Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Science
versión On-line ISSN 1996-7489
versión impresa ISSN 0038-2353
S. Afr. j. sci. vol.118 no.11-12 Pretoria nov./dic. 2022
http://dx.doi.org/10.17159/sajs.2022/12560
RESEARCH ARTICLE
A tetracycline hydrochloride-loaded SiO2/polycaprolactone composite from bamboo stem for controlled drug release study
Enobong R. Essien; Violette N. Atasie; Samson T Scott; Opeyemi A. Ajayi
Department of Chemical and Food Sciences, Bells University of Technology, Ota, Ogun State, Nigeria
ABSTRACT
A controlled drug delivery system is preferable to traditional drug administration because it can supply the drug continuously and ensure on-demand bioavailability. The production of silica/polymer composite delivery material is expensive due to the use of alkoxysilane silica precursors. As bamboo is an abundant plant in Africa, we investigated the use of bamboo stems as an alternative silica starting material. The ash from the bamboo stem was mixed with polycaprolactone (PCL) solution to produce a (SiO2/PCL) composite, which was then loaded with the drug, tetracycline hydrochloride (TCH), to test in vitro degradability and controlled-release in phosphate-buffered saline (PBS). Scanning electron microscopy, X-ray diffractometry and Fourier transform infrared spectroscopy were used to examine the structure, phase composition, and chemical bond properties of the material. The TCH release profile was determined using an ultraviolet (UV) spectrophotometer. The SiO2/PCL composite showed a high capacity for drug loading. The composite released TCH in a consistent and sustained way, and showed regulated degradability in PBS. As a result, the use of bamboo stem-derived silica in the formulation of SiO2/PCL for continuous TCH delivery shows considerable cost-benefit potential for a safe, regulated drug delivery strategy.
SIGNIFICANCE:
• This study shows the benefit of using bamboo stem as an alternative silica source to alkoxysilanes.
• SiO2/PCL composites can be employed for the sustained delivery of drugs while providing congruent degradation.
• This study can serve as a benchmark for further utilisation of bamboo stem as a low-cost silica precursor.
Keywords: bamboo stem, polycaprolactone, composites, controlled drug release, silica, tetracycline hydrochloride
Introduction
A drug delivery system is a formulation or material capable of injecting a medication into the body while managing the speed, duration, and location of its release to preserve its effectiveness and safety. Drug delivery systems are important in drug pharmacology because they can impact drug release and speed, as well as drug distribution inside the body and reduce the occurrence of side effects. A good drug delivery system guarantees that an active drug is released at the proper moment and exerts its effects while still in the body.1 Only a small portion of the drug reaches the right spot in the body during normal use, and the majority of it is eliminated through metabolism and enzyme secretion. Each drug has a therapeutic range in terms of concentration, with the greatest safe concentration being toxic and the lowest effective concentration being ineffective.2
Wen and Park2 point out that a more regulated or zero-order release from microcontainers is desirable because controlling the drug release rate has several benefits, including boosting therapeutic impact, decreasing side effects, and reducing administration frequency. A system with continuous drug release must be biodegradable and biocompatible to release a therapeutic agent into the body and enhance the degree of effectiveness and safety of the treatment by modifying the rate, time, and place of release.
The drug can be embedded in a polymer matrix and released in a regulated manner due to the presence of the polymer matrix. Poly(lactide-co-glycolide), polylactide, polyesters, polyglycolide, and poly(hydroxybutyrate) have all been explored extensively as materials for drug administration.3,4
Polycaprolactone (PCL) is a synthetic polyester that has a low glass transition temperature (T) of about 60 °C. PCL has a high permeability due to its semi-crystallinity caused by its low T which makes it useful for delivering drugs with a low molecular weight. PCL degrades at a slower rate than other biocompatible polymers like poly(L-lactide) and poly(lactide-co-glycolide) due to its semi-crystalline form. As a result, drug diffusion often dictates drug release from PCL matrices, making PCL ideal for regulated and sustained drug release. However, because drug release from PCL is primarily driven by diffusion, drug distribution within the polymer matrix has a significant impact on drug release rate. The influence of drug characteristics and polymer composition on drug release from PCL microspheres and thin films has been explored in previous work by Wang et al.5 and Schlesinger et al.6
PCL has a low biodegradation rate, but good biocompatibility and high steroid permeability7, which explains its use in an implanted, 1-year contraceptive delivery system for levonorgestrel8. Progesterone (Mw 314Da) has been successfully loaded into microporous PCL matrices by co-dissolving the steroid with PCL in acetone before precipitation casting.9 In phosphate-buffered saline (PBS), high drug loadings of progesterone/mg matrix/day were possible after 11 days. Furthermore, anti-proliferative action of the steroid on breast cancer cells demonstrated that it was released with preserved activity. To include hydrophilic drug species like gentamicin sulfate into microporous PCL matrices made by precipitation casting, the drug powder must first be dispersed in a PCL solution. This formulation technique may sustain gentamicin sulfate release for 11 weeks in vitro, revealing the development of an interconnected pore network within the matrix that allows for efficient diffusion of low molecular weight water-soluble species.10
PCL has previously been found to have good ocular tolerability in the form of nanocapsule drug carriers for carteolol chlorhydrate.11 This finding, combined with the ability of microporous PCL matrices to sustain gentamicin sulfate release and the inherent flexibility of the PCL carrier (5-10 N/m2 compressive strength)12,13, suggests that highly water-soluble antibiotics such as tetracycline antibiotics could be delivered topically.
