Services on Demand
Journal
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
African Entomology
On-line version ISSN 2224-8854Print version ISSN 1021-3589
AE vol.31 Pretoria 2023
https://doi.org/10.17159/2254-8854/2023/a12637
RESEARCH ARTICLE
Palm wine as a food-based bait for monitoring adult Ceratitis ditissima (Munro) (Diptera: Tephritidae) in citrus orchards
John AbrahamI; Carlos AmissahI; Felix Osei KuffourI; Janice Dwomoh AbrahamII
IDepartment of Conservation Biology and Entomology, School of Biological Sciences, College of Agriculture and Natural Sciences, University of Cape Coast, Ghana
IIDepartment of Biological Sciences Education, Faculty of Science Education, Akenten Appiah-Menka University of Skills Training and Entrepreneurial Development, Mampong-Ashanti, Ghana
ABSTRACT
Tephritid fruit flies, including Ceratitis ditissima, often invade citrus orchards. These flies cause economic losses to farmers and can prevent farmers from exporting their fruits to foreign markets. To detect the presence of fruit flies in citrus orchards, traps are baited with synthetic lures, which are often expensive for smallholder farmers. Farmers in developing or financially less-endowed countries have to import such synthetic baits, raising the cost of pest monitoring and control. Therefore, we evaluated the potential of palm wine and three other food-based mixtures for trapping C. ditissima and the proportion of non-target flies they trap. Transparent deli cup traps were baited with four different food-based baits, namely palm wine, sugarcane spirit-wine mixture, apple cider vinegar and yeast-sugar mixture. The traps were placed within a citrus orchard on fruit-bearing trees. The content of each trap was collected after one week and evaluated. This was repeated for eight consecutive weeks. Traps baited with palm wine captured more C. ditissima than those with the other baits. Furthermore, the proportion of non-target insects, Bactrocera dorsalis and Drosophila spp., in palm wine-baited traps was less than the other baited traps. This study indicates that palm wine, a cheap beverage across Africa, Asia and South America, could be used to monitor the presence of C. ditissima in citrus orchards. Smallholder farmers who cannot afford expensive synthetic baits could make use of palm wine to monitor fruit flies in their farms.
Keywords: fermented-food attractant, field trapping, pest control, smallholder farmers, tephritid fruit flies
INTRODUCTION
Fruit flies belonging to the family Tephritidae are among the most economically important insect pests of horticultural crops (Badii et al. 2015; Ekesi et al. 2016; Yazid et al. 2020). They attack and cause economic damage to a variety of crops including citrus (Lloyd et al. 2010; Vayssieres et al. 2010), mango (Ansari et al. 2012; Gnanvossou et al. 2017; Hanna et al. 2020), guava (Birke et al. 2015), melon (Dhillon et al. 2005; Ansari et al. 2012; Mokam et al. 2018) and papaya (Ansari et al. 2012). About 200 species of known tephritid fruit flies are pests, of which some are polyphagous and may switch host plants depending on their location, season and available host plants (Lloyd et al. 2010; Vayssiéres et al. 2010; Ansari et al. 2012; Kambura et al. 2018). The gravid female fruit fly lays eggs underneath the epicarp of fruits, the eggs hatch and the larvae feed on the pulp, causing fruit damage, while the oviposition wound serves as an entry point for pathogens (Walsh et al. 2011; Thomas et al. 2013; Birke et al. 2015). The losses due to tephritid fruit flies could reach 100% (Dhillon et al. 2005; Thomas et al. 2019). For instance, studies in Papua New Guinea have shown that Bactrocera cucurbitae (Coquillett) could damage 95% of bitter gourd fruits and 90% of snake gourd (Hollingsworth et al. 1997). Due to such losses, trade restrictions are often imposed on fruits originating from areas with known fruit fly infestation, leading to fruits being quarantined (Lloyd et al. 2010; Yazid et al. 2020). These restrictions, while necessary, delay the transport of fruits and often results in increased spoilage and further economic losses to farmers.
Citrus is considered as a major cash crop in many countries including Ghana where this study was conducted (Anno-Nyarko et al. 1998; Ofosu-Budu et al. 2007). However, citrus orchards are affected by fruit fly infestation from Ceratitis ditissima (Munro) (Diptera: Tephritidae) (Foba et al. 2012). The Ceratitis genera contains several multivoltine species (Chen et al. 2006; Ansari et al. 2012), of which one is C. ditissima. Ceratitis ditissima produces several generations per year making them a major concern in citrus orchards. Although C. ditissima has been identified as an abundant tephritid fruit fly species in citrus orchards in Ghana (Foba et al. 2012), it has also been sighted in mango orchards, albeit in smaller numbers (Hanna et al. 2020; Zida et al. 2020; J. Abraham, unpubl.).
Olfaction in insects enables them to navigate to their host plants (Robacker & Heath 1996; Siderhurst & Jang 2010; Abraham et al. 2014, 2015, 2022). Many studies have demonstrated that insects orientate toward odours they perceive as food (e.g. Kimbokota et al. 2013; Cha et al. 2014; Epsky 2015; Knight et al. 2016; Cloonan et al. 2018). In Drosophila spp. it is known that fermentation products are particularly attractive and have been used to monitor their presence in fruit farms (Landolt et al. 2012; Cha et al. 2014; Cloonan et al. 2018). Among tephritid fruit flies, it is known that the oriental fruit fly, Bactrocera dorsalis Drew, Tsura & White (Diptera: Tephritidae) is attracted to host fruit odours (Kimbokota et al. 2013). Such food odours could potentially be harnessed to monitor fruit flies and subsequently optimised for control (Biasazin et al. 2018). Generally, fruit flies are controlled with insecticides, with some farmers following strict calendar-based spraying, which may impact negatively on the environment (Beers et al. 2011; Badii et al. 2012; Abraham et al. 2015).
