Osman et al., 2020). Moreover, the presence of E. coli in pasteurized milk is a faecal contamination indicator, which may be attributed to an inappropriate sanitary environment in the post-pasteurization process (Samet-Bali et al., 2016; Ryu et al., 2021). This contamination could be associated with foodborne poisoning. In pasteurized milk samples, Listeria was detected in only one sample that was contaminated by L. monocytogenes. The work performed by Bogdanovicové et al. (2016) detected the presence of L. monocytogenes in three samples of different raw milks (cow, goat, and sheep milk) studied in the Czech Republic. This bacterium is responsible for listeriosis, manifested mainly in pregnant women and immune-compromised people (Davis et al., 2014; Lee et al., 2019).
The present study of the commercial sampled yoghurts showed relatively low contamination by total and faecal coliforms (TC and FC), with 83% and 91% of samples following EC limits for TC and FC, respectively. However, 54% and 85% of traditional dairy yoghurts exceeded the standard limits in TC and FC, respectively (Table 3). In traditional yoghurts, the coliform counts at 37 and 44 °C showed no marked difference between all the studied samples, which can be explained by the lack of pasteurization or by the failure in the process, especially in the inadequacy of the temperature and time needed. According to the microbiological criteria for yoghurts recommended by the European Commission, counts of TC and FC should not exceed 10 cfu/g and 0.1 cfu/g, respectively. Pal et al. (2016) mentioned that the exposure of yoghurt to microbial contamination during processing, storage, and transport without basic sanitary practices and temperature control, could rapidly lead to the product spoilage, making it unacceptable for human consumption. In all analysed samples, Salmonella and Listeria were not detected, and consequently all the investigated products were safe from these pathogenic bacteria.
The microbiological analysis of the 31 Lben and Raieb samples is presented in Table 4. According to the EC standard limits, the analysis involved the counts of total and faecal coliforms, Salmonella sp., and L. monocytogenes. The results revealed the absence of Salmonella sp. and L. monocytogenes in all the analysed samples. However, all of these exceeded the European Commission limits of total and faecal coliforms (10 cfu/g and 0.1 cfu/g, respectively), confirming the high microbial contamination and the doubtful safety of the traditional fermented milks (Lben and Raeib). There was no marked difference between TC and FC in the Lben and Raeib samples (P >0.05).
Abbas et al. (2016), when studying the chemical characterization and microbial quality of "Laban Rayeb," as it is called in Egypt, found the same results. Furthermore, Samet-Balti et al. (2016) reported that 66.66 % of traditional Raieb sampled were contaminated by coliforms in Tunisia. Al-Otaibi (2009) recorded low concentrations of coliforms because of acidic pH values (pH <4.5) in the fermented milk; these results were confirmed by Samet-Balti et al. (2016), who mentioned a fermented milk of pH 3.8 - 4.7.
Coliforms can cause rapid spoilage of milk because they ferment lactose, producing acid and gas and are able to degrade milk proteins. Coliforms are associated with poor hygiene, and their occurrence in the product may indicate a potential health risk (Ryu et al., 2021). The dairy product's original contamination is linked to the lack of hygiene along the production chain, starting with its management to the preservation of the final product, from milking and processing (including lack of hand hygiene, potable water unavailability, equipment cleaning, and lack of disinfection) (Samet-Balti et al., 2016; Ryu et al., 2021).
The microbiological analysis of 18 traditional soft cheeses was concerned with the total coliform (TC), E. coíi, S. aureus, Salmonella spp., and L. monocytogenes counts (Table 5), in accordance with EC standard limits. The results showed that 61% of these samples were acceptable in accordance with EC standard limits of total coliforms, and 94% were acceptable in terms of E. coíi and S. aureus. Only one sample of traditional cheese was contaminated with E. coíi and S. aureus, while Salmonella and Listeria were not detected in all the studied samples. No difference (P >0.05) was revealed between growth germs in all the traditional soft cheese samples.
