Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Dental Journal
versão On-line ISSN 0375-1562
versão impressa ISSN 0011-8516
S. Afr. dent. j. vol.78 no.7 Johannesburg Ago. 2023
http://dx.doi.org/10.17159/sadj.v78i07.16425
RESEARCH
Bacterial contamination of curing light guides: prevalence and students' knowledge and awareness of measures to maintain sterility
D MotlhakeI; SR MthethwaII; EM SekatiIII
IBSc, BChD, Thabazimbi Hospital, Thabazimbi, Limpopo, South Africa. ORCID: https://orcid.org/0009-0000-2305-9783
IIBDS, MPH, PhD, Sefako Makgatho Health Sciences University, Pretoria, South Africa. ORCID: https://orcid.org/0000-0003-0420-808X
IIIND Med Tech, BTech, BSc Med Honours, MSc ORCID: https://orcid.org/0009-0000-0395-940X
ABSTRACT
INTRODUCTION: It is generally accepted that inadequately sterilised dental curing light guides pose risks of infection and cross-contamination.
AIMS AND OBJECTIVES: To determine the presence and level of bacterial contamination among curing light guides used by students during patient care at a dental school in South Africa and to describe students' knowledge and awareness of measures used to maintain their sterility.
DESIGN: A two-part descriptive study consisting of microbiological testing and a cross-sectional survey.
Methods: Swabs were collected from curing light guide tips before and after use for aerobic culture and a questionnaire was used to collect data pertaining to students' knowledge and awareness of measures used to maintain their sterility.
RESULTS: The prevalence of contamination increased after use (54.5% vs 45.5%). Grades of bacterial growth higher than 1+ were not detected. Isolated bacteria were contaminants. The response rate for the questionnaire was 42.5%. Fifth-year students were overall more knowledgeable than fourth-year students (81.6% vs 67.5%) and were more aware of the existence of the different types of disposable barriers (27.5% vs 12.8%) and the impact of infection control barriers on curing light intensity (52.4% vs 15%).
CONCLUSION: Contamination occurred despite high levels of knowledge and awareness of the risk.
INTRODUCTION AND BACKGROUND
Healthcare-associated infections are a major global safety concern for both patients and healthcare professionals.1-3 In dental settings, infections may be acquired directly through contact with blood, oral fluids or other secretions; indirectly through contact with contaminated instruments, operating equipment or environmental surfaces; and through inhalation or contact with microorganisms present in aerosols or spatters of oral and respiratory fluids or in dental unit waterlines.4,5 The cross-contamination potential of light-curing units, defined by Dolly and Sasa (2019) as "handheld devices that are used for the polymerisation of visible light-activated dental materials"6, has long been recognised.7 However, very few studies have investigated their microbial contamination. Janoowalla and colleagues (2010) found bacterial contamination on the base button, fan and handle of the light-curing units before and after use. The isolated bacteria included Staphylococcus aureus.8Bacterial contamination of the light guide has not previously been described.
The four types of light-curing units that are currently available include quartz-tungsten-halogen (QTH), light-emitting diode (LED), plasma arc curing (PAC) and Argon laser units.6 The two most commonly used are halogen and LEDs. They are generally provided to the user as nonsterile devices. ANSI/ ADA and ISO standards recommend manufacturers provide appropriate cleaning and disinfection directions to use on the unit which should be followed between each patient.9
Light-curing units are categorised as semicritical in the Spaulding classification scheme of patient-care items. Light guides come into direct contact with mucous membranes and have the potential for saliva or blood contamination.10 Just as with other instruments that come into contact with bodily fluids, light guides must be disinfected to control for infection and cross-contamination.9 Ideally, the curing lights should have removable, autoclavable light guides and easily disinfected surfaces. However, an autoclavable light guide is impractical for many curing lights that do not use a light guide and instead have the LED emitter at the light tip.11
Numerous studies researching the use and maintenance of curing lights recommend that: light guides be routinely autoclaved or soaked in disinfectant after each patient or (to avoid damage by these processes) the light curing tips may be covered with disposable sheaths or barriers; the disinfection of the unit body should be done by spraying an appropriate disinfectant on to a 4 x 4 gauze and using this to wipe the LCU. The disinfectant should not be sprayed on to the hand piece or charger surface to avoid internal damage.7,12-14
There is a large volume of published studies describing the impact of infection control barriers on light output from dental curing lights.15-18 Recent evidence suggests that the method of application of plastic barriers significantly influences the amount of reduction in light output. Soares and colleagues (2020) found that correctly applied plastic barriers reduced the light output by 5-8% compared to the incorrectly applied (14-26%).18
This study was intended to indirectly assess compliance with infection control practices at the dental school.