Furthermore, porous silica particles as an encapsulation material for drugs offer an interesting advantage in drug delivery due to their small size, ability to manage surface charge, optical properties, high surface area, low density and adsorption capacity delivery.13,14
Several biomasses have been investigated as silica sources based on their renewability and eco-friendliness.15-17 The bamboo plant can thrive in any kind of climate and in very poor soils. Besides its renewability, the preponderance of bamboo, which is one of the most productive and fastest-growing natural resources18, makes it an attractive natural resource. In Africa where it is widely available, it is regarded as 'a potential green gold'19, implying that it could offer huge economic benefit if used as a substitute silica precursor to expensive alkoxysilanes on a large scale. Therefore, in this study, we aimed to investigate the use of silica derived from bamboo stem for the development of a controlled drug delivery system based on polycaprolactone.
Materials and methods
Materials
Bamboo (Bambusa vulgaris) stems (Figure 1) were obtained from a swampy area at the University of Lagos in Lagos, South-West, Nigeria. Authentication of the sample was performed by the Herbarium at the University of Lagos (number: LUH5493). The analytical grade reagents used for this experiment which were purchased from Sigma-Aldrich included: polycaprolactone (PCL, average molecular weight = 80 000); chloroform (HPLC grade, assay 99%); tetracycline hydrochloride (TCH, 95%); double-distilled de-ionised water, sodium chloride (98%), sodium dihydrogen phosphate (99%), potassium chloride (99%) and disodium hydrogen phosphate (99%).

Biosilica preparation
The method used to extract the biosilica from the bamboo stem was similar to the approach by Kow et al.20 Accordingly, the stem was cut into pieces, washed with double-distilled de-ionised water and sun-dried. Afterwards, the stem pieces were subjected to further drying in a hot air oven at 105 °C for 72 h and then ground to form a powder to increase the surface area. The powdered material was transferred into a muffle furnace and burned at 800 °C for 6 h at a heating rate of 10 °C/ min to form the biosilica.
SiO2/PCL composite preparation
To prepare the SiO2/PCL composite via solvent casting, 0.5 g PCL powder was dissolved in chloroform (50 mL) by sonication (ultrasonic cleaner, CLEAN 120HD) at 40 W, 30 °C for 5 min. Following this, the as-prepared biosilica (1.0 g) was dispersed in the solution and the temperature was adjusted to 45 °C while the mixture was sonicated for another 2 h to give a uniform colloidal solution. After completing this procedure, the mixture was immediately cast into five cylindrical moulds of dimension 12 mm X 6 mm (height x diameter) and kept to dry at ambient condition for 5 days. The sample was tagged SiPCL after removal from the mould.
SiO2/PCL-TCH composite preparation
To load the TCH onto the SiO2/PCL, the procedure for obtaining the SiO2-PCL mixture was repeated with a similar concentration. However, 2 min after adding the biosilica, 150 mg of TCH was dispersed in the mixture and sonicated at 45 °C for 2 h to give a homogeneous solution. The obtained mixture was then cast into five cylindrical moulds measuring 12 mm X
6 mm (height x diameter) and allowed to dry at room temperature for 5 days. When the drying process was completed, the samples were removed from the mould and labelled SiPCL/TCH.
Characterisation
The drug-loaded and unloaded composites, in quintuplicate, were assessed based on their tensile and compressive strengths by a universal testing machine (MODEL BAB-200, Transcell Technology) with a 100 kgf load, at a crosshead rate of 50 mm/min. The test was conducted under a temperature of 24±1 °C and relative humidity of 45±5% in accordance with ASTM standard D5034-95. The dimensions of the samples were 22 mm in nominal length and 5 mm in width based on ASTM standard D1708-96. With the aid of Instron pneumatic clamps, the tensile tests were performed at a strain rate of 0.01/s from where the load and displacement data were obtained. The data were analysed using a two-way analysis of variance (ANOVA; Prism, GraphPad).