Moreover, it is expensive for many smallholder farmers to buy synthetic insecticides to apply in their farms regularly on a calendar-basis. It is therefore necessary to find cheap and environmentally friendly ways of monitoring the presence of C. ditissima in citrus orchards. This could help farmers to detect the insect pest early and subsequently facilitate its control (Ekesi et al. 2006; Suszkiw 2014; Abraham et al. 2015; Marakhan et al. 2017). The volume of synthetic insecticides to be applied per year could be reduced if monitoring is done to know when and where to apply insecticides (Zehnder et al. 1995; Stewart et al. 2002).
In this study, we evaluated the potential of palm wine and three other food-based mixtures for monitoring the presence of C. ditissima in citrus orchards. In addition, we investigated the percentage capture of non-target flies compared to the target fly, C. ditissima. The tested baits, based largely on locally available materials were palm wine, sugarcane spirit-wine mixture, apple cider vinegar and yeast-sugar mixture. These were tested in an attempt to provide other viable alternative baits to commercial baits, which are mostly imported.
MATERIALS AND METHODS
Study area
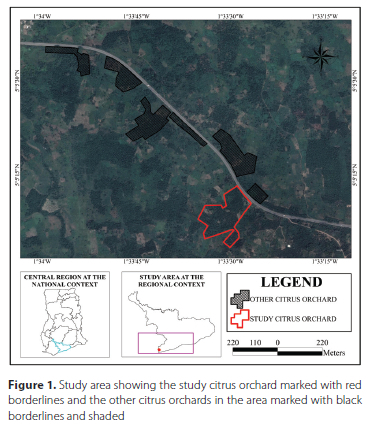
The study was conducted in a 3.68 ha citrus orchard (Citrus sinensis (L.) Osbeck cv. Valencia late) located in the Central Region of Ghana (5.085607°, -1.558507°). The citrus trees were planted in rows. The distance between the trees in the rows was 4 m. Furthermore, the study orchard was surrounded by other citrus orchards (Figure 1). The study was conducted over an 8-week period between November through to January, which coincides with the major fruiting season of citrus in Ghana (Lawson et al. 2017). The study commenced two weeks before the harvesting period (i.e. 21 November) and ended three weeks after harvesting (i.e. 9 January) when C. ditissima populations had reduced drastically.
Tested food-based baits
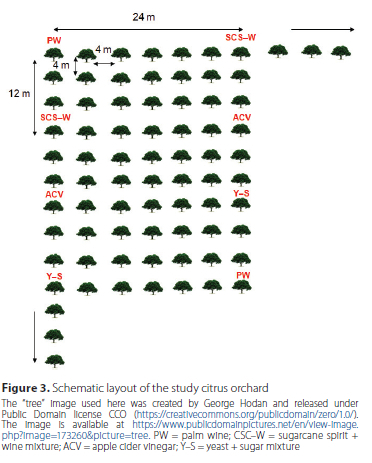
We tested the potential of the following food-based baits: palm wine (PW), sugarcane spirit + wine mixture (SCS-W), apple cider vinegar (ACV) (Heinz, Heinz North America, Pittsburgh, PA) and yeast + sugar mixture (Y-S) as attractants of C. ditissima.
Fresh PW was obtained from the sap of the inflorescence of the oil palm tree (Elaeis guineensis Jacq.; Arecaceae: Cocoeae) (Stringini et al. 2009; Karamoko et al. 2012). Palm wine was chosen because it is easily available in Ghana, several other African countries, Asia and South America (Uzochukwu et al. 2007; Ukwuru & Awah 2013, Shet & Belur 2015). The sugarcane spirit (ca 50% v/v) + wine mixture (13% v/v) was prepared by modifying the recipe for Droskidrink (Grassi et al. 2014; Burrack et al. 2015) by mixing 675 ml of sugarcane spirit with 230 ml of red wine (Rey Don Garcia, Rioja, Spain) and 18 g of white sugar (CSR sugar, Sugar Australia, Yarraville, VIC) to obtain ~900 ml of liquid. The sugarcane spirit was obtained from a local brewer who distilled fermented sugarcane juice. The Y-S mixture was prepared following the guidelines published in Walsh et al. (2011) and Burrack et al. (2015) by mixing 10.14 g of yeast (Saccharomyces cerevisiae; S.I. Lesaffre, Marcq., France), 50.70 g of white sugar (CSR sugar) and 900 ml of water. Apple cider vinegar (Heinz) was obtained from a grocery store (Sonturk Supermarket, Cape Coast, Ghana). These particular food-based baits were chosen because similar baits have previously been used in monitoring other fruit flies e.g. Bactrocera minax (Enderlein), Ceratitis capitata Wiedemann, Drosophila suzukii (Matsumura) (Beers et al. 2011; Landolt et al. 2012; Zhou et al. 2012; Lee et al. 2013; Burrack et al. 2015; Guillemain et al. 2021). Palm wine and sugarcane spirit were particularly chosen because they are common and relatively cheap in most parts of Africa, Asia and South America (Alcarde et al. 2014; Bortoletto & Alcarde 2015). Moreover, fruit flies are generally known to be attracted to fermented products (Cha et al. 2015; Pinero et al. 2015; Candia et al. 2018).
