Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.114 n.4 Pretoria Apr. 2024
http://dx.doi.org/10.7196/SAMJ.2024.v114i4.1667
RESEARCH
Prevalence and predictors of severe Crohn's disease at a tertiary hospital in South Africa
C L GoundenI, II; V G NaidooIII, IV; Y MoodleyV, VI
IMB ChB, FCP (SA), Cert Gastroenterology (Phys); Department of Gastroenterology, Nelson R. Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
IIMB ChB, FCP (SA), Cert Gastroenterology (Phys); Gastrointestinal Cancer Research Group, Nelson R. Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
IIIFCP (SA) Cert Gastroenterology (Phys) MMed; Department of Gastroenterology, Nelson R. Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
IVFCP (SA) Cert Gastroenterology (Phys) MMed; Gastrointestinal Cancer Research Group, Nelson R. Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
VPhD; Division of Health Systems and Public Health, Department of Global Health, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
VIPhD; Gastrointestinal Cancer Research Group, Nelson R. Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
ABSTRACT
BACKGROUND: Predicting severe Crohn's disease (SCD) can assist in planning risk reduction therapy for SCD, thereby improving disease outcomes
OBJECTIVE: To determine the prevalence and predictors of SCD in a sample of South African (SA) patients
METHODS: This was a retrospective chart review of patients with Crohn's disease (CD) attending the gastroenterology unit at a tertiary hospital in Durban, SA. Demographic and clinical variables at diagnosis of CD were collected and analysed for statistical associations with SCD (defined as the presence of >1 of the following over the course of CD: complex perianal disease, colonic resection, >2 small-bowel resections, a single small-bowel resection >50cm, or construction of a definitive stoma). The prognostic utility of statistically significant variables was investigated by establishing their sensitivity, specificity and predictive values for SCD
RESULTS: The study sample consisted of 93 patients. The rate of SCD was 64.5%, with 63.3% of patients developing SCD within 1 year of CD diagnosis. Ileocolonic location (p=0.046) and penetrating disease at initial diagnosis of CD (p=0.021) were statistically associated with SCD. The sensitivity, specificity, positive predictive value and negative predictive value of ileocolonic location for SCD were 72.7%, 47.4%, 66.7% and 54.6%, respectively. The sensitivity, specificity, positive predictive value and negative predictive value of penetrating disease for SCD were 85.7%, 41.7%, 30.0% and 91.0%, respectively
CONCLUSION: Most patients with CD developed SCD within 1 year of their CD diagnosis. CD with a penetrating phenotype at diagnosis is a good predictor for the development of SCD and should be further investigate
Crohn's disease (CD) is a subtype of inflammatory bowel disease, involving inflammation of the gastrointestinal tract. CD results from an interaction between genetic and environmental factors. It tends to affect the distal small intestine and colon.[1] Inflammation in CD is discontinuous along the intestine, and can involve all layers from mucosa to serosa.[1] Clinical presentation may depend on location of disease, and outcomes are based on individualised factors.[2]
Corticosteroids, immunomodulators or biological agents are usually used to treat CD.[2] A proportion of patients may fail to respond to therapy, and complications will occur in half of these patients, resulting in the development of severe Crohn's disease (SCD). [3-6] Age, gender, disease location and behaviour, use of corticosteroids and smoking have been identified as predictors for the development of SCD.[6] However, these findings should be interpreted with a degree of caution, as limitations regarding the reliability of existing published data are the inconsistent definitions used for SCD, heterogeneity of study designs, as well as conclusions based on these analyses.[6]
Loly et al.[7] defined SCD as the presence of >1 of the following over the entire disease course of CD: complex perianal disease, any colonic resection, two or more small-bowel resections (or a single small-bowel resection more than 50 cm in length) or construction of a definitive stoma. Using this definition, Loly et al.[7] and Watermeyer and Thomson[8] determined the rate of development and predictors of SCD. The advantage of this definition is that it is uncomplicated, easy to apply and already validated in a South African (SA) setting. Loly et al.[7] identified weight loss and stricturing disease at diagnosis of CD to be independently associated with time to development of SCD. Watermeyer and Thomson[8] identified perianal disease and granulomas on endoscopic mucosal biopsy as predictors of SCD. Even though the criteria for SCD were the same in both studies, the study designs and conclusions varied. Furthermore, the study by Watermeyer and Thomson was the only local research conducted on predictors of SCD. Therefore, further research in a SA setting is required to understand SCD in a local context.