The elemental composition of the biosilica was determined in an energy dispersive X-ray fluorescence (EDXRF) spectrometer (Minipal 4). The samples were converted into fine homogeneous particles by sieving through a 150-micron mesh sieve. The voltage used for oxides was 14 kV with a Kapton filter, while for trace elements an Ag/Al thin-film filter was set at 20 kV.
To examine the microstructure of the composites, a scanning electron microscope was used (Phenom ProX, Phenomworld). The samples were distributed over carbon conductive tape and sputter-coated with 5 nm gold using a quorum-Q150R Plus E sputter coater.
For phase composition and crystallinity assessment, an X-ray diffractometry (XRD; Empyrean Panalytical) system, using Cu-Ka radiation with a wavelength of 1.5418 nm and a tube current of 40 mA to generate the X-ray, was employed. The samples were scanned through the diffraction angle from 4° to 75° with a two-step of 0.026261 at 8.67 s for each step.
A Fourier transform infrared spectrometer (FTIR; Agilent Technology Cary 630) in transmittance mode with attenuated total reflectance was used to obtain the spectra depicting the chemical bond properties of the composites. To obtain the spectra, an average of 30 scans at 8 cm-1 resolutions were performed over the spectral scan range of 4000-650 cm-1.
In vitro drug release experiment
To study the in vitro drug release, the SiPCL/TCH composite (0.25 g) was immersed in 100 mL PBS release medium at a pH of 7.4 in a clear glass vial. Afterwards, it was incubated at 37 °C with constant agitation at 100 rpm for a maximum of 28 days. During the incubation period, the PBS solution was withdrawn at pre-defined time intervals to measure the absorbance with a UV/visible spectrophotometer (T70 + UV/Vis Spectrometer, PG Instruments Ltd.) at a wavelength of 358 nm to determine the concentration of TCH released from the composite, in accordance with previous studies.21,22 The TCH stock concentration of 5-100 μg/mL in PBS solution was initially used to establish the calibration curve from which the drug concentration was extrapolated using an absorbance-concentration calibration curve. The cumulative TCH released from the SiPCL/TCH composite was computed relative to the original drug weight in the polymer matrix and plotted against time. The drug release testing was carried out in triplicate.
In vitro degradation test
The weight loss of the SiPC/TCH composite was investigated for a maximum of 28 days by incubating 0.5 g of the sample in 30 mL of PBS (pH 7.4) at 37 °C with continual stirring at 100 rpm. The samples were kept in glass vials with a cover to prevent the PBS buffer from evaporating. After removal from PBS at intervals of 7, 14, 21 and 28 days, the samples were washed with double-distilled de-ionised water and dried at 105 °C until they reached a consistent weight, then cooled and kept in a desiccator at room temperature for 60 min. The degradation (weight loss) in % was calculated according to Equation 1.23 The dry weights of the original and degraded specimens are W0 and Wt, respectively, and the degradation rate is D.

Statistical analysis
One-way ANOVA was used to determine the difference between the drug cumulative amounts released from the tested samples at each immersion time; values are mean±standard deviation. This analysis was followed by an appropriate post-hoc test, where p<0.05 was taken as a criterion for a statistically significant difference.
Results and discussion
Composition of the bamboo stem-derived silica
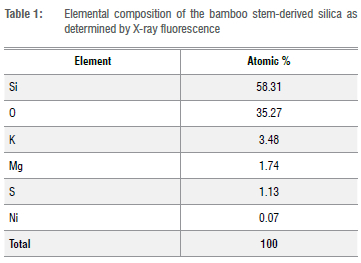
The silica production was 37.16% based on the dry weight of the bamboo leaf biomass. The elemental composition of the calcined bamboo leaves as determined by EDXRF is given in Table 1. The analysis shows that the abundance of silicon is 58.31%. This finding contrasts with the study of Dirna et al.24 who obtained 42.84% silicon from bamboo stem after burning it at 700 °C for 6 h in a muffle furnace. From the result presented, SiO2 was present in the bamboo stem up to 93.58%. No heavy metals were found, thus indicating that the silica is safe for medical use.
Mechanical properties
The mechanical properties assessment of SiPCL gave 1.4462±0.31 MPa as the compressive strength and 8.327±0.43 MPa as the tensile strength. Based on the results of the stress-strain curves of typical composite scaffolds, these low values could be attributed to the porous nature of the microstructure. These pore structures could enable the encapsulation and diffusion of drugs if applied in controlled drug release. For the SiPCL/TCH, the compressive strength was 1.236±0.22 MPa whereas the tensile strength was 7.776±0.38 MPa. Previous studies25,26 have shown that, at certain thresholds, drug loading could cause a reduction in mechanical properties. This finding has been attributed to a decrease in the polymer crystallinity25 due to the impregnation of the drug into the polymer matrix.