Field trapping
One hundred transparent deli cup traps were constructed from 370 ml disposable cups (Everpack, Accra, Ghana) by drilling five holes (diameter ~12 mm) equidistant to each other and ~3 cm from the top of the cup (Figure 2). The traps were baited with the four food-based baits, so that there were 25 cups each baited with 30 ml of PW, SCS-W mixture, ACV or Y-S mixture. These were deployed systematically in the orchard by hanging them singly at the distal ends of branches of the citrus trees so that same baits do not repeat directly after each other. The distance between traps was 12 m x 24 m and they covered ~2 ha of the citrus orchard (Figure 3). Contents of baited traps were checked weekly.

Collection of trapped insects
At the end of each week, the traps were taken down and captured flies were emptied into a labelled vial. This was done so that all insects trapped by the respective baits were placed in separate vials, thus, there were separate vials for PW, SCS-W, ACV and Y-S. The deli cup traps were cleaned and fresh baits were placed in them before hanging on the trees for the following week. Vials containing the captured flies were transported to the laboratory where flies were confirmed as C. ditissima or other flies by viewing their features under an optical microscope (Leica, Leica Microsystems Ltd, Heerbrugg, Switzerland) and comparing them with those described in Ekesi & Billah (2007). This was repeated for eight consecutive weeks.
Statistical analysis
Captured C. ditissima were sorted by sex and counted. The count of all C. ditissima for each treatment (bait) per week was treated as one data set (n = 1/per week). The non-target flies B. dorsalis and Drosophila spp. were also counted but not separated by sex. One-way analysis of variance (Minitab 17, Minitab Inc., State College, PA) was performed on the number of insects captured. The significance level, a, was 0.05 at a confidence interval of 95%. Where statistical differences occurred, Tukey's test was performed (Minitab).
RESULTS
A total of 2942 adult C. ditissima were captured over the entire 8-week period of monitoring with the four different baits in deli cup traps. There were significant differences in the capture of C. ditissima by the different baited traps (F = 6.21; df = 3; p = 0.002; Table 1). The average capture of adult C. ditissima per week by PW-baited traps was significantly higher than the captures by ACV- and Y-S-baited traps. The capture by PW-baited traps was numerically higher than the capture by SCS-W-baited traps but not statistically higher. A similar observation was made between the numbers of C. ditissima captured by ACV- and Y-S-baited traps (Table 1).
Total adult female C. ditissima captured over the monitoring period was 1540. There were significant differences in the number of adult females that were captured by the different baited traps (F = 5.72; df = 3; p = 0.003; Table 1). Significantly more females were captured by PW-baited traps than ACV- and Y-S-baited traps. The number of females captured by PW-baited traps was also higher than that captured by SCS-W-baited traps but not statistically different. Almost equal numbers were captured by ACV and Y-S-baited traps (Table 1).
The total number of adult male C. ditissima captured over the 8-week study period was 1402. The different baited traps captured significantly different numbers of adult males (F = 6.01; df = 3; p = 0.003; Table 1). The number of males captured by PW-baited traps was significantly higher than those captured by ACV- and Y-S-baited traps. Moreover, the number of C. ditissima captured by PW-baited traps was higher than that of SCS-W-baited traps but not statistically different. Almost the same number of males was captured by ACV- and Y-S-baited traps (Table 1).
Besides C. ditissima, non-target flies were also captured in the baited traps. A total of 136 adult B. dorsalis were captured among all four baits. There were significant differences in the overall capture of B. dorsalis by the different baited traps (F = 6.19; df = 2; p = 0.002; Table 1). The number of B. dorsalis captured by SCS-W and PW-baited traps were similar and both were significantly higher than the number captured by Y-S-and ACV-baited traps. Yeast + sugar-baited traps captured an average of one per week and the total capture of B. dorsalis by ACV-baited traps for the entire 8-week study period was one individual.
A total of 3296 Drosophila spp. were captured as non-target flies in the traps. The number of Drosophila spp. captured by the different baited traps were significantly different (F = 5.36; df = 3; p = 0.005; Table 1). The number of Drosophila spp. captured by both SCS-W and PW-baited traps were significantly higher than that captured by Y-S-baited traps.
Though numerically higher, captures by SCS-W and PW-baited traps were not significantly higher than those of ACV-baited traps (Table 1).
When the captures of each bait were evaluated based on the percentage of target and non-target flies captured, SCS-W and Y-S mixtures captured significantly more Drosophila spp. than C. ditissima (Table 2). The percentage of Drosophila spp. captured by ACV-baited traps was about 12% higher than the C. ditissima captured but this was not significantly different. Furthermore, PW-baited traps captured only 2% more Drosophila spp. than C. ditissima, the target fly. In all cases, the tested baits captured more C. ditissima than B. dorsalis (Table 2).