OBJECTIVES OF THE STUDY
To determine the presence and level of bacterial contamination among curing light guides used by students during patient care at a dental school in South Africa.
To describe students' knowledge and awareness of measures used to maintain the sterility of curing light guides.
MATERIALS AND METHODS
Study design
This two-part descriptive study consisted of microbiological testing of the light guides of curing light units and a cross-sectional survey.
Target populations
The two populations studied over a period of one week during the 11h00 to 13h00 clinic session in June and August 2022 were dental students who provided care to patients and the curing light units they used.
Dental student population
A tiny fraction of the population size of 143 third-year (49), fourth-year (71) and fifth-year (23) dental students had clinic sessions.
Curing lights population
A total of 20 sequentially numbered quartz-tungsten-halogen curing light units were available to be used by third- and fourth-year students. Half the population size was actually used. A lone fifth-year student used the individually issued portable LED curing light unit.
Data collection
Microbiological testing
Sterile swabs, premoistened with sterile saline, were used to collect samples for aerobic culture from the tip of the light guide of numbered curing light units at the clinics over a period of one week during the 11h00 to 13h00 session before the units had been used and after they had been used. The third- and fourth-year clinics were held separately in the same floor of the hospital. Consequently, students signed for the same curing lights which were kept in the common control room. Samples were collected separately three months apart. All samples reached the laboratory within 2 hours of collection and were processed immediately upon arrival. Each labelled swab was uncapped and lightly rolled over the entire surface of a blood agar plate with the same label and incubated at 37°C for 48 hours. Resultant colonies were graded on a scale of 0 to 4+ based on the number of quadrants on each plate that showed positive growth according to the procedure used by Bible and colleagues (2009).19 They were classified according to the Gram stain procedure of Engelkirk and Duben-Engelkirk (2008).20 A selection of colonies was sub-cultured in blood agar and the bacteria identified in VITEK®2, an automated instrument used for identification and antimicrobial susceptibility testing.
Cross-sectional survey
A self-administered, structured closed questionnaire was used to collect data from the classes of third-, fourth- and fifth-year dental students enrolled at a dental school in Gauteng, South Africa in 2022. The questionnaire surveyed students' knowledge and perception of infection control measures used to disinfect curing light guides. It consisted of 19 questions. The response variable of the questionnaire consisted mainly of a dichotomous or multiple choice of items.
Definition of variables and terms
Overwhelming majority refers to a majority that is around 70% or more. Vast majority refers to a majority that is 85% or more.
Ethical considerations
The study protocol was approved by the University Ethics Committee (SMREC/D/209/2020:PG). Permission to conduct the study was granted by the Chief Executive Officer (CEO) of the Oral Health Centre.
Statistical analysis/Hypothesis testing
Collected data was captured and analysed in SPSS software. Means and proportions (percentages) were calculated. The Chi-squared tests was performed to test for the statistical significance of the differences in proportions of responses to questions on knowledge and perception. McNemar's test was performed to test the hypothesis that the proportions of contaminated curing light guides were equal before and after use. The chosen significance level for the tests was a p-value equal to or less than 0.05.
Results
The results of microbiological testing and the cross-sectional survey are presented separately.
Microbiological testing
Data obtained from microbiological testing of swabs were analysed.
Bacterial contamination was detected from nine out of 21 (42.9%) light guides before use. Grades of contamination higher than 1+ were not observed.
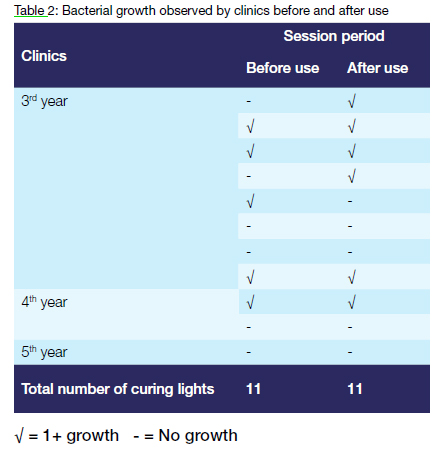
Bacterial growth (1+) was observed from 36.4% (4/11) light guides before and after use. The overwhelming majority (75%) of these curing lights were used in the third-year clinic. Two curing lights, which were uncontaminated before use, were contaminated after use.