Additionally, early aggressive treatment[9,10] may prevent poor outcomes associated with SCD. These drugs, however, are expensive and associated with adverse events such as serious infections and development of malignancies.[11,12] In addition, optimisation of a patient's nutritional state, achievement of mucosal healing and treatment of existing sepsis may prevent morbidity and mortality associated with complex abdominal surgery for CD.[13] Consequently, it is imperative to appropriately select patients for a targeted treatment approach.
This study aimed to determine the prevalence of SCD and applicability of various clinical characteristics for the prediction of SCD in patients attending a SA tertiary hospital.
Methods
Study setting
The study was a retrospective chart review of patients with a diagnosis of CD at the gastroenterology unit at a tertiary hospital in Durban, SA. The gastroenterology unit manages both inpatients and outpatients referred from secondary public-sector hospitals in the region.
Study eligibility criteria
All patients aged >12 years with a diagnosis of CD registered from 1 January 2003 to 31 December 2019 were included. Patients who had a diagnosis that was revised and those with incomplete data were excluded from analysis.
Data collection
Each patient medical chart was reviewed, and relevant data were recorded on an Excel spreadsheet (Microsoft, USA) for the following variables at diagnosis of CD: age, gender (self-reported by patient), smoking status, presenting symptoms and presence of non-caseating granulomas on endoscopic mucosal biopsy. The presence of extraintestinal manifestations (EIMS), namely primary sclerosing cholangitis (PSC), erythema nodosum (EN), pyoderma gangrenosum (PG), uveitis, scleritis, peripheral arthritis, axial arthropathies, other EIMS at diagnosis and follow-up, medical treatments (use of corticosteroids, immunomodulators and biological agents) and hospitalisations was recorded. Disease phenotype was assessed according to the Montreal classification for CD.[14] Age at diagnosis was classified as A1: <16 years; A2: between 17 and 40 years; or A3: >40 years. A simplified age cut-off of >40 years was used for analysis. CD location was classified as follows: L1: ileal; L2: colonic; L3: ileocolonic; L4: upper gastrointestinal CD. CD behaviour was classified as follows: B1: non-stricturing/non-penetrating; B2: stricturing; B3: penetrating; p: perianal disease. The development of SCD was defined according to the definition proposed by Loly et al.[7] Those patients who did not meet criteria for SCD were classified as having non-severe Crohn's disease (NSCD).
Statistical analysis
Data were analysed using SPSS version 27.0 (IBM Corp., USA). Categorical variables are summarised using frequencies and percentages. Depending on distribution of continuous variables, medians (interquartile ranges (IQRs)) were calculated to reflect their central tendency. Standard deviations or interquartile ranges were calculated to reflect their dispersion. Pearson's x2 test was used to test for statistical associations between clinical characteristics and the development of SCD. P<0.05 was considered statistically significant. Furthermore, the predictive accuracy of characteristics statistically associated with SCD was estimated using sensitivity, specificity, positive predictive values and negative predictive values. Where applicable, 95% confidence intervals (95% CI) are provided for estimates.
Ethical approval
Ethical approval for this study was obtained from the Biomedical Research Ethics Committee of the University of KwaZulu-Natal (ref. no. BREC/00004596/2022).