DISCUSSION
All the tested baits were food-based and so were attractive to C. ditissima. Food is often incorporated in many commercially available baits because of the importance of nutrients in the survival of insects. Baits that incorporate food include CeraTrap, Mazoferm E802, Torula yeast, GF-120, Hymlure and Nulure meant for monitoring and capture of tephritid fruit flies (Epsky et al. 2011; Ekesi et al. 2014; Perea-Castellanos et al. 2015; Pinero et al. 2015; Hanna et al. 2020). Recent findings indicate that locally available materials such as beer waste (Pinero et al. 2015) and poultry manure (Filgueiras et al. 2016) are effective in monitoring tephritid fruit flies in orchards. This fact, coupled with the relatively high cost of commercially available baits to smallholder farmers (Ekesi et al. 2014; Filgueiras et al. 2016; Candia et al. 2018) has made it necessary to test other locally available materials that could help detect and control C. ditissima is citrus orchards.
Our findings show that PW is effective in detecting C. ditissima in citrus orchards. Moreover, PW-baited traps captured relatively less non-target flies, particularly Drosophila spp. This makes it a better bait than the others tested. In fact, ACV is said to be a non-selective bait because it captured only 26-31% of the target fly in a study (Lee et al. 2012). In this study, PW-baited traps captured 42-54% of the target fly, C. ditissima. Moreover, it captured 1-3% of B. dorsalis. This indicates that, palm wine is relatively selective in attracting C. ditissima but could also detect B. dorsalis which was not a target for PW-baited traps. This is a plus because it is known that B. dorsalis is a serious pest in citrus orchards elsewhere (Li et al. 2019; Faye et al. 2021).
Palm wine emits several volatile organic compounds including acetic acid, benzyl alcohol, ethyl hexanoate, ethyl octanoate, 3-methylbutan-1-ol and nonanal (Uzochukwu et al. 1997). These compounds are known to elicit attractive responses in several tephritid fruit flies. For instance, acetic acid, one of the first volatiles emitted from PW as it ferments (Uzochukwu et al. 1997) elicits antennal responses from B. cucurbitae and could be used as one of the components in an attractive bait for monitoring it in cucumber cultivations (Siderhurst & Jang 2010). Likewise, benzyl alcohol and nonanal elicit antennal responses from B. cucurbitae with acetic acid and nonanal acting in synergy with other volatiles as a bait for B. cucurbitae (Siderhurst & Jang 2010). Moreover, ethyl hexanoate and ethyl octanoate in combination with hexanol and 1,8-cineole attracts Anastrepha ludens Leow in citrus orchards (Robacker & Heath 1996). In
addition, ethyl octanoate elicits antennal responses from both male and female C. capitata (Cossé et al. 1995). The antennal response to these chemicals is very important in the process of discovering and formulating baits for fruit fly monitoring. Another important physiologically active compound in PW, 3-methylbutan-1-ol, has been found to act in synergy with other volatiles such as 4,8-dimethyl-1,3(E),7-nonatriene, butyl hexanoate, and dihydro-β-ionone to elicit attraction response from Rhagoletis pomonella (Nojima et al. 2003). Palm wine may have been very attractive to C. ditissima probably because of the presence of these compounds in the volatiles emitted and a possible synergy with other compounds present in it.
In previous studies, wine has either been used alone or in combination with other baits to monitor fruit flies (Landolt et al. 2012; Burrack 2015; Beers et al. 2021). Fruit fly catch was improved by the combination of wine and other attractants. The combination of sugarcane spirit and wine likely accounts for the relatively good performance of this bait. This bait captured more C. ditissima than ACV and Y-S mixture. The ACV and Y-S mixture-baited traps on the other hand captured more Drosophila spp. reaching 46-66% and 60-72%, respectively. This is not surprising because Drosophila spp. are normally attracted to vinegar and yeast which are products associated to fermentation and rotten fruits (Epsky et al. 2015; Keesey et al. 2015; Cloonan et al. 2018). The highest proportion of Drosophila spp. captured in the Y-S-baited traps is in agreement with an earlier study that showed that adding the yeasts, Saccharomyces cerevisiae or Aureobasidium pullulans, and sugar to insecticides in the class diamides and spinosyns, increased the proportion of Drosophila suzukii that were killed significantly by the insecticides (Knight et al. 2016).
It is desirable to have a selective bait when monitoring a particular insect pest. In our study, though PW was the most selective among the tested baits, it still had a relatively high proportion of Drosophila spp. The progressive fermentation of PW may account for the proportion of Drosophila spp. it captured (Epsky et al. 2015). In spite of the relatively high proportion of non-target flies in the catches of the PW, it is a promising bait which could be optimised. For example, a 5-component bait for D. suzukii was optimised to perform better in a 4-component bait (Cha et al. 2014). Today, that 4-component bait is the basis for the commercial bait Pherocon SWD (Suszkiw 2014).
It is worth noting two important findings that have come out of this study, thus 1) locally available materials could be used as baits in traps to monitor C. ditissima and that PW, a relatively cheap beverage, was the most effective among tested materials (SCS-W mixture, Y-S mixture and ACV); 2). Palm wine attracted relatively less non-target flies i.e., Drosophila spp., compared to the other baits tested. The overall effectiveness of the baits for monitoring C. ditissima could therefore be ranked in descending order as: PW > SCS-W mixture > ACV > Y-S mixture.