Morphology
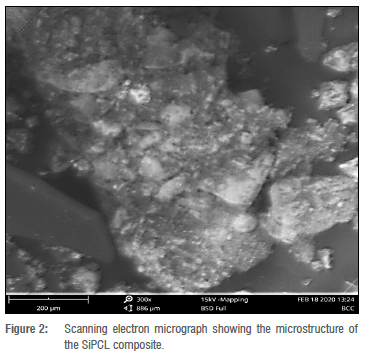
The microstructure of SiPCL (Figure 2) demonstrates the presence of SiO2 crystal aggregates contained in the polymer matrix. These spots can be found everywhere throughout the surface of the polymer matrix. The surfaces where the particles are embedded appear flat and broad, resulting in a vast surface area. The surface became more compact with a larger number of particle aggregates, an increase in the number of embedment sites, and a substantially bigger surface area after loading with TCH (SiPCL/TCH) (Figure 3). A large surface area is necessary to provide the high specific capacity required to establish an enhanced drug diffusion gradient in the composite.27

Diffraction patterns
Figure 4 shows the diffractograms that illustrate the phase identification of the crystallographic structure of the composites. Peaks in the SiPCL diffraction pattern match both the PCL and SiO2 components in the sample. When indexed with the standard reference file ICDD # -01-0771 31728, prominent peaks at 26 21.5°, 26.5°, 28.5°, 31.5°, 36.5°, 39.7°, and 60° match cristobalite (SiO2) in both angular position and intensity; those corresponding to PCL are situated at 26 21.3° and 23.6°.29
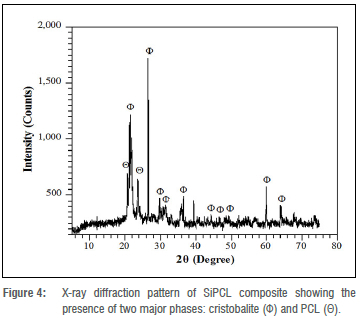
The appearance of the cristobalite peaks at high intensity validates the X-ray fluorescence result, which shows a high content of SiO2 in the bamboo stem ash. The sharpness and intensity of the diffraction peaks, as well as the nature of their pattern at the baseline, indicate that the SiPCL sample is semi-crystalline.
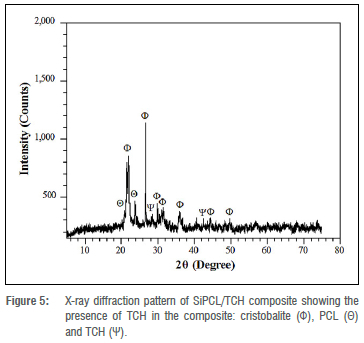
Figure 5 shows the XRD pattern after drug loading of the SiPCL composite (SiPCL/TCH). All SiO2 and PCL peaks that were found in the SiPCL spectra are still present. In addition, two additional peaks corresponding to TCH crystal plane reflections appear at angular coordinates 28.69° and 42.47°.30 In the spectrum for SiPCL/TCH, there was a small drop in baseline intensity. This is due to impregnation of the drug into the polymer matrix31, and hence, supports the reduction in the mechanical strength after TCH loading of the composite discussed earlier.
Chemical bond characteristics
The vibrational modes of the sample without the drug (SiPCL) are shown in the FTIR spectrum presented in Figure 6. The spectrum contains bands at 1428 cm-1 and 1368 cm-1 with low intensity considered for the methylene (CH2) groups in amorphous regions in the PCL.32 The small peak near 1238 cm-1 is attributed to the asymmetric vibrational stretching of C-O-C bonds of ester, which is further supported by an intense band that splits into two sharp peaks at 1001 cm-1 and 988 cm-1, corresponding to the bending mode of C-O-C bonds in ester.32 The sharp nature of these peaks signifies the development of crystallinity. The presence of PCL in the sample is further indicated by the C-H stretching vibration at 2937 cm-1.
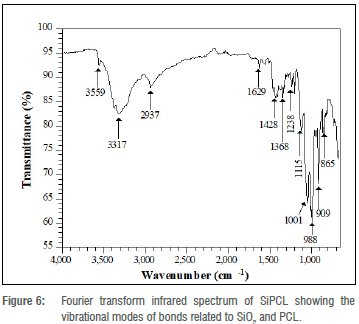
The characteristic vibrational stretching mode of the carbonyl (C=O) ester bond in PCL usually observed around 1720 cm-1,33 could not be found in the spectrum. This may be due to its involvement in a crosslinking reaction with an oxygen atom of the SiO2 via nucleophilic attack on the PCL carbonyl to form a network structure. Accordingly, two O-H peaks at 3559 cm-1 and 3317 cm-1 are observed in the spectrum, which are considered for the stretching vibrations of O-H group in the PCL and that result from the crosslinking reaction. The peak at 1115 cm-1 corresponds to the asymmetric stretching vibration of Si-O-Si and the ones at 909 cm-1 and 865 cm-1 are ascribed to symmetric stretching vibrations of Si-O-Si bonds in the silica and hence confirm the successful incorporation of silica into the polymer.34
The hydrophilicity of the silica was demonstrated by the angular vibration surface hydroxyl in the sample observed around 1629 cm-1.35 The appearance of the hydroxyl group can be attributed to the silanol bonds solely responsible for the adsorptive behaviour of hydrophilic silica and silicate surfaces.