Results
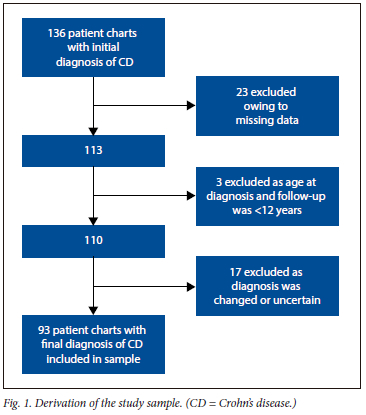
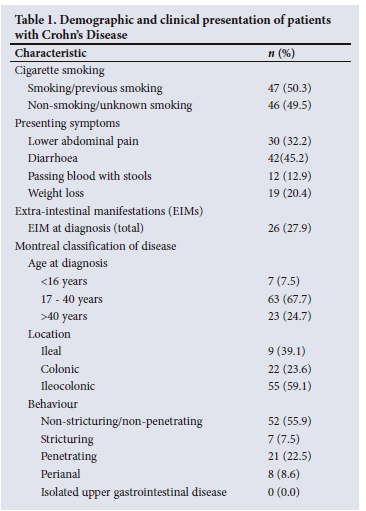
One hundred and thirty-six charts with an initial diagnosis of CD were identified. Fig. 1 provides a summary of the number of patients who were included and excluded from this study. There were 43/136 patients who were excluded, with the most common reason for exclusion being missing data (23/43 excluded patients). Therefore, the final study sample consisted of 93 patients with CD. Most patients (62.3%) were female (male: female ratio 1:1.65). The median (IQR) age at diagnosis was 30 (23 - 40) years. The median (IQR) duration of disease and follow-up were 264 (84 - 307) months and 84 (36 -120) months, respectively. Diarrhoea (n=42, 45.2%) was the main presenting complaint. Other presenting complaints, smoking status and disease phenotype according to Montreal classification are shown in Table 1. Twenty-six patients had EIMS, the most common of which were peripheral arthritis (n=13, 13.9%) and axial arthritis (n=7, 7.5%). Thirty-nine patients (41.9%) developed EIMs after the diagnosis of CD, the most common of which was anaemia (n=20, 21.5%).
Sixty patients (64.5%) met the criteria for development of SCD. The remaining 33 patients (35.5%) were classified as NSCD patients. Events meeting the criteria for SCD included: any colonic resection (n=51, 54.8%); 2 or more small bowel resections (n=10, 10.7%); single small bowel resection >50 cm in length (n=11, 11.8%); construction of a definite stoma (n=10, 10.7%); and complex perianal disease (n=10, 10.7%). More than one of the criteria for SCD were recorded in 26 (27.9%) patients. Median (IQR) time to development of SCD was 12 (1 - 60) months. In patients who developed SCD (n=60), the event occurred within 1 year in 38 patients (63.3%), within 1 - 5 years in 8 patients (13.3%) and after 5 years in 14 patients (23.3%).
The comparison of demographic and clinical features between SCD and NSCD patient groups is shown in Table 2. There was no difference between age at diagnosis of CD between the SCD and NSCD group (p=0.054). There was a significantly (p=0.046) higher proportion of patients with ileocolonic location at diagnosis of CD in the SCD group (n=40, 43.0%) when compared to the NSCD group (n=15, 16.1%). A significantly higher (p=0.02) proportion of patients with penetrating disease in the SCD group (n=18, 19.4%) when compared with the NSCD group (n=3, 3.2%) was observed. Other clinical variables analysed were not significantly different between the SCD and NSCD groups (Table 2).
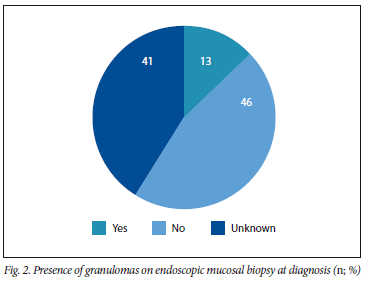
As per Fig. 2, the presence of granulomas on mucosal biopsy at diagnosis was recorded in 12 patients (12.9%). No granulomas were identified in 43 patients (46.2%). There was no statistical difference (p=0.867) in the prevalence of granulomas between the SCD (n=8, 8.6%) and NSCD (n=4, 4.3%) groups. Of the 12 patients with non-caseating granulomas on endoscopic mucosal biopsies, 9 patients (75.0%) had non-stricturing/non-penetrating disease, and 3 patients (25.0%) had penetrating disease at diagnosis of CD.
The predictive accuracy of ileocolonic disease location and penetrating disease at diagnosis for subsequent SCD is shown in Table 3. Sensitivity for ileocolonic location was fair (72.7%, 95% CI 59.0 - 83.9%). Sensitivity for penetrating disease was good (85.7%, 95% CI 63.7 - 97.0%). Specificity and the positive predictive values obtained for ileocolonic location and penetrating disease were low. The negative predictive value for ileocolonic location was low (54.6%, 95% CI 36.4 - 72.0%), but excellent for penetrating disease (91.0%, 95% CI 75.7 - 98.1%).