In conclusion, this study has shown that PW is attractive to C. ditissima and that, when placed in simple homemade deli cup traps, it could attract and trap adult C. ditissima. Therefore, raw PW could be utilised to monitor the presence of C. ditissima in citrus orchards. The advantage of using PW is that it is relatively inexpensive and abundantly available in several countries across Africa, Asia and South America, where citrus is cultivated. Smallholder farmers could therefore access it easily. For large commercial orchards, the use of PW for monitoring could indicate which parts of the orchard are under threat of tephritid fly infestation for remedial action.
ACKNOWLEDGEMENT
We are grateful to Mr Alhassan Inusah for allowing us access to his citrus orchard to conduct this study.
AUTHORS' CONTRIBUTIONS
Conceptualisation - JA; Data curation - JA, CA, FOK; Formal analysis - JA; Funding acquisition - JA, CA, FOK, JDA; Investigation - JA, CA, FOK; Methodology - JA; Project administration - JA, JDA; Resources: JA, CA, FOK, JDA; Supervision - JA, JDA; Validation - JA; Visualisation - JA; Writing original draft: JA, CA; Writing review and editing - JA, CA, FOK, JDA.
ORCID IDs
John Abraham - https://orcid.org/0000-0001-6049-6042
Janice Dwomoh Abraham - https://orcid.org/0000-0002-9908-275X
Carlos Amissah - https://orcid.org/0000-0002-3971-0005
REFERENCES
Abraham J, Opuni-Frimpong E, Weissbecker B, Schütz S, Angeli S. 2014. Olfactory cues of mahogany trees to female Hypsipyla robusta. Bulletin of Insectology 67: 21-30. [ Links ]
Abraham J, Zhang A, Angeli S, Abubeker S, Michel C, Feng Y, Rodriguez-Saona C. 2015. Behavioral and antennal responses of Drosophila suzukii (Diptera: Drosophilidae) to volatiles from fruit extracts. Environmental Entomology 44: 356-367. https://doi.org/10.1093/ee/nvv013 [ Links ]
Abraham J, Angeli S, Antwi JB, Rodriguez-Saona C. 2022. Editorial: Research advances on Drosophila suzukii. Frontiers in Ecology and Evolution 10: 897222. https://doi.org/10.3389/fevo.2022.897222 [ Links ]
Alcarde AR, Souza LM, Bortoletto AM. 2014. Formation of volatile and maturation-related congeners during the aging of sugarcane spirit in oak barrels. Journal of the Institute of Brewing 120: 529-536. https://doi.org/10.1002/jib.165 [ Links ]
Anno-Nyarko FO. 1998. The major citrus production constraints: Status of citrus research and the future of the citrus industry in Ghana. Paper presented at the first Biennial National Agricultural Research Systems Workshop, Accra, Ghana. 1, 18.
Ansari MS, Hasan F, Ahmad N. 2012. Threats to Fruit and Vegetable Crops: Fruit flies (Tephritidae) - Ecology, behaviour, and management. Journal of Crop Science and Biotechnology 15: 169-188. [ Links ]
Badii KB, Billah MK, Afreh-Nuamah K, Obeng-Ofori D, Nyarko G. 2015. Review of the pest status, economic impact and management of fruit-infesting flies (Diptera: Tephritidae) in Africa. African Journal of. Agricultural Research 10: 1488-1498. [ Links ]
Beers EH, Smith TJ, Walsh D. 2021. Spotted wing drosophila. Available at: https://treefruit.wsu.edu/crop-protection/opm/spotted-wing-drosophila/ [Accessed 20 October 2021].
Beers EH, van Steenwyk RA, Shearer PW, Coates WW, Grant JA. 2011. Developing Drosophila suzukii management programs for sweet cherry in the western United States. Pest Management Science 67: 1386-1395. [ Links ]
Biasazin TD, Chernet HT, Herrera SL, Bengtsson M, Karlsson MF, Lemmen-Lechelt JK, Dekker T. 2018. Detection of volatile constituents from food lures by tephritid fruit flies. Insects 9(3): 119. https://doi.org/10.3390/insects9030119 [ Links ]
Birke A, Acosta E, Aluja M. 2015. Limits to the host range of the highly polyphagous tephritid fruit fly Anastrepha ludens in its natural habitat. Bulletin of Entomological Research 105: 743-753. [ Links ]
Bortoletto AM, Alcarde AR. 2015. Assessment of chemical quality of Brazilian sugar cane spirits and cachaças. Food Control 54: 1-6. [ Links ]
Burrack HJ, Asplen M, Bahder L, Collins J, Drummond FA, Guédot C, Isaacs R, Johnson D, Blanton A, Lee JC, Loeb G, Rodriguez-Saona C, van Timmeren S, Walsh D, McPhie, DR. 2015. Multistate comparison of attractants for monitoring Drosophila suzukii (Diptera: Drosophilidae) in blueberries and caneberries. Environmental Entomology 44: 704-712. https://doi.org/10.1093/ee/nvv022 [ Links ]