Soft cheeses made from pasteurized milk are frequently contaminated during the manufacturing process, especially during the ripening process; or cold storage may pose a hygienic hazard. This is particularly the case of the microorganisms belonging to the coliform groups, which are very often found in soft cheeses. This includes E. coli species, which are considered responsible for food poisoning.
The presence of coliform and E. coli generally indicates exogenous contamination of faecal origin, which demonstrates poor hygiene conditions during or after product processing. In addition, the presence of non-faecal coliforms indicates the risk of prevalence of other pathogenic bacteria (Ben Hassine et al., 2015). Ryu et al. (2021) detected coliform concentrations exceeding the standard in cheese produced in one of six Korean farms, suggesting that hygiene management is the main prevention method for faecal coliform contamination. Raw milk cooling temperature affects the cheese microbial quality. In this case, Franceschi et al. (2021) mentioned that cooling temperatures of 9 °C reduced the number of spoilage bacteria (total count bacteria and psychrophilic bacteria) of Parmigiano Reggiano cheese compared to 20 °C.
Microbiological analysis of traditional butter indicated high percentages of TC and FC contamination of 57% and 29%, respectively. One butter sample (14%) showed contamination with S. aureus (Table 6). In the traditional butter samples, detection of Salmonella spp. and L. monocytogenes was negative. Ahmed et al. (2014) reported high coliform counts in all the studied butter samples in Egypt, with a mean value of 107-108 cfu/g. Staphylococcus aureus is mastitic bacteria which may contaminate bulk milk and is a public health concern because it is a zoonotic pathogen (Karzis et al., 2018). In Tunisia, traditional butter is obtained after spontaneous fermentation of milk, followed by churning, which concentrates the fat ~20 times. According to Samet-Bali et al. (2009), the traditional Tunisian butter shelf life is 12 days at 4 °C and 2 days when stored at 10 °C. Prolonged storage time and improper plant sanitation are the major sources of traditional butter spoilage by microorganisms (Samet-Bali et al., 2009; Mehdizadeh et al., 2019).
Conclusion
In the present study, the majority of the investigated commercial dairy products showed compliance of greater than 80% in accordance with the EC limits. Traditionally manufactured products were contaminated by several pathogenic organisms affecting human health, with the majority of the investigated samples being out of the acceptable standards. The lack of hygiene and deficiency of awareness among the employees working at the farm (i.e., hygiene, milking practice) and those implicated during the milk processing could be responsible for the initial raw milk contamination and spoilage of the derivatives. Traditional producers must implement good manufacturing practices and HACCP management systems to ensure safety and quality of dairy products. Furthermore, the Government is responsible for protecting consumers and ensuring food safety by strengthening food control systems and working to implementing laws and inciting the application of appropriate preventive methods, such as HACCP, to hold industries more accountable and regulate food hazards.
Author Contributions: R.J.; writing-original draft preparation, H.GH.; formal analysis, S.S.; methodology and investigation, A.E.; supervision and writing-review and editing. All authors have read and agreed to the published version of the manuscript.
Acknowledgments: The authors are grateful to the personnel of the Laboratory of Hygiene in Sfax for their help in the microbiological analysis.
Conflict of interest statement: The authors declare no conflict of interest that are directly or indirectly related to the research.