Discussion
This retrospective study identified that most patients with CD developed SCD. In addition, most patients developed SCD within 1 year of initial diagnosis of CD. Disease location and disease behaviour at diagnosis of CD were associated with the development of SCD. Penetrating phenotype at diagnosis had a good sensitivity and excellent NPV for predicting the development of SCD. Ileocolonic location had a fair sensitivity but low specificity for predicting the developed SCD.
The prevalence of SCD (64.5%) in this study was much higher than that in two previous studies that used the same definition for SCD.[7,8] The rates of SCD identified by Loly et al.[7] and Watermeyer and Thomson[8] were similar at 37.4% and 33.7%, respectively. The difference between findings by Loly et al. and this study may be attributed to the variation in sample size. The study by Watermeyer and Thomson had a similar sample size to this study, but excluded patients with perianal, stricturing and penetrating disease at diagnosis. Owing to differing study designs, results from the two previous studies[7,8] and the current study cannot be directly compared. Discrepant rates for major abdominal surgery at 5 years of diagnosis of CD was shown earlier in North America and Europe, at 43.7% v. 59.0%, respectively.[15] Diagnostic and treatment options were similar in these countries, therefore authors attribute differences in surgical rates to unidentified environmental and genetic factors.[15] Unidentified genetic or environmental factors and variable therapeutic options may be a reason for the high rate of SCD in this study. The tertiary setting where patients with more severe disease are inevitably treated may further contribute to high prevalence of SCD in this study.
More than half (63.3%) of the 60 patients who developed SCD in this study met this outcome early, within 1 year of diagnosis of CD. Penetrating disease at diagnosis, a more aggressive phenotype,[16] may explain the rapid progression to SCD. In the present study, the sensitivity for penetrating disease at diagnosis as a predictor for the development of SCD was good. Penetrating lesions include fistulas, phlegmons or abscesses, and may reflect development of significant damage to the bowel.[17] The association with a penetrating phenotype and the need for early abdominal surgery for CD identified in this study is consistent with the literature.[3,15,18-22]
The association between ileocolonic location and poor outcomes in CD was shown by Beaugerie et al.,[9] who used a combination of criteria to define disabling CD. Several other studies identified ileal or ileocolonic location as independent risk factors for need for surgery.[15,18,19,23] Similarly to this study, Loly et al.[7] identified a significant association between ileocolonic disease and the development of SCD. The finding was expected for small bowel disease as this location was frequently associated with penetrating and stenosing disease.[3] The ileocolonic location of disease had a lower positive and negative predictive value when compared with penetrating disease.
Young age at diagnosis of CD has been reported in previous studies as a predictor for early surgery,[24] requirement for stoma,[22] relapse of CD after surgery[18] and complex abdominal surgery.[25] The present study failed to establish an association between age at diagnosis of CD and SCD. This is consistent with studies by Loly et al.[7] and Watermeyer and Thomson[8] The literature is inconsistent with respect to age as a predictor of SCD. One study identified older age of diagnosis, between 45 and 59 years old, to be a risk for abdominal surgery in CD,[26] while other studies did not find any association.[21,27-29]
One-third of patients in this study smoked cigarettes. An association between smoking and SCD was not identified in this study. This was not unusual, as there is an inconsistent association between smoking and poor outcomes in CD reported in the current literature.[30] Some studies show a strong association between smoking and requirement for surgery,[31,32] early surgery[3] and post-surgical recurrence.[33] There are other studies that show that there is no association between smoking and poor outcomes with respect to requirement for complex abdominal surgery.[34-36]
In the present study, the presence of granulomas on colonic mucosal biopsies was not a useful predictor for the development of SCD. This was different to the only local study analysing the presence of intestinal non-caseating granulomas on histology at diagnosis of CD as a predictor for development of SCD.[8] The difference probably relates to the rate of granulomas identified. While the majority of patients had granulomas in the study by Watermeyer and Thomson,[8] it was rare in our study. A previous large study of 10 456 patients with CD also found that granulomas were rarely found (9%).[37] A recent meta-analysis of 19 studies identified that the presence of granulomas was significantly associated with hospitalisations, but not surgery.[38] This meta-analysis also identified specific factors preventing applicability to a clinical setting. These factors include a wide variation in prevalence of CD patients with granulomas, several retrospective and observational study designs, unclear method for tissue acquisition and inclusion of granulomas from both mucosal biopsies and surgical specimens.[38] Therefore, the significance of granulomas on the outcome of CD is uncertain, as conclusions made by previous studies are variable and therefore not easily applicable.