Candia IF, Bautista V, Larsson Herrera S, Walter A, Ortuno Castro N, Tasin M, Dekker T. 2019. Potential of locally sustainable food baits and traps against the Mediterranean fruit fly Ceratitis capitata in Bolivia. Pest Management Science 75(6): 1671-1680. https://doi.org/10.1002/ps.5286 [ Links ]
Cha DH, Adams T, Werle CT, Sampson BJ, Adamczyk JJ Jr, Rogg H, Landolt PJ. 2014. A four component synthetic attractant for Drosophila suzukii (Diptera: Drosophilidae) isolated from fermented bait headspace. Pest Management Science 70: 324-331. [ Links ]
Cha DH, Gill MA, Epsky ND, Werle CT, Adamczyk Jr, JJ, Landolt PJ. 2015. From a non-target to a target: identification of a fermentation volatile blend attractive to Zaprionus indianus. Journal of Applied Entomology 139: 114-122. [ Links ]
Chen P, Ye H, Liu J. 2006. Population dynamics of Bactrocera dorsalis (Diptera: Tephritidae) and analysis of the factors influencing the population in Ruili, Yunna Province, China. Acta Ecologica Sinica 26: 2801-2808. [ Links ]
Cloonan KR, Abraham J, Angeli S, Syed Z, Rodriguez-Saona C. 2018. Advances in the chemical ecology of the Spotted Wing Drosophila (Drosophila suzukii) and its applications. Journal of Chemical Ecology 44(10): 922-939. https://doi.org/10.1007/s10886-018-1000-y [ Links ]
Cossé AA, Todd JL, Millar JG, Martinez LA, Baker TC. 1995. Electroantennographic and coupled gas chromatographic-electroantennographic responses of the Mediterranean fruit fly, Ceratitis capitata, to male-produced volatiles and mango odor. Journal of Chemical Ecology 21: 1823-1836. [ Links ]
Dhillon MK, Singh R, Naresh JS. Sharma HC. 2005. The melon fruit fly, Bactrocera cucurbitae: A review of its biology and management. Journal of Insect Science, 5(1): 40. [ Links ]
Ekesi S, Billah MK. 2007. A field guide to the management of economically important Tephritid fruit flies in Africa (2nd Edition). The International Centre of Insect Physiology and Ecology. icipe Science Press, Nairobi, Kenya.
Ekesi S, De Meyer M, Mohamed SA, Virgilio M, Borgemeister C, 2016. Taxonomy, ecology, and management of native and exotic fruit fly species in Africa. Annual Review of Entomology 61: 219-238. [ Links ]
Ekesi S, Mohamed S, Tanga, CM. 2014. Comparison of food-based attractants for Bactrocera dorsalis (Diptera: Tephritidae) and evaluation of Mazoferm-Spinosad bait spray for field suppression in mango. Journal of Economic Entomology 107: 299-309. [ Links ]
Epsky ND, Gill MA, Robert ML 2015. Grape juice as a bait for Anastrepha suspensa (Diptera: Tephritidae) and Zaprionus indianus (Diptera: Drosophilidae). Journal of Economic Entomology 108: 2065-2073. [ Links ]
Epsky ND, Kendra PE, Pena J, Heath RR, 2011. Comparison of synthetic food-based lures and liquid protein baits for capture of Anastrepha suspensa (Diptera: Tephritidae) adults. Florida Entomologist 94: 18-185. [ Links ]
Filgueiras RMC, De Azevedo FR, Azevedo R, De Farias RB, Coutinho CR. 2016. Livestock manure as an alternative attractant for fruit flies (Diptera: Tephritidae) in guava tree. Pesquisa Agropecuária Tropical 46: 51-56. [ Links ]
Faye PD, Bal AB, Ndiaye NM, Diop F, Sangaré YK, Haddad C, Coly EV, Dieng EO, Niassy S. 2021. Field efficacy of Metarhizium acridum (Hypocreales: Clavicipitaceae) in the control of Bactrocera dorsalis (Diptera: Tephritidae) in citrus orchards in Senegal. International Journal of Tropical Insect Science 41(2):1185-1195. [ Links ]
Foba CN, Afreh-Nuamah K, Billah MK, Obeng-Ofori D. 2012. Species composition of fruit flies (Diptera: Tephritidae) in the citrus Museum at the Agricultural Research Centre (ARC), Kade, Ghana. International Journal of Tropical Insect Science 32: 12-23. https://doi.org/10.1017/S1742758412000045 [ Links ]
Gnanvossou D, Hanna R, Goergen G, Salifu D, Tanga CM, Mohamed SA, Ekesi S, 2017. Diversity and seasonal abundance of tephritid fruit flies in three agro-ecosystems in Benin, West Africa. Journal of Applied Entomology 141(10): 798-809. https://doi.org/10.1111/jen.12429 [ Links ]
Grassi A, Anfora G, Maistri S, Maddalena G, De Cristofaro A., Savini G, Ioriatti C. 2014. Development and efficacy of Droskidrink, a food bait for trapping Drosophila suzukii. In: IOBC VIII Workshop on Integrated Soft Fruit Production. 26-28 May 2014, Vigalzano di Pergine, Italy. Available at https://openpub.fmach.it/retrieve/handle/10449/23692/10378/2014%20IOBC%20Vigalzano%20Anfora.pdf. [Accessed 20 October 2021].