References
Abbas, A.A., Amer, A.A. & Mohamed, S.I.S., 2016. Physical, chemical, and microbiological properties of Laban Rayeb. Alexandria J. Veter. Sci. 51, 269-274. DOI:10.5455/ajvs.230003 [ Links ]
Ahmed, L.I., Morgan, S.D., Hafez, R.S. & Abdel-All, A.A.A., 2014. Hygienic quality of some fermented milk products. Int. J. Dairy Sci. 9, 63-73. DOI: 10.3923/ijds.2014.63.73 [ Links ]
Al-Otaibi, M.M., 2009. Evaluation of some probiotic fermented milk products from Al-Ahsa Markets, Saudi Arabia. Am. J. Food Technol. 4, 1-8. DOI: 10.3923/ajft.2009.1.8 [ Links ]
Anonymous, 2004. Regulation (EC) No 853/2004 of the European Parliament and of the council of 29 April 2004 laying down specific hygiene rules for on the hygiene of foodstuffs. Official Journal of European Commission L139, 1-55. [ Links ]
Belli, P., Cantafora, A.F.A., Stella, S., Barbieri, S. & Crimella, C., 2013. Microbiological survey of milk and dairy products from a small-scale dairy processing unit in Maroua (Cameroon). Food Control 32, 366e370. DOI:10.1016/J.FOODCONT.2012.12.021 [ Links ]
Ben Hassine, S., Ben Ameur, W., Eljarrat, E., Barceló, D., Touil, S. & Driss, M.R., 2015. Methoxylated polybromin-ated diphenyl ethers (MeO-PBDE) in human milk from Bizerte, Tunisia. Env. Res. 138, 32-37. DOI:10.1016/j.envres.2015.01.016 [ Links ]
Bogdanovicová, K., Vyletëlová-Klimesová, M., Babák, V., Kalhotka, L., Kolácková, I. & Karpísková, R., 2016. Microbiological quality of raw milk in the Czech Republic. Czech Journal of Food Science 34,189-196. DOI: 10.17221/25/2016-CJFS [ Links ]
Chawla, S., 2018. Role of water activity in dairy industry. International Journal of Trend in Research and Development, 5(2), ISSN: 2394-9333. [ Links ]
Codex Alimentarius, Standard CAC-RCP57-2004: Code on Hygienic Practice for Milk and Milk Products. 2004. Available online: http://codexalimentarius.org (accessed on 31 August 2017). [ Links ]
Davis, B.J., Li, C.X. & Nachman, K.E., 2014. A literature review of the risks and benefits of consuming raw and pasteurized cow's milk; A response to the request from the Maryland House of Delegates' Health and Government Operations Committee; John Hopkins Report; Johns Hopkins University: Baltimore, MD, USA. [ Links ]
Debbabi, H., Gliguem, H. & Ben Salah, A., 2018. Effect of milk pre-treatments on chemical composition, and sensory quality of traditional fermented milk, rayeb. J. New Sci. 56(1), 3653- 3659. [ Links ]
Deshapriya, R.M.C., Silva, K.F.S.T. & Wilbey, R.A., 2006. Investigation of suitable pasteurization conditions for raw milk in Srilanka. Sri Lanka Veter. J. 53,1-6. DOI:10.29322/JJSRP.8.7.2018.p7938 [ Links ]
European Commission, EC, 2004. Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules on the hygiene of foodstuffs. L 139, 30.4, 55-200. [ Links ]
Fitouhi, N., Gmar, M., Sboui, N. & Debbabi, H., 2018. Correlations between quality, enzymatic activities, and storage stability of UHT milk in Tunisia. Nature Technol. J. Vol. B, Agronomic & Biological Sciences 18, 36-41. http://www.univ-chlef.dz/revuenatec/issue-18/Article_B/Article_478.pdf [ Links ]
Franceschi, P., Brasca, M., Malacarne, M., Formaggioni, P., Faccia, M., Natrella, G. & Summer, A., 2021. Effects of the cooling temperature at the farm on milk maturation and cheese making process in the manufacture of Parmigiano Reggiano PDO Cheese. Animals 11, 2835. DOI: 10.3390/ani11102835 [ Links ]