Previous studies have suggested various other predictors of poor outcomes in CD, namely gender,[15,22] weight loss,[7,37] presence of EIMS,[27] use of immunosuppressant drugs[4,27,39] and the need for repeated courses of steroids.[3,27] The present study failed to establish an association between any of these variables and SCD. The small sample size may be the main reason for an absence of a statistical association between the various clinical characteristics and development of SCD in this study. In addition, risk factors from other populations may not be applicable to the population in this study. Further studies with larger sample sizes are required to detect an association between some of the variables and the development of SCD. Comparably with recent studies, we recommend investigating additional variables such as blood parameters,[28] faecal calprotectin,[28] patterns of use of biological agents,[27,28] findings on intestinal ultrasound[28,40,41] and genotypic variables[40] to improve prediction of SCD.
A limitation of this study is the retrospective design that is more likely to have missing data. Patients were excluded because of missing data, contributing to a small sample size. Therefore, predictors identified in previous studies could not be replicated. Given the tertiary setting, patients with less severe disease may have been managed at other secondary hospitals and did not present to the gastroenterology unit at the study site for care.
This study highlights the poor outcome of patients with ileocolonic and penetrating CD. As described in literature, patients with moderate to severe ileocaecal disease who had an initial response to corticosteroids may benefit from early anti-tumour necrosis factor (TNF) agents.[17] However, variable efficacy of biological therapy is seen in patients with external and internal intestinal fistulae.[42] Response rates for external and internal intestinal fistulae to ant-TNF agents vary between 14 and 25%.[43-45] Therefore, medical therapy should be individualised in patients presenting with penetrating disease to improve outcomes. Biological therapy, specifically infliximab, was shown to be more effective in treating fistulising disease, especially perianal fistulae.[42] Abscesses and phlegmons may require initial treatment with antibiotics.[46,47] Low-output fistulae without abscess may respond to immunomodulator and biological therapy.[48] In patients with fistulising CD, management of malnutrition, mucosal inflammation and abdominal sepsis to prevent morbidity associated with subsequent bowel surgery is recommended. [13,42] While both ileocolonic location and penetrating disease at diagnosis of CD have a predictive value for the development of SCD in this study, penetrating disease is a better option. Further research is required to confirm the role of penetrating phenotype as a predictor for the development of SCD.
Conclusion
In conclusion, the prevalence of SCD in this study was high, and occurred within 1 year of initial diagnosis of CD in most patients. Penetrating disease at diagnosis of CD may be a prognostic marker for SCD at this study setting. However, further research and validation are necessary to establish the clinical utility of this prognostic marker. If validated, penetrating phenotype at diagnosis of CD may be utilised as an indicator to initiate risk reducing measures earlier.
Declaration. This research was a component of the Master's degree studies of CLG.
Acknowledgements. The authors acknowledge the contributions of the gastroenterology unit's staff towards this research.
Author contributions. CLG conceptualised and designed the research study with guidance from VGN. CLG was responsible for data acquisition and management. YM analysed data with guidance from CLG and VGN. CLG was responsible for writing the manuscript. All authors substantially contributed to manuscript revision and approved the final version.
Funding. None.
Conflicts of interest. None.