After drug loading, the sample (SiPC/TCH) gave some shifts in vibrational frequencies as observed in Figure 7. The C-O-C ester bond stretching became visible around 1245 cm-1 while the twin sharp bending modes fused to develop a band around 1029 cm-1. Several bands around that region disappeared, including the symmetric stretching Si-O-Si vibrational peaks around 909 cm-1 and 865 cm-1; while the asymmetric stretching vibrational peak observed at 1001 cm-1 in SiPCL collapses into the band at 1029 cm-1. Furthermore, the C-H stretching band was shifted to 2919 cm1 and the peak due to the CH2 group was observed around 1375 cm-1.
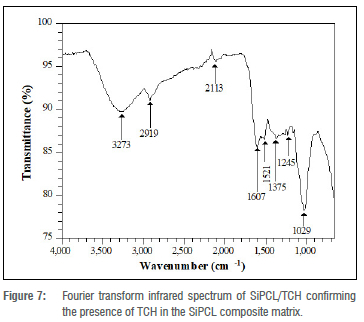
The impregnation of the drug into the polymer matrix was responsible for these manifestations. The absence of some FTIR peaks also explains the drop in the baseline intensity of the XRD peaks reported previously in the XRD spectrum of SiPCL/TCH (Figure 5) and, as a result, SiPCL/TCH exhibited lower mechanical properties.
The increase in the intensity of the OH broad band centred at 3273 cm-1, which was attributable to the joint contribution of the OH vibrational stretching of the hydroxyl group in surface water and that in TCH, confirms the presence of TCH in the composite. In addition, due to C=O stretching in the tetracycline rings, a significant peak at 1607 cm-1 and a smaller one at 1521 cm-1 emerged.36
Biodegradability assessment of the SiO2/PCL-TCH composite
The degradation of SiPCL/TCH after immersion in PBS was observed at 37 °C for a total of 28 days. The relationship in Equation 1 was used to investigate the weight loss and degradability after immersion. Figure 8 shows how the composite degraded over time after immersion in PBS for 28 days. With a loss of about 7.73±0.87% of its starting weight in 24 h, degradation was found to be minor at the start of the investigation, indicating gradual hydrolytic degradation of the sample. On Day 7, the weight reduction climbed to 35.13±1.12%, to 52.18±2.11% on Day 14, 62.33±2.45% on Day 21, and 65.46±1.31% on Day 28.
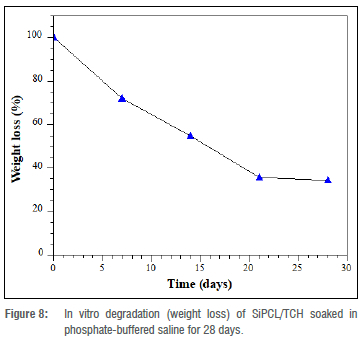
After soaking in PBS for 28 days, the degradation result indicates that the majority of the components were lost. PCL is a low-degrading polymer that can be used to construct long-term implanted drug delivery devices.37 This is frequently determined by the hydrophilic-hydrophobic interactions between the drug and the polymer. The structure and layout of the polymer matrix, pore diameters, and degradation media all influence degradation behaviour. When exposed to water, the PCL, a degradable semi-crystalline synthetic polymer, typically shows surface deterioration. The amorphous portions are targeted first, followed by the crystalline regions for hydrolysis.38 Random hydrolytic cleavages are also possible in PCL with high molecular weights because they are aliphatic polyesters in nature.39
In vitro drug release evaluation
Figure 9 shows the release curve of SiPCL/TCH in PBS at pH 7.4 and 37 °C as assessed by UV-visible spectroscopy at 358 nm. The TCH cumulative release curves showed a biphasic pattern (Figure 9) at the beginning (0-1 day), which is normally associated with a drug incorporated into a polymer as an encapsulating material.40 At 0-1 h, a release of 9.24±0.93 μg/mL was detected, corresponding to 6.14% of the total concentration of the encapsulated drug. The cumulative release after 3 h was 12.34±1.18 μg/mL, and after 1 day was 65.63±2.13 μg/mL, indicating 12.34% and 43.61%, respectively. After 24 h, the controlled release began and lasted until Day 28. The cumulative releases for 7, 14, and 21 days were 74.10%, 84.43%, and 85.26%, respectively, as shown in Figure 9. The cumulative release peaked at 129.74±4.36 ug/mL after Day 28, accounting for 86.35% of the drug.