International Organization for Standardization, ISO 11290-1, 2017. Microbiology of food and animal feeding stuffs- Horizontal method for the detection and enumeration of Listeria monocytogenes - Part 1: Detection method. Geneva. [ Links ]
International Organization for Standardization, ISO 16649- 1, 2019. Microbiology of food and animal feeding stuffs - Horizontal method for the enumeration of beta-glucuronidase-positive Escherichia coli - Part 1: Colony-count technique at 44 °C using membranes and 5-bromo-4-chloro-3-indolyl beta-D-glucuronide. 2001. [ Links ]
International Organization for Standardization, ISO 6579:2002/AMD 1, 2017. Microbiology of food and animal feeding stuffs-Horizontal method for the detection of Salmonella spp.- Amendment 1: Annex D: Detection of Salmonella spp. in animal faeces and in environmental samples from the primary production stage. 2007. https://www.iso.org/standard/42109.html. [ Links ]
International Organization for Standardization, ISO 6888-1, 2000. Microbiology of food and animal feeding stuffs -Horizontal method for the enumeration of coagulase positive staphylococci (Staphylococcus aureus and other species) (ISO 6888-1:1999) - Part 1: Technique using Baird-Parker agar medium. International Organization for Standardization, ISO 7218, 2007. Microbiology of food and animal feeding stuffs -General requirements and guidance for microbiological examinations. International Standards Organisation. Geneva. [ Links ]
Karzis, J., Petzer, I.-M., Donkin, E.F. & Naidoo, V., 2018. Proactive udder health management in South Africa and monitoring of antibiotic resistance of Staphylococcus aureus in dairy herds from 2001 to 2010. J. South Afric. Veter. Ass. 89, a1490. DOI: 10.4102/ jsava.v89i0.1490 [ Links ]
Keshtkar, M., Momtaz, H., Ghavamizadeh, M. & Rahimi, E., 2016. Detection of enterotoxin gene profiles of Bacillus cereus in pasteurized and sterile milk, baby food, and dairy products. Inter. Medical J. 23(2), 110- 113. [ Links ]
Klibi, A., Maaroufia, A., Torres, C. & Jouini, A., 2018. Detection and characterization of methicillin-resistant and susceptible coagulase-negative staphylococci in milk from cows with clinical mastitis in Tunisia. Inter. J. Antimicrobial Agent 52, 930-935. DOI:10.1016/j.ijantimicag.2018.07.026 [ Links ]
Koushki, M., Koohy-Kamaly, P., Azizkhani, M. & Hadinia, N., 2015. Microbiological quality of pasteurized milk on expiration date in Tehran, Iran. J. Dairy Sci. 99, 1796-1801. DOI: 10.3168/jds.2015-10400 [ Links ]
Lamas, A., Regal, P., Vázquez, B., Miranda, J.M., Cepeda, A. & Franco, C.M., 2018. Influence of milk, chicken residues and oxygen levels on biofilm formation on stainless steel, gene expression and small RNAs in Salmonella enterica. Food Control, 90:1-9. https://doi.org/10.1016/j.foodcont.2018.02.023 [ Links ]
Lee, S.H.I., Cappato, L.P., Guimarães, J.T., Balthazar, C.F., Rocha, R.S., Franco, L.T., da Cruz, A.G., Corassin, C.H. & de Oliveira, C.A.F., 2019. Listeria monocytogenes in milk: Occurrence and recent advances in methods for inactivation. Beverages, 5, 14; doi:10.3390/beverages501001 [ Links ]
Machado, S.G., Baglinière, F., Marchand, S., Van Coillie, E., Vanetti, M.C.D., De Block, J. & Heyndricks, M., 2017. The biodiversity of the microbiota producing heat-resistant enzymes responsible for spoilage in processed bovine milk and dairy products. Front. Microbiol. 8, 302. doi: 10.3389/fmicb.2017.00302 [ Links ]