References
1. Feldman M, Friedman L, Brandt L. Sleisenger and Fortran's Gastrointestinal and Liver Disease. 10th ed. Philadelphia: Elsevier, 2016. [ Links ]
2. Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn's disease. Lancet 2017;389(10080):1741-1755. https://doi.org/10.1016/s0140-6736(16)31711-1 [ Links ]
3. Sands BE, Arsenault JE, Rosen MJ, et al. Risk of early surgery for Crohn's disease: Implications for early treatment strategies. Am J Gastroenterol 2003;98(12):2712-2718. https://doi.org/10.1111/j.1572-0241.2003.08674.x [ Links ]
4. Cosnes J, Nion-Larmurier I, Beaugerie L, Afchain P, Tiret E, Gendre JP. Impact of the increasing use of immunosuppressants in Crohn's disease on the need for intestinal surgery. Gut 2005;54(2):237-241. https://doi.org/10.1136/gut.2004.045294 [ Links ]
5. Beaugerie L, Rahier JF, Kirchgesner J. Predicting, preventing, and managing treatment-related complications in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2020;18(6):1324-1335.e2. https://doi.org/10.1016/j.cgh.2020.02.009 [ Links ]
6. Torres J, Caprioli F, Katsanos KH, et al. Predicting outcomes to optimise disease management in inflammatory bowel diseases. J Crohns Colitis 2016;10(12):1385-1394. https://doi.org/10.1093/ecco-jcc/jjw116 [ Links ]
7. Loly C, Belaiche J, Louis E. Predictors of severe Crohn's disease. Scand J Gastroenterol 2008;43(8):948-954. https://doi.org/10.1080/00365520801957149 [ Links ]
8. Watermeyer GA, Thomson SR. Granulomas at initial diagnosis of Crohn's disease signal a poor outcome. S Afr Med J 2015;105(6):480-483. https://doi.org/10.7196/samj.9093 [ Links ]
9. Beaugerie L, Seksik P, Nion-Larmurier I, Gendre JP, Cosnes J. Predictors of Crohn's disease. Gastroenterology 2006;130(3):650-656. https://doi.org/10.1053Zj.gastro.2005.12.019 [ Links ]
10. Ungaro RC, Aggarwal S, Topaloglu O, Lee WJ, Clark R, Colombel JF. Systematic review and metaanalysis: Efficacy and safety of early biologic treatment in adult and paediatric patients with Crohn's disease. Aliment Pharmacol Ther 2020;51(9):831-842. https://doi.org/10.1111/apt.15685 [ Links ]
11. Lichtenstein GR, Feagan BG, Cohen RD, et al. Infliximab for Crohn's Disease: More than 13 years of real-world experience. Inflamm Bowel Dis 2018;24(3):490-501. https://doi.org/10.1093/ibd/izx072 [ Links ]
12. Toruner M, Loftus EV, Jr., Harmsen WS, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology 2008;134(4):929-936. https://doi.org/10.1053/j.gastro.2008.01.012 [ Links ]
13. Adamina M, Bonovas S, Raine T, et al. European Crohn's and Colitis Organisation (ECCO). ECCO guidelines on therapeutics in Crohn's disease: Surgical treatment. J Crohns Colitis 2020;14(2):155-168. https://doi.org/10.1093/ecco-jcc/jjz187 [ Links ]
14. Satsangi J, Silverberg M, Vermeire S, Colombel J. The Montreal classification of inflammatory bowel disease: Controversies, consensus, and implications. Gut 2006;55:749-753. https://doi.org/10.1136/gut.2005.082909 [ Links ]
15. Peyrin-Biroulet L, Harmsen WS, Tremaine WJ, Zinsmeister AR, Sandborn WJ, Loftus EV Jr. Surgery in a population-based cohort of Crohn's disease from Olmsted County, Minnesota (1970 - 2004). Am J Gastroenterol 2012;107(11):1693-1701. https://doi.org/10.1038/ajg.2012.298 [ Links ]
16. Cosnes J, Gower-Rousseau C, Seksis P, Cortot A. Epidemiology and natural history of inflammatory bowel disease. Gastroenterology 2011;140:1785-1794.e4. https://doi.org/10.1053/j.gastro.2011.01.055 [ Links ]
17. Gomollón F, Dignass A, Annese V, et al 3rd European evidence-based consensus on the diagnosis and management of Crohn's disease 2016: Part 1: Diagnosis and medical management. J Crohns Colitis 2016;11(1):3-25. https://doi.org/10.1093/ecco-jcc/jjw168 [ Links ]