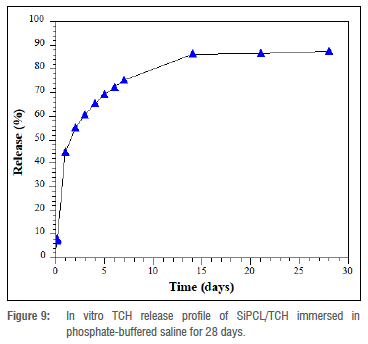
On-demand bioavailability of controlled drug release systems gives them a huge advantage over traditional drug administration. The fact that the silica was derived from biomass is significant because, apart from being a cheap source, it can compare to alkoxysilanes as silica precursors.
The silica/PCL composite was made using silica extracted from a bamboo stem at a low cost. The antibiotic tetracycline hydrochloride (TCH) was put into the composite material to act as a drug carrier system for controlled drug release research. The results show that the SiO2/PCL composite was successfully formed, and the TCH was encapsulated into the composite's matrix. The drug release test yielded a sustained release profile, with the SiO2 component playing a key role in determining the TCH release kinetics. The rate of degradation of the composite in PBS over 28 days allowed for controlled drug release, which is ideal for a material intended to be used as an in vivo drug release vehicle.
The preparation technique should pose no health or environmental risks when compared to the similar analytical grade silica starting materials based on alkoxysilanes. It is also worth noting that the bamboo plant is abundantly available in Africa and it is underutilised in most regions where it grows, making it an appealing large-scale silica starting material for preparing SiO2/polymer composites for drug delivery.
Acknowledgements
We thank the Department of Chemical and Food Sciences at Bells University of Technology in Ota, Nigeria, for providing the facilities to undertake this study.
Competing interests
We have no competing interests to declare.
Authors' contributions
E.R.E.: Conceptualisation; student supervision; writing; project leadership; initial draft writing. V.N.A.: Project management; funding acquisition. S.T.S.: Sample analysis; data analysis; validation. O.A.A.: Methodology; data collection.
References
1. Jain KK. Drug delivery systems - An overview. Totowa, NJ: Humana Press; 2008. https://doi.org/10.1007/978-1-59745-210-6_1 [ Links ]
2. Wen H, Park K. Oral controlled release formulation design and drug delivery: Theory to practice. Hoboken, NJ: John Wiley & Sons; 2011. https://doi.org/10.1002/9780470640487 [ Links ]
3. Nair LS, Laurencin CT. Biodegradable polymers as biomaterials. Prog Polym Sci. 2007;32(8-9):762-798. https://doi.org/10.1016/j.progpolymsci.2007.05.017 [ Links ]
4. Dash TK, Konkimalla VB. Poly-e-caprolactone based formulations for drug delivery and tissue engineering: A review. J Controlled Release. 2012;58(1):15-33. https://doi.org/10.1016/j.jconrel.2011.09.064 [ Links ]
5. Wang X, Wang Y Wei K, Zhao N, Zhang S, Chen J. Drug distribution within poly (E-caprolactone) microspheres and in vitro release. J Mater Process Technol. 2009;209(1):348-354. https://doi.org/10.1016/j.jmatprotec.2008.02.004 [ Links ]
6. Schlesinger E, Ciaccio N, Desai TA. Polycaprolactone thin-film drug delivery systems: Empirical and predictive models for device design. Mater Sci Eng C. 2015;57:232-239. https://doi.org/10.1016/j.msec.2015.07.027 [ Links ]
7. Pitt CG, Gratzl MM, Jeffcoat AR, Zweidinger RA, Schindler A. Sustained drug delivery systems II: Factors affecting release rates from polycaprolactone and related biodegradable polyesters. J Pharm Sci. 1979;68(12):1534-1538. https://doi.org/10.1002/jps.2600681219 [ Links ]
8. Darney PD. Hormonal implants: Contraception for a new century. Am J Obstet Gynecol. 1994;170(5Pt 2):1536-1543. https://doi.org/10.1016/S0002-9378(12)91812-8 [ Links ]
9. Chang HI, Williamson MR, Perrie Y Coombes AG. Precipitation casting of drug-loaded microporous PCL matrices: Incorporation of progesterone by co-dissolution. J Controlled Release. 2005;106(2):263-272. https://doi.org/10.1016/j.jconrel.2005.05.013 [ Links ]