Guillemain MJ, Díaz Nieto, LM, Suárez L, Rull J, Ovruski S, Acosta JC, Molina D, Murua F. 2021. Offseason Medfly Trapping Using Makeshift Fruit-Based and Wine Vinegar Baits. Neotropical Entomology 50: 289-297. https://doi.org/10.1007/s13744-020-00844-0 [ Links ]
Hanna R, Gnanvossou D, Goergen G, Bokonon-Ganta AH, Mohamed SA, Ekesi S, Agnontchémè AI. 2020. Efficiency of food-based attractants for monitoring tephritid fruit flies diversity and abundance in mango systems across three west African agro-ecological zones. Journal of Economic Entomology 113(2): 860-871. https://doi.org/10.1093/jee/toz338 [ Links ]
Hollingsworth R, Vagalo M, Tsatsia F. 1997. Biology of melon fly, with special reference to the Solomon Islands. In: Allwood AJ, Drew RAI (editors). Management of fruit flies in the Pacific. Proceedings of Australian Country Industrial Agricultural Research 76: 140-144.
Kambura C, Tanga CM, Kilalo D, Muthomi J, Salifu D, Rwomushana I, Ekesi S. 2018. Composition, host range and host suitability of vegetable-infesting tephritids on cucurbits cultivated in Kenya. African Entomology 26(2): 379-397. https://doi.org/10.4001/003.026.0379 [ Links ]
Karamoko D, Djeni NT, N'Guessan KF, Bouatenin KMJ-P, Dje KM. 2012. The biochemical and microbiological quality of palm wine samples produced at different periods during tapping and changes which occured during their storage. Food Control. 26: 504-511. [ Links ]
Keesey IW, Knaden M, Hansson BS. 2015. Olfactory specialization in Drosophila suzukii supports an ecological shift in host preference from rotten to fresh fruit. Journal of Chemical Ecology 41: 121-128. [ Links ]
Kimbokota F, Njagi PGN, Torto B, Ekesi S, Hassanali A. 2013. Responses of Bactrocera dorsalis (Diptera: Tephritidae) to volatile emissions of fruits from three hosts. Journal of Biology, Agriculture and Healthcare 3: 53-60. [ Links ]
Knight AL, Basoalto E, Yee W, Hilton R, Kurtzman CP. 2016. Adding yeasts with sugar to increase the number of effective insecticide classes to manage Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) in cherry. Pest Management Science 72: 1482-1490 [ Links ]
Landolt PJ, Adams T, Rogg H. 2012. Trapping spotted wing drosophila, Drosophila suzukii (Matsumura) (Diptera: Drosophilidae), with combinations of vinegar and wine, and acetic acid and ethanol. Journal of Applied Entomology 136: 148-154 [ Links ]
Lawson LEV, Brentu FC, Cornelius EW, Oduro KA, Sedano ME, Vicent A. 2017. Crop loss and control of Pseudocercospora fruit and leaf spot of citrus in Ghana. European Journal of Plant Pathology 147: 167-180. https://doi.org/10.1007/s10658-016-0990-y [ Links ]
Lee JC, Burrack HJ, Barrantes LD, Beers EH, Dreves AJ, Hamby KA, Haviland DR, Isaacs R, Richardson TA, Shearer PW, Stanley CA, Walsh DB, Walton VM, Zalom FG, Bruck DJ. 2012. Evaluation of monitoring traps for Drosophila suzukii (Diptera: Drosophilidae) in North America. Journal of Economic Entomology 105: 1350-1357. [ Links ]
Lee JC, Shearer PW, Barrantes LD, Beers EH, Burrack HJ, Dalton DT, Dreves AJ, Gut LJ, Hamby KA, Haviland DR, Isaacs R, Nielsen AL, Richardson T, Rodriguez-Saona CR, Stanley CA, Walsh DB, Walton VM, Yee WL, Zalom FG, Bruck DJ. 2013. Trap designs for monitoring Drosophila suzukii (Diptera: Drosophilidae). Environmental Entomology 42: 1348-1355. [ Links ]
Li, X, Yang H, Wang T, Wang J, Wei H, 2019. Life history and adult dynamics of Bactrocera dorsalis in the citrus orchard of Nanchang, a subtropical area from China: implications for a control timeline. ScienceAsia, 45(3): 212-220. [ Links ]
Lloyd AC, Hamacek EL, Kopittke RA, Peek T, Wyatt PM, Neale CJ, Eelkema M, Gu H. 2010. Area-wide management of fruit flies (Diptera: Tephritidae) in the Central Burnett district of Queensland, Australia. Crop Protection 29: 462-469. [ Links ]
Manrakhan A, Daneel JH, Beck R, Virgilio M, Meganck K, De Meyer M. 2017. Efficacy of trapping systems for monitoring of Afrotropical fruit flies. Journal of Applied Entomology 141(10): 825-840. https://doi.org/10.4001/003.026.0379 [ Links ]
Mokam DG, Djiéto-Lordon C, Bilong CB, Lumaret JP. 2018. Host susceptibility and pest status of fruit flies (Diptera: Tephritidae) attacking cucurbits in two agroecological zones of Cameroon, Central Africa. African Entomology 26(2): 317-332. https://doi.org/10.4001/003.026.0317 [ Links ]
Nojima S, Linn Jr C, Morris B, Zhang A, Roelofs W. 2003. Identification of host fruit volatiles from Hawthorn (Crataegus spp.) attractive to Hawthorn-origin Rhagoletis pomonella flies. Journal of Chemical Ecology 29: 321-336. [ Links ]
Ofosu-Budu KG, Monney EO, Quaye E, Amankwah A, Mintah P, Mpere-Asare C, Agboka M. 2007. Citrus production in Ghana. Horticulture Export Industry Initiatve (HEII), Ministry of Food and Agriculture, Accra, Ghana. 108 pp.