18. Solberg IC, Vatn MH, H0ie O, et al. Clinical course in Crohn's disease: Results of a Norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol 2007;5(12):1430-1438. https://doi.org/10.1016/j.cgh.2007.09.002 [ Links ]
19. Magro F, Portela F, Lago P, et al. Crohn's disease in a southern European country: Montreal classification and clinical activity. Inflamm Bowel Dis 2009;15(9):1343-1350. https://doi.org/10.1002/ibd.20901 [ Links ]
20. Moon CM, Park DI, Kim ER, et al. Clinical features and predictors of clinical outcomes in Korean patients with Crohn's disease: A Korean Association for the Study of Intestinal Diseases multicenter study. J Gastroenterol Hepatol 2014;29(1):74-82. https://doi.org/10.1111/jgh.12369 [ Links ]
21. Wewer MD, Zhao M, Nordholm-Carstensen A, Weimers P, Seidelin JB, Burisch J. The incidence and disease course of perianal Crohn's Disease: A Danish nationwide cohort study, 1997 - 2015. J Crohns Colitis 2020;15(1):5-13. https://doi.org/10.1093/ecco-jcc/jjaa118 [ Links ]
22. Nacer S, Haddad F, Tahiri M, et al. P51 Predictive factors of surgery in Crohn's disease. Gut 2022;71(Suppl 1):A64-A65. https://doi.org/10.1136/gutjnl-2022-bsg.111 [ Links ]
23. Szamosi T, Banai J, Lakatos L, et al. Early azathioprine/biological therapy is associated with decreased risk for first surgery and delays time to surgery but not reoperation in both smokers and nonsmokers with Crohn's disease, while smoking decreases the risk of colectomy in ulcerative colitis. Eur J Gastroenterol Hepatol 2010;22(7):872-879. https://doi.org/10.1097/meg.0b013e32833036d9 [ Links ]
24. Brant SR, Picco MF, Achkar JP, et al. Defining complex contributions of NOD2/CARD15 gene mutations, age at onset, and tobacco use on Crohn's disease phenotypes. Inflamm Bowel Dis 2003;9(5):281-289. https://doi.org/10.1097/00054725-200309000-00001 [ Links ]
25. Romberg-Camps MJ, Dagnelie PC, Kester AD, et al Influence of phenotype at diagnosis and of other potential prognostic factors on the course of inflammatory bowel disease. Am J Gastroenterol 2009;104(2):371-383. https://doi.org/10.1038/ajg.2008.38 [ Links ]
26. Bernell O, Lapidus A, Hellers G. Risk factors for surgery and postoperative recurrence in Crohn's disease. Ann Surg 2000;231(1):38-45. https://doi.org/10.1097/00000658-200001000-00006 [ Links ]
27. Lujan R, Atia O, Gili F, et al. DOP58 predictors of complicated disease course in CD in an administrative database: A nationwide study from the epi-IIRN. J Crohns Colitis 2023;17(Supplement_1):i128-i30. https://doi.org/10.1093/ecco-jcc/jjac190.0098 [ Links ]
28. Attauabi M, Madsen GR, Bendtsen F, et al. P293 correlation between clinical measures to capture disease burden and prediction of the initial course of Crohn's disease - findings from a Copenhagen IBD inception cohort study (IBD Prognosis Study). J Crohns Colitis 2023;17(Supplement_1):i437-i40. https://doi.org/10.1093/ecco-jcc/jjac190.0423 [ Links ]
29. Atia O, Asayag N, Focht G, et al. Perianal Crohn's disease is associated with poor disease outcome: A nationwide study from the epiIIRN cohort. Clinical Gastroenterol Hepatol 2022;20(3): e484-e495. https://doi.org/10.1016/j.cgh.2021.04.007 [ Links ]
30. Parkes GC, Whelan K, Lindsay JO. Smoking in inflammatory bowel disease: Impact on disease course and insights into the aetiology of its effect. J Crohns Colitis 2014;8(8):717-725. https://doi.org/10.1016/j.crohns.2014.02.002 [ Links ]
31. Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG clinical guideline: Management of Crohn's disease in adults. Am J Gastroenterol 2018;113(4):481-517. https://doi.org/10.1038/ajg.2018.27 [ Links ]
32. Jasper RA, Chen P-H, Patel R, Joseph S, Miller SD, Hutfless S. Tobacco use in Crohn's disease patients and association with disease outcomes in the United States Medicaid population, 2010 - 2019. JGH Open 2023;7(4):291-298. https://doi.org/10.1002/jgh3.12893 [ Links ]