Mallappa, R.H., Balasubramaniam, C., Nataraj, B.H., Ramesh, C., Kadyan, S., Pradhan, D., Muniyappa, S.K. & Grover, S., 2021. Microbial diversity and functionality of traditional fermented milk products of India: Current scenario and future perspectives. Inter. Dairy J. 114, 104941. DOI: 10.1016/j.idairyj.2020.104941 [ Links ]
Mehdizadeh, T., Mohammadipour, N., Langroodi, A.M. & Raeisi, M., 2019. Effect of walnut kernel septum membranes hydroalcoholic extract on the shelf life of traditional butter. Heliyon, 5, e01296. doi: 10.1016/j.heliyon.2019. e01296 [ Links ]
Melini, F., Melini, V., Luziatelli, F., & Ruzzi, M., 2017. Raw and heat-treated milk: From public health risks to nutritional quality. Beverages, 3, 54; doi:10.3390/beverages3040054 [ Links ]
Osman, E.O.M., Yahya, A.A. & Mohamed, A.M.O., 2020. Microbiological quality of pasteurized milk and stirred yoghurt during the stages of processing. World J. Advanced Res. Rev. 06(2), 120128. DOI: 10.30574/wjarr.2020.6.2.0149 [ Links ]
Pal, M., Mulu, S., Tekle, M., Pintoo, S.V. & Prajapati, J.P., 2016. Bacterial contamination of dairy products. Beverage and Food World 43(9), 40-43. [ Links ]
Ryu, S., Shin, M., Yun, B., Lee, W., Choi, H., Kang, M., Oh, S. & Kim, Y., 2021. Bacterial quality, prevalence of pathogens, and molecular characterization of biofilm-producing Staphylococcus aureus from Korean dairy farm environments. Animals 11, 1306. DOI: 10.3390/ani11051306 [ Links ]
Salman, A.M.A. & Hagar, M.E., 2013. Some bacterial and physical quality of pasteurized milk in Khartoum. J. App. Ind. Sci. 1, 30-37. [ Links ]
Samet-Bali, O., Ayadi, M.A. & Attia, H., 2009. Traditional Tunisian butter: Physicochemical and microbial characteristics and storage stability of the oil fraction. LWT - Food Sci. Technol., 42, 899-905. DOI:10.1016/j.lwt.2008.11.007. [ Links ]
Samet-Bali, O., Felfoul, I., Lajnaf, R., Attia, H. & Ayadi, M.A., 2016. Hygienic quality of "Rayeb", a traditional Tunisian fermented cow's milk. Int. Food Res. J. 23, 366-369. [ Links ]
Tadielo. L.E., Bellé. T.H., Rodrigues dos Santos, E.A., Schmiedt. J.A., Cerqueira-Cézar, C.K, Nero LA, Yamatogi, R.S., Gonçalves Pereira, J. & dos Santos Bersot, L., 2022. Pure and mixed biofilms formation of Listeria monocytogenes and Salmonella typhimurium on polypropylene surfaces. LWT, 162, 113469. https://doi.org/10.1016/j.lwt.2022.113469 [ Links ]
Weckman, B.G. & Catlin, B.W., 1957. Deoxyribonuclease activity of micrococci from clinical sources. J. Bacteriol. 73, 747-753. DOI: 10.1128/jb.73.6.747-753.1957 [ Links ]
Woldemariam, H.W. & Asres, A.M., 2017. Microbial and physico-chemical qualities of pasteurized milk. J. Food Process Technol. 8, 1-5. DOI: 10.4172/2157-7110.1000651 [ Links ]
Yu, S., Xu, Q., Huang, J., Yi, B., Aguilar, Z.P. & Xu, H., 2021. Rapid and sensitive detection of Salmonella in milk based on hybridization chain reaction and graphene oxide fluorescence platform. J. Dairy Sci. 104:122951230. https://doi.org/10.3168/jds.2021-20713 [ Links ]
Zhang, H., Xu, Y., Zhao, C., Xue, Y., Tan, D., Wang, S., Jia, M., Wu, H., Ma, A., Chen, G., 2022. Milk lipids characterization in relation to different heat treatments using lipidomics. Food Res. Inter. 157, 111345. https://doi.org/10.1016/j.foodres.2022.111345 [ Links ]
Submitted 8 October 2022
Accepted 10 April 2023
Published 9 July 2023
# Corresponding author: rajajarboui2000@yahoo.fr