Perea-Castellanos C, Pérez-Staples D, Liedo P, Díaz-Fleischer F. 2015. Escape of Mexican fruit flies from traps baited with CeraTrap and effect of lure feeding on reproduction and survival. Journal of Economic Entomology 108: 1720-1727. [ Links ]
Pinero JC, Souder SK, Smith TR, Fox AJ, Vargas RI. 2015. Ammonium acetate enhances the attractiveness of a variety of protein-based baits to female Ceratitis capitata (Diptera: Tephritidae). Journal of Economic Entomology 108: 694-700. [ Links ]
Robacker DC, Heath RR, 1996. Attraction of Mexican fruit flies (Diptera: Tephritidae) to lures emitting host-fruit volatiles in a citrus orchard. Florida Entomologist 79: 600-602. [ Links ]
Shet VB, Belur PD. 2015. Production of concoction wine using palm sap and raisin through batch fermentation. Journal of Microbiology, Biotechnology and Food Sciences 5: 293-296. https://doi.org/10.15414/jmbfs.2015/16.5.3.293-296 [ Links ]
Siderhurst MS, Jang EB. 2010. Cucumber volatile blend attractive to female melon fly, Bactrocera cucurbitae (Coquillett). Journal of Chemical Ecology 36: 699-708. https://doi.org/10.1007/s10886-010-9804-4 [ Links ]
Stewart CD, Braman SK, Sparks BL, Williams-Woodward JL, Wade GL, Latimer JG. 2002. Comparing an IPM Pilot Program to a Traditional Cover Spray Program in Commercial Landscapes, Journal of Economic Entomology 95(4): 789-796. [ Links ]
Stringini M, Comitini F, Taccari M, Ciani M. 2009. Yeast diversity during tapping and fermentation of palm wine from Cameroon. Food Microbiology 26: 415-420. [ Links ]
Suszkiw J. 2014. Pest's taste for fine wine may prove its undoing. In: Sowers R, Kendall S. (Eds). Agricultural Research. AgResearch Magazine 62, 9. https://agresearchmag.ars.usda.gov/AR/archive/2014/Oct/October2014.pdf. [Accessed 20 October 2021].
Thomas MC, Heppner JB, Woodruff RE, Weems HV, Steck GJ, Fasulo TR. 2019. Mediterranean fruit fly, Ceratitis capitata (Wiedemann) (Insecta: Diptera: Tephritidae). Institute of Food and Agricultural Sciences Extension. University of Florida. http://edis.ifas.ufl.edu/pdffiles/IN/IN37100.pdf (Accessed 13 July 2022).
Ukwuru MU, Awah JI. 2013. Properties of palm wine yeasts and its performance in wine making. African Journal of. Biotechnology 12: 2670-2677. [ Links ]
Uzochukwu SVA, Balogh E, Tucknott O, Lewis MJ, Ngoddy PO. 1997. Volatiles of palm wine using solvent extracts. Journal of Food Quality 20: 483-494. https://doi.org/10.1111/j.1745-4557.1997.tb00489.x [ Links ]
Vayssieres J-F, Adandonon A, Sinzogan A, Korie S. 2010. Diversity of fruit fly species (Diptera: Tephritidae) associated with citrus crops (Rutaceae) in southern Benin in 2008-2009. International Journal of Biological Sciences 4: 1881-1897. [ Links ]
Walsh DB, Bolda MP, Goodhue RE, Dreves AJ, Lee J, Bruck DJ, Walton VM, O'Neal SD, Zalom FG. 2011. Drosophila suzukii (Diptera: Drosophilidae): Invasive pest of ripening soft fruit expanding its geographic range and damage potential. Journal of Integrated Pest Management 2: 1-7. [ Links ]
Yazid JB, Chafik Z, Imane BIBI, Bousamid A, Kharmach EZ. 2020. Key fruit flies species (Diptera, Tephritidae) reported in Africa and presenting a biosecurity concern in Morocco: An Overview. Moroccan Journal of Agricultural Sciences. 1(4). [ Links ]
Zehnder GW, Sikora EJ, Goodman WR. 1995. Treatment decisions based on egg scouting for tomato fruitworm, Helicoverpa zea (Boddie), reduce insecticide use in tomato. Crop Protection 14(8): 683-687. [ Links ]
Zida I, Nacro S, Dabiré R, Somda I. 2020. Seasonal abundance and diversity of fruit flies (Diptera: Tephritidae) in three types of plant formations in Western Burkina Faso, West Africa. Annals of the Entomological Society of America. https://doi.org/10.1093/aesa/saaa004
Zhou X-W, Niu C-Y, Han P, Desneux N. 2012. Field evaluation of attractive lures for the fruit fly Bactrocera minax (Diptera: Tephritidae) and their potential use in spot sprays in Hubei Province (China). Journal of Economic Entomology 105(4): 1277-1284 [ Links ]
 Correspondence:
Correspondence:
John Abraham
Email:jabraham@ucc.edu.gh
Received: 24 October 2021
Accepted: 19 September 2022