33. Cottone M, Rosselli M, Orlando A, et al. Smoking habits and recurrence in Crohn's disease. Gastroenterology 1994;106(3):643-648. https://doi.org/10.1016/0016-5085(94)90697-1 [ Links ]
34. Aldhous MC, Drummond HE, Anderson N, Smith LA, Arnott IDR, Satsangi J. Does cigarette smoking influence the phenotype of Crohn's disease? Analysis using the Montreal classification. The Am J Gastroenterol 2007;102(3):577-588. https://doi.org/10.1111/j.1572-0241.2007.01064.x [ Links ]
35. Odes HS, Fich A, Reif S, et al. Effects of current cigarette smoking on clinical course of Crohn's disease and ulcerative colitis. Dig Dis Sci 2001;46(8):1717-1721. https://doi.org/10.1023/A:1010609722315 [ Links ]
36. Van der Heide F, Dijkstra A, Weersma RK, et al. Effects of active and passive smoking on disease course of Crohn's disease and ulcerative colitis. Inflamm Bowel Dis 2009;15(8):1199-1207. https://doi.org/10.1002/ibd.20884 [ Links ]
37. Turner K, Genta RM, Lujan G, Robiou C, Sonnenberg A. Significance of the epithelioid granuloma in biopsies of Crohn's colitis. Inflamm Bowel Dis 2014;20(12):2271-2275. https://doi.org/10.1097/mib.0000000000000196 [ Links ]
38. Hong SW, Yoon H, Shin CM, et al Clinical significance of granulomas in Crohn's disease: A systematic review and meta-analysis. J Gastroenterol Hepatol 2020;35(3):364-373. https://doi.org/10.1111/jgh.14849 [ Links ]
39. Cosnes J, Bourrier A, Laharie D, et al. Early administration of azathioprine vs conventional management of Crohn's Disease: A randomised controlled trial. Gastroenterology 2013;145(4):758-765.e2. https://doi.org/10.1053/j.gastro.2013.04.048 [ Links ]
40. Sarter H, Savoye G, Marot G, et al. A novel 8-predictors signature to predict complicated disease course in pediatric-onset Crohn's disease: A population-based study. Inflamm Bowel Dis 2023;29(6):izad090, https://doi.org/10.1093/ibd/izad090 [ Links ]
41. De Cristofaro E, Montesano L, Lolli E, et al. Echopattern parameter as an aid to profile Crohn's disease patients. Dig Liver Dis 2023;55(12):1654-1657. https://doi.org/10.1016/j.dld.2023.05.018 [ Links ]
42. Hirten RP, Shah S, Sachar DB, Colombel J-F. The management of intestinal penetrating Crohn's disease. Inflamm Bowel Dis 2018;24(4):752-765. https://doi.org/10.1093/ibd/izx108 [ Links ]
43. Parsi MA, Lashner BA, Achkar JP, Connor JT, Brzezinski A. Type of fistula determines response to infliximab in patients with fistulous Crohn's disease. Am J Gastroenterol 2004;99(3):445-449. https://doi.org/10.1111/j.1572-0241.2004.04083.x [ Links ]
44. Kim SH, Yang S, Kim KJ, et al Efficacy of infliximab in the treatment of Korean patients with Crohn's disease. Korean J Gastroenterol 2009;54(2):108-116. https://doi.org/10.4166/kjg.2009.54.2.108 [ Links ]
45. Nunes J, Santos PM, Tavares L. Complete resolution of enterocolic fistulas with infliximab. BioDrugs 2010;24(Suppl 1):S28-S30. https://doi.org/10.2165/11586260-000000000-00000 [ Links ]
46. Cullen G, Vaughn B, Ahmed A, et al. Abdominal phlegmons in Crohn's disease: Outcomes following antitumor necrosis factor therapy. Inflamm Bowel Dis 2012;18(4):691-696. https://doi.org/10.1002/ibd.21783 [ Links ]
47. Feagins LA, Holubar SD, Kane SV, Spechler SJ. Current strategies in the management of intraabdominal abscesses in Crohn's disease. Clin Gastroenterol Hepatol 2011;9(10):842-850. https://doi.org/10.1016/j.cgh.2011.04.023 [ Links ]
48. Gionchetti P, Dignass A, Danese S, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn's disease 2016: Part 2: Surgical management and special situations. J Crohns Colitis 2017;11(2):135-149. https://doi.org/10.1093/ecco-jcc/jjw169 [ Links ]
 Correspondence:
Correspondence:
C L Gounden
drcathrinegounden@gmail.com
Accepted 4 March 2024