10. Chang HI, Perrie Y Coombes AG. Delivery of the antibiotic gentamicin sulphate from precipitation cast matrices of polycaprolactone. J Controlled Release. 2006;110(2):414-421. https://doi.org/10.1016/j.jconrel.2005.10.028 [ Links ]
11. Marchal-Heussler L, Sirbat D, Hoffman M, Maincent P. Poly(epsilon-caprolactone) nanocapsules in carteolol ophthalmic delivery. Pharm Res. 1993;10:386-390. https://doi.org/10.1023/A:1018936205485 [ Links ]
12. Chang HI, Wang Y Perrie Y Coombe AGA. Microporous polycaprolactone matrices for drug delivery and tissue engineering: The release behaviour of bioactives having extremes of aqueous solubility. J Drug Del Tech. 2010;20(3):207-212. https://doi.org/10.1016/S1773-2247(10)50031-5 [ Links ]
13. Sakai-Kato K, Hasegawa T, Takaoka A, Kato, M, Toyo'oka T, Utsunomiya-Tate N, et al. Controlled structure and properties of silicate nanoparticle networks for incorporation of biosystem components. Nanotechnology. 2011;22(20), Art. #205702. https://doi.org/10.1088/0957-4484/22/20/205702 [ Links ]
14. Bitar A, Ahmad NM, Fessi H, Elaissari A. Silica-based nanoparticles for biomedical applications. Drug Discov Today. 2012;17(19-20):1147-1154. https://doi.org/10.1016/j.drudis.2012.06.014 [ Links ]
15. Kalapathy U, Proctor A, Shultz J. A simple method for production of pure silica from rice hull ash. Bioresour Technol. 2000;73(3):257-262. https://doi.org/10.1016/S0960-8524(99)00127-3 [ Links ]
16. Carmona VB, Oliveira RM, Silva WTL, Mattosoa LHC, Marconcini JM. Nanosilica from rice husk: Extraction and characterization. Ind Crop Prod. 2013;43(1):291-296. https://doi.org/10.1016/j.indcrop.2012.06.050 [ Links ]
17. Wong DP Suriyaprabha R, Yuvakumar R, Rajendran V Chen Y-T, Hwang B-J, et al. Binder-free rice husk-based silicon-graphene composite as energy efficient Li-ion battery anodes. J Mater Chem A. 2014;2(33):13437-13441. https://doi.org/10.1039/C4TA00940A [ Links ]
18. Rangaraj S, Venkatachalam R. A lucrative chemical processing of bamboo leaf biomass to synthesize biocompatible amorphous silica nanoparticles of biomedical importance. Appl Nanosci. 2017;7:145-153. https://doi.org/10.1007/s13204-017-0557-z [ Links ]
19. Musau Z. Bamboo: Africa's untapped potential. The new economic force is generating income, creating jobs and protecting the environment. African Renewal. 2016;April. Available from: https://www.un.org/africarenewal/magazine/april-2016/bamboo-africa%E2%80%99s-untapped-potential [ Links ]
20. Kow KW, Yusoff R, Abdul Aziz AR, Abdullah EC. Physicochemical properties of bamboo leaf aerogels synthesized via different modes of gelation. Appl Surf Sci. 2014;301:161-172. https://doi.org/10.1016/j.apsusc.2014.02.031 [ Links ]
21. Sumitha NS, Prakash P Nair BN, Sailaja GS. Degradation-dependent controlled delivery of doxorubicin by glyoxal cross-linked magnetic and porous chitosan microspheres. ACS Omega. 2021;6(33):21472-21484. https://doi.org/10.1021/acsomega.1c02303 [ Links ]
22. Harting R, Johnston K, Petersen S. Correlating in vitro degradation and drug release kinetics of biopolymer-based drug delivery systems. Int J Biobased Plast. 2019;1(1):8-21. https://doi.org/10.1080/24759651.2018.1563358 [ Links ]
23. Yu L, Li Y Zhao K, Tang Y Cheng Z, Chen J, et al. A novel injectable calcium phosphate cement-bioactive glass composite for bone regeneration. PLoS ONE. 2013;8(4), e62570. https://doi.org/10.1371/journal.pone.0062570 [ Links ]
24. Dirna FC, Rahayu I, Maddu A, Darmawan W, Nandika D. Nanosilica synthesis from Betung bamboo sticks and leaves by ultrasonication. Nanotech Sci Appl. 2020;13:131-136. https://doi.org/10.2147/NSA.S282357 [ Links ]
25. Wong RSH, Dodou K. Effect of drug loading method and drug physicochemical properties on the material and drug release properties of poly (ethylene oxide) hydrogels for transdermal delivery. Polymers. 2017;9(7):286. https://doi.org/10.3390/polym9070286 [ Links ]