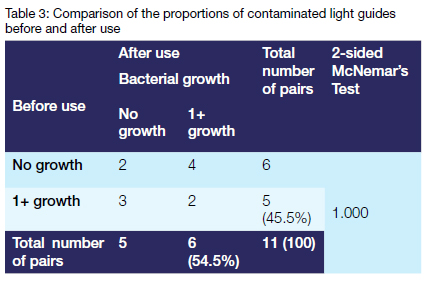
The prevalence of contamination increased after use (54.5% vs 45.5%). However, there was insufficient evidence (p=1.00) to reject the null hypothesis of no difference in the prevalence of contamination before and after use in the population.
Microscopy
A mixed picture was observed on stained slides under the light microscope. The overwhelming majority of the bacteria were Gram positive i.e. they stayed purple, and only a few were Gram negative i.e. they turned pink. Cocci in pairs, clusters or chains predominated. Rod-shaped single cells were also seen.
Bacterial identification
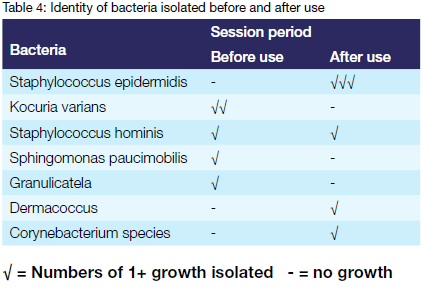
Bacteria of the genus staphylococcus were the most common isolates. Staphylococcus epidermidis was isolated solely after use. Staphylococcus hominis was the only bacteria isolated before and after use.
Cross-sectional survey
Survey data collected from the fourth- and fifth-year classes were analysed. The response rates were 32.8% and 91.3% respectively. The third-year class was excluded from the survey after they failed to return the completed questionnaire two months after being issued with them. The frequency of responses to questions on the topics of knowledge, attitude and behaviour are reported separately.
Demographic characteristics of study participants
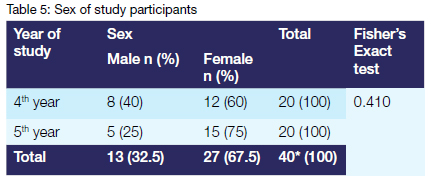
More than twice as many females as males pwarticipated in the study. The difference in proportions between male and female students in the fourth and fifth years of study was, however, not statistically significant (p=0.410)
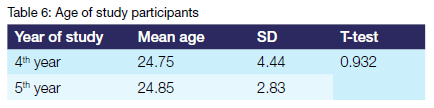
The mean age of the fifth-year class was 0.1 year larger than that of the fourth-year class. However, the difference was not statistically significant (p=0.932).
Questionnaire responses
The differences in the proportions of responses to the question about the categories of patient-care items between the fourth- and fifth-year classes were statistically significant (p=0.021).
A vast majority of students from both classes understood clearly that: curing light could cause transmission of infection among patients; universal precautions should always be used when working on a patient using a curing light; it was their responsibility to ensure that the light guide was sterile at the start of the day, between patients, and at the end of the day and that wiping the light guide with a suitable disinfectant or applying barrier protection between patients was necessary for infection control.
Almost thirty-eight percent (37.4%) more fifth-year students than fourth-year students understood that the light intensity of the light-curing unit affected disposable infection control barriers. The differences in the proportions of responses to the question about whether disposable infection control barriers affect the light intensity of the light-curing unit was statistically significant (p=0.027).
An overwhelming majority of students from both classes were not aware of the manufacturer's recommendation for the maintenance of sterility of the curing light guide.
Almost twice as many fifth-year students as fourth-year students (38.1% vs 20%) had seen or were aware of different types of disposable barriers available to cover the curing light guide such as tip sleeve and plastic wrap. The difference in the proportions of fourth- and fifth-year students who had seen the plastic wrap was statistically significant (p=0.029).
DISCUSSION
This study set out to determine the presence and level of bacterial contamination of curing light guides used by dental students during patient care and describe students' knowledge and awareness of measures used to maintain their sterility.
Microbiological testing
Microbial contamination of the light guides has not previously been described - comparable studies were not found. Bacterial contamination of the base button, fan and handle of the curing light has, however, been established.7
The results of this study show that bacterial contamination before use was prevalent at 42.9%. The findings of the current study are consistent with those of Janoowalla and colleagues (2010) who detected bacteria on the base button, fan and handle of almost 40% of the curing lights sampled in the morning before use.7 This combination of findings suggests that disinfecting curing lights before use might be a problem at dental teaching hospitals.
This study did not detect levels of bacterial contamination higher than 1+. These results are somewhat encouraging considering the fact that light guides have the potential for saliva or blood contamination.
The most interesting result was that bacterial contamination was detected from 36.4% of the curing light guides sampled before and after use. These findings are rather disappointing. They suggest poor compliance with the manufacturer's instruction to disinfect the curing light between each patient.
Another important finding was that the prevalence of contamination increased after use compared to before use (54.5% vs 45.5% respectively). This was, however, not statistically significant (p=1.00). The findings of the current study are consistent with those of Janoowalla and colleagues (2010) who detected contamination on almost 40% of the curing lights before use and 64% after use.7
Microscopic identification
The results of this study show that Gram positive cocci were the predominant organisms. These findings accord with those of Janoowalla and colleagues (2010).7
Biochemical identification
The results of this study indicate that bacteria detected were contaminants. The isolated bacteria included Kocuria varians, Staphylococcus epidermidis, Staphylococcus hominis, Granulicatella, Dermacocus and Sphingomonas paucimobilis. It seems possible that these organisms could have originated from hand contact or oral contamination as they naturally inhabit the skin and mucous membranes.21,22
Cross-sectional survey
Students' awareness of measures used to maintain the sterility of curing light guides has not previously been researched.
Response rate
The results of this study indicate that the response rate of the fourth-year class was 32.8%. This low response rate accords with that found in questionnaire-based studies in dental literature.23 The data collected in this study needs to be interpreted with caution because the sample is not representative of the population as a whole. There are several possible explanations for this result. They include: students were away on off-campus rotations; patients did not honour appointments; and the days of data collection for the study coincided with test dates.
Demographic information
The results of this study indicate that more than twice as many female students as male students participated. This finding is not representative of the gender distribution within the classes. It is due to the fact that more female than male students were available to participate in the study. The results of this study indicate that the mean age of the fifth-year class was 0.1 year larger than that of the fourth-year class. This marginal difference in mean age between the classes was contrary to expectations. It could be attributed to the presence of outliers in the fourth-year class as evidenced by the large standard deviation.
Awareness
The current study found that awareness of the existence of the different types of disposable barriers among students was poor. This was demonstrated by the results that show that: 29.1% of all students had seen or were aware of different types of disposable barriers available to cover the curing light such as tip sleeve and plastic wrap; the difference in the proportions of fourth- and fifth-year students who had seen the plastic wrap was statistically significant (p=0.029) (Table 8). These results were not very encouraging. They indicate that students are not keeping up with the latest technological developments in the field.
Knowledge
The current study found a huge discrepancy in the knowledge of Spaulding classification scheme of patient-care items between the fourth- and fifth-year year classes. Nearly three times as many (2.86 times) fifth-year as fourth-year students knew that curing lights belonged to the semicritical category (p=0.021). These findings are rather disappointing considering that this classification scheme is a universal framework used by infection control professionals and others when planning methods for disinfection or sterilisation.24,25
The results of this study show that a vast majority of students from both classes understood clearly that: a curing light could cause transmission of infection among patients; universal precautions should always be used when working on a patient using a curing light; it was their responsibility to ensure that the light guide was sterile before use, between patients, and at the end of the day and that wiping the light guide with a suitable disinfectant or applying barrier protection between patients was necessary for infection control. This combination of findings is encouraging. It indicates that students are aware of the potential risk of infection transmission from contaminated curing light guides. The most interesting finding was that 52.4% of the fifth-year class and only 15% of the fourth-year class was aware of the impact of infection control barriers on curing light intensity. This finding reflects the difference in the level of clinical experience between the fourth-year and fifth-year classes. It is rather disappointing considering that a great deal of the previous work has reported a reduction of light intensity related to the use of gloves or other opaque barriers.15-18
Limitations of the study
The small sample size and low response rate threatens the internal validity of this study.