26. Gao Y, Li J, Xu C, Hou Z, Yang L. Mechanical properties and drug loading rate of a polycaprolactone 5-fluorouracil controlled drug delivery system. Mater Res Express. 2021;8(9), Art. #095302. https://doi.org/10.1088/2053-1591/ac1fb5 [ Links ]
27. Pang Q, Nazar LF. Long-life and high-areal-capacity Li-S batteries enabled by a light-weight polar host with intrinsic polysulfide adsorption. ACS Nano. 2016;10(4):4111-4118. https://doi.org/10.1021/acsnano.5b07347 [ Links ]
28. Hao R, Yan Z, Huamin Z, Jinxiang C. Production and evaluation of biodegradable composites based on polyhydroxybutyrate and polylactic acid reinforced with short and long pulp fibres. Cellulose Chem Technol. 2015;49(7-8):641-652. https://www.cellulosechemtechnol.ro/pdf/CCT7-8(2015)/p.641-652.pdf [ Links ]
29. Petersen RS, Nielsen LH, Rindzevicius T, Boisen A, Keller SS. Controlled drug release from biodegradable polymer matrix loaded in microcontainers using hot punching. Pharmaceutics. 2020;12(11):1050. https://doi.org/10.3390/pharmaceutics12111050 [ Links ]
30. Blanchard FN. The space group and reference powder diffraction data for tetracycline hydrochloride, C22H24N2O8HCl. Powder Diffr. 1989;4(2):103-105. https://doi.org/10.1017/S088571560001647X [ Links ]
31. Basu S, Adhiyaman, R. Preparation and characterization of nitrendipine-loaded eudragit RL 100 microspheres prepared by an emulsion-solvent evaporation method. Trop J Pharm Res. 2008;7(3):1033-1041. https://doi.org/10.4314/tjpr.v7i3.14688 [ Links ]
32. Phillipson K, Hay J, Jenkins M. Thermal analysis FTIR spectroscopy of poly(-caprolactone). Thermochim Acta. 2014;595:74-82. https://doi.org/10.1016/j.tca.2014.08.027 [ Links ]
33. Janarthanan G, Kim IG, Chung E-J, Noh I. Comparative studies on thin polycaprolactone-tricalcium phosphate composite scaffolds and its interaction with mesenchymal stem cells. Biomater Res. 2019;23:1. https://doi.org/10.1186/s40824-018-0153-7 [ Links ]
34. Liu X, Ding C, Chu PK. Mechanism of apatite formation on wollastonite coatings in simulated body fluids. Biomaterials. 2004;25(10):1755-1761. https://doi.org/10.1016/j.biomaterials.2003.08.024 [ Links ]
35. Adams LA, Essien ER, Adesalu AT, Julius ML. Bioactive glass 45S5 from diatom biosilica. J Sci Adv Mater Devices. 2017;2(4):476-482. https://doi.org/10.1016/j.jsamd.2017.09.002 [ Links ]
36. Caminati G, Focardi C, Gabrielli G, Gambinossi F, Mecheri B, Nocentini M, et al. Spectroscopic investigation of tetracycline interaction with phospholipid Langmuir-Blodgett films. Mater Sci Eng C. 2002;22(2):301-305. https://doi.org/10.1016/S0928-4931(02)00217-5 [ Links ]
37. Agrawal CM, Ray RB. Biodegradable polymeric scaffolds for musculoskeletal tissue engineering. J Biomed Mater Res. 2001;55(2):141-150. https://doi.org/10.1002/1097-4636(200105)55:2<141::AID-JBM1000>3.0.CO;2-J [ Links ]
38. Bikiaris DN. Nanocomposites of aliphatic polyesters: An overview of the effect of different nanofillers on enzymatic hydrolysis and biodegradation of polyesters. Polym Degrad Stab. 2013;98(9):1908-1928. https://doi.org/10.1016/j.polymdegradstab.2013.05.016 [ Links ]
39. Chasin M, Langer R. Biodegradable polymers as drug delivery systems. Drugs Pharm Sci. 1999;92:45. [ Links ]
40. Swornakumari C, Meignanalakshmi S, Legadevi R, Palanisammi A. Preparation of microspheres using poly-3-hydroxybutyrate biopolymer and its characterization. J Environ Biol. 2018;39(3):331-338. https://doi.org/10.22438/jeb/39/3/MRN-315 [ Links ]
 Correspondence:
Correspondence:
Enobong Essien
Email: reggiessien@gmail.com
Received: 14 Oct. 2021
Revised: 30 July 2022
Accepted: 02 Aug. 2022
Published: 30 Nov. 2022
Editors: Priscilla Baker Amanda-Lee Manicum
Funding: None