CONCLUSION
The current study found that contamination occurred despite high levels of students' knowledge and awareness of the risk of infection transmission from contaminated curing light guides
REFERENCES
1. Burke JP. Infection control - a problem for patient safety. N Engl J Med 2003; 348: 651-6 [ Links ]
2. Van Kleef, E, Robotham JV, Jit M, Deeny SR, Edmunds WJ. Modelling the transmission of healthcare associated infections: a systematic review. BMC Infect Dis 2013; 13(1):1-13 [ Links ]
3. Irek EO, Amupitan AA, Aboderin AO, Obadare TO. A systematic review of healthcare-associated infections in Africa: An antimicrobial resistance perspective. Afr J Lab Med 2018; 7(2): 1-9 [ Links ]
4. Mutters NT, Hagele U, Hagenfeld D, Hellwig E, Frank U. Compliance with infection control practices in a university hospital dental clinic. GMS Hyg Infect Control 2014; 9(3) [ Links ]
5. Cristina ML, Spagnolo AM, Sartini M etal. Investigation of organizational and hygiene features in dentistry: a pilot study. J Prev Med Hyg 2009; 50(3): 175-80 [ Links ]
6. Donly KJ, Sasa IS. Dental Materials. In: Nowak AJ, Christensen JR, Mabry TR, Townsend JA, Wells MH, eds. Pediatric Dentistry,6th ed. Elsevier, 2019:293-303 [ Links ]
7. Janoowalla Z, Porter K, Shortall A, Burke F, Sammons R. Microbial contamination of light curing units: a pilot study. J Infect Prev 2010; 11(6):217-21 [ Links ]
8. Caughman WF, O'Connor RP, Volkmann KR, Schuster GS, Caughman GB. Oper Den Visible-light-curing devices: a potential source of disease transmission. Oper Dent 1987; 12(1):10-4 [ Links ]
9. American Dental Association. Dental Curing Lights. Available https://www.ada.org/en/resources/research/science-and-research-institute/oral-health-topics/dental-curing-lights [Accessed 14/06/2023] [ Links ]
10. Kohn WG, Harte JA, Malvitz DM, et al. Guidelines for infection control in dental health care settings - 2003. J Am Dent Assoc 2004;135(1): 33-47 [ Links ]
11. Price RB, Ferracane JL, Hickel R, Sullivan B. The light-curing unit: An essential piece of dental equipment. Int Dent J 2020;70(6):407-17 [ Links ]
12. Mitton BA, Wilson NH. The use and maintenance of visible light activating units in general practice. Br. Dent. J. 2001; 191:82-6 [ Links ]
13. Pollington S, Kahakachchi N, van Noort R. The influence of plastic light cure sheaths on the hardness of resin composite. Oper Dent 2009; 34(6):741-5 [ Links ]
14. Santini A. Current status of visible light activation units and the curing of light-activated resin-based composite materials. Dent Update 2010; 37(4): 214-27 [ Links ]
15. Hodson NA, Dunne SM, Pankhurst CL. The effect of infection-control barriers on the light intensity of light-cure units and depth of cure of composite. Prim. Dent. Care 2005; 12:61-7 [ Links ]
16. Scott BA, Felix CA, Price RB. Effect of disposable infection control barriers on light output from dental curing lights. J. Can. Dent. Assoc 2004; 70:105-10 [ Links ]
17. McAndrew R, Lynch CD, Pavli M, Bannon A, Milward P. The effect of disposable infection control barriers and physical damage on the power output of light curing units and light curing tips. Br. Dent. J 2011;210: E12 [ Links ]
18. Soares CJ, Braga SSL, Ribeiro MTH, Price RB. Effect of infection control barriers on the light output from a multi-peak light curing unit. J Dent 2020; 103:103503 [ Links ]
19. Bible JE, Biswas D, Whang PG, et al. Which regions of the operating gown should be considered most sterile? Clin Orthop Relat Res 2009; 467(3): 825-30 [ Links ]
20. Engelkirk PG, Duben-Engelkirk J. Laboratory Diagnosis of Infectious Diseases: Essentials of Diagnostic Microbiology. Baltimore: Wolters Kluwer/Lippincott Williams & Wilkins, 2008 [ Links ]
21. Williams AN, MacLea KS. Draft Genome Sequence of Dermacoccus nishinomiyaensis TSA37, Isolated from Wood Ash. Microbiol Resour Announc 2019; 8(50): e01370-19 [ Links ]
22. Kandi V, Palange P, Vaish R, et al. Emerging bacterial infection: identification and clinical significance of Kocuria species. Cureus 2016;8(8): e731 [ Links ]
23. Al Khalaf K, O'Dowling Keane S, da Mata C, McGillycuddy CT, Chadwick BL, Lynch CD. Response rates to questionnaire-based studies in the contemporary dental literature: A systematic review. J Dent. 2022; 126:104284 [ Links ]
24. Simmons BP. CDC Guidelines for the prevention and control of nosocomial infections. Guidelines for hospital environmental control. Am. J. Infect. Control 1983; 11:97-120 [ Links ]
25. Rutala WA, 1994,1995,1996 APIC Guidelines Committee. APIC guidelines for selection and use of disinfectants. Association for Professionals in Infection Control and Epidemiology, Inc. Am. J. Infect. Control 1996; 24:313-42 [ Links ]
 Correspondence:
Correspondence:
Dr SR Mthethwa
Tel: (012) 521 5888
Email: rocky.mthethwa@smu.ac.za
Author's contribution:
1. D Motlhake 30%
2. SR Mthethwa 40%
3. EM Sekati 30%














