Services on Demand
Journal
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Chemistry
On-line version ISSN 1996-840XPrint version ISSN 0379-4350
S.Afr.j.chem. (Online) vol.79 Durban 2025
https://doi.org/10.17159/0379-4350/2025/v79a01
RESEARCH ARTICLE
Unary Adsorption of Phthalates from Wastewater onto Water Hyacinth Biochar: Parameters, Drivers and Mechanism
Elkanah N. OgoraI; Zachary M. GetengaII; Joel M. GichumbiI; Victor O. ShikukuIII,
IDepartment of Physical Sciences, Chuka University, Kenya
IIDepartment of Physical Sciences, Machakos University, Machakos, Kenya
IIIDepartment of Physical Sciences, Kaimosi Friends University, Kaimosi, Kenya
ABSTRACT
In this study, water hyacinth root-derived biochar (WHB) was prepared as a low-cost adsorbent for the removal of three phthalates, namely, benzyl butyl phthalate (BBP), dimethyl phthalate (DMP) and bis(2-ethylhexyl) phthalate (BEHP) from single solute aqueous solutions. The equilibrium data were best described by the adsorption isotherm models in the order Freundlich>Langmuir>Dubinin-Radushkevich-Kaganer (D-R-K) isotherms. The maximum monolayer adsorption capacity (Qo) was 1.83, 1.77, and 1.62 mg/g for DMP, BBP, and BEHP, respectively. The adsorption of the phthalates was diminished by increased molecular weight and molar volume of the molecules but compensated by their hydrophobicity. The kinetic data were best described by the pseudo-second order (PSO) model and pore diffusion was not the sole operative rate-determining step. The calculated thermodynamic functions, changes in Gibb's free energy (ΔG<0), enthalpy (ΔΗ<0), and entropy (ΔS<0) demonstrate the adsorption of DMP, BBP, and BEHP onto WHB is energetically favorable, exothermic, spontaneous and of a physical type controlled by hydrophobic interactions. The comparative adsorption capacities imply that WHB would sequester phthalates regardless of their physicochemical profiles.
Keywords: Phthalates, Water hyacinth biochar, adsorption, physicochemical properties
INTRODUCTION
Endocrine-disrupting chemicals (EDCs) are chemical substances that disturb the functionality of the endocrine system in both animals and humans. They interfere with natural hormone cycles, specifically affecting reproduction, development, metabolism, and growth.1 Most endocrine-disrupting chemicals are man-made.2 Unfortunately, these EDCs have been reported in groundwater, oceans, lakes, marine, and food products, posing a potential risk to both aquatic and terrestrial life forms.3,4 They have negative health effects on male and female reproductive systems (natural estrogen and androgens), thyroid and breast development, and cause birth defects and obesity.5 Examples of EDCs reported in water resources include persistent organic pollutants (POPs), pesticides, pharmaceuticals and personal care products (PPCPs), and per- and polyfluoroalkyl substances (PFAS).6 Complete removal of these contaminants from drinking water is therefore critical. Among the POPs with endocrine disruption potential reported in effluents from wastewater treatment plants (WWTPs) are phthalates.4 This indicates that WWTPs are point sources of phthalates loading into recipient water bodies, a testament that these traditional WWTPs are not designed to sequester phthalates from water. Alternative approaches are required. Phthalates are potentially carcinogenic, have been linked to infertility and even birth defects are some of the harmful effects.7 Approaches for the removal of water contaminants include adsorption, membrane filtration, ion exchange treatment, advanced oxidation process (AOPs), precipitation, and solvent extraction.8 Unfortunately, some of these techniques, such as AOPs, require intensive capital investment thus making them unsustainable, especially in rural areas in low-income countries.9 Some of the materials reported as possible adsorbents for the removal of phthalates include activated carbons,10 polymer resins,11 carbon nanotubes,12 chitosan13 and seaweed biosorbent14 among others. Adsorption onto biomass waste-derived adsorbents has been demonstrated as an alternative and sustainable method for the removal of organic micropollutants from water due to its eco-friendliness, efficiency, cost-effectiveness, simplicity, and availability of these feedstocks even in remote places.15 The aforementioned studies show that the adsorption characteristics of biomass-based adsorbents depend on the type of feedstock and the adsorbent preparation conditions. Furthermore, the adsorption kinetics and adsorption capacity are also a function of the molecular properties such as molecular weight, kinetic diameter, solubility, and functional group density of the phthalates.16 For a given adsorbent, it is important to evaluate the interplay of these factors to optimize the performance.
Water hyacinth (WH), an aquatic weed prevalent on the Kenyan side of the Lake Victoria basin, presents a suitable candidate for biomass-based adsorbent development with concomitant environmental benefits. While water hyacinth-derived biochar (WHB) has been reported as a suitable material for the removal of antibiotics from water,17 heavy metals,18 pesticides,19 and industrial chemicals,20 to date, there has been no study of the adsorption characteristics (rates and capacity) of WHB for the removal of phthalates as influenced by both environmental conditions and the physicochemical properties of the phthalates. For WHB to be considered a next-generation adsorber, its ability to adsorb a broad spectrum of pollutants and the associated drivers must be evaluated.
The objective of this study was to symmetrically evaluate the adsorption of three phthalates, namely, dimethyl phthalate (DMP), benzyl butyl phthalate (BBP), and Bis(2-ethylhexyl) phthalate (BEHP), onto WHB. The compounds were selected as probe molecules for their recalcitrance to conventional WWTPs techniques and occurrence in treated effluents21 and for their varied chemical structures and molecular properties as shown in Table 122 that influence their environmental partitioning.
MATERIALS AND METHODS
Chemicals, reagents and apparatus
The standards (99.9% purity) of DMP, BBP, and BEHP, analytical grade methanol, de-ionized water, glass wool filter papers, and 0.45μm glass micro filters were purchased from Kobian Scientific Ltd, Kenya.
Adsorbent preparation
The water hyacinth (Eichhornia crassipes) was collected from Lake Victoria, in Kisumu City (0°5'30.1" S, 34°46'4.8" E), Kenya. The roots were cut into pieces and washed with distilled water to remove all the dirt and air dried. Biochar preparation was done through a slow-pyrolysis temperature of 350 °C, at a heating rate of 10 °C/minute for 1 hour using a furnace. The sample was then washed using distilled water. It was oven-dried at the temperature of 1000C for a period of 2 hours.23 The resulting sample (WHB) was sieved through a 212 μm sieve and stored for adsorption experiments.
Adsorbent characterization
Elemental analysis of WHB was carried out using an XRF (Brucker, S1 TITAN, Germany). The surface functional groups in the WHB were inspected using FTIR (IRAffinity-1S, Shimadzu) between 4000 and 400 cm-1 wavenumbers. Crystallinity and mineral phases were determined by XRD.
Effect of contact time
A mass of 0.1 g ofWHB was placed in a 250 mL conical flask containing 50 mL of a 10 mg/L phthalate solution and agitated at 125 rpm using an orbital shaker at room temperature.. At regular time intervals of 5 minutes (5, 10, 15, 20, 25, 30, 35, 40, and 50 min), the residual concentrations of phthalate in the solution were determined by HPLC (Thermos Scientific Dionex UltiMateTM 3000 HPLC system). The mobile phase consisted of methanol and water in a 80:20 v/v ratio, operating in isocratic mode. A, a C18 reverse phase HPLC column maintained at 35 °C and, a mobile phase flow rate of 10 μL/min was used, and column temperature maintained at 35 °C. The amounts of phthalate (mg/g) adsorbed onto WHB per unit mass (qe) at any given time (t) were determined as:

where C0 and Ce are the initial and equilibrium phthalate concentrations (mg/L), respectively. m is the mass (g) of the WHB used, and V is the volume of the solution (L). The experimental data obtained were fitted to three kinetic models:
Pseudo-first-order (PFO):24

Pseudo-second-order (PSO):25

Intra-particle diffusion (IPD) model:26

where t (minutes) and qt (mg/g) are the time and amount adsorbed at equilibrium time, respectively, while qeis the equilibrium adsorption capacity. Also, k, k2, and kpare rate constants for PFO, PSO, and IPD models, respectively. The intercept C is related to the mass transfer across the boundary.
Effect of initial concentration
Masses of 0.1 g of WHB were separately, put into 50 ml of phthalate solutions with varying concentrations ranging between 4 to 12 mg/L (4, 6, 8, 10, and 12 mg/L) done at 298 K and then agitated at 125 rpm for 25 min. The residual phthalate in the solution was then determined.
The experimental data were then fitted to linearized Langmuir, Freundlich isotherm and Dubinin-Radushkevich-Kaganer (D-R-K) isotherm models,27-30 shown in Table 2. In Table 2, qe (mg/g) and Ce (mg/L) are the solute concentration on the adsorbent and in the bulk solution at equilibrium, respectively. Qo (mg/g) is the maximum monolayer adsorption capacity, while qs and ε are theoretical isotherm saturation capacity (mg/g) and Polanyi potential, respectively. RL is the dimensionless Langmuir separation constant. The KL (L/g), Kf and Kads (mol2/kJ2) are Langmuir, Freundlich, and D-R-K isotherm constants, respectively. The exponential factor IMAGEMAQUI is related to the adsorption affinity and surface heterogeneity.31 The constants R and T represent the universal gas constant (8.314 J/K.mol) and temperature (K), respectively.
Effect of temperature
The effect of temperature change on the adsorption of selected phthalates onto WHB was studied in the range of 298-338 K. A mass of 0.1 g of WHB was put into 50 mL of 10 mg/L solutions of each phthalate compound and the solutions in triplicates agitated at different temperatures (298, 308, 318, 328 and 338 K) until equilibration. The thermodynamic parameters, AG, AH and AS, were estimated using the equations below:

where Kc is the equilibrium constant (dimensionless), Ce is the equilibrium concentration in the solution (mg/L) and Cads is the equilibrium solid phase concentration (mg/g). Kd is the distribution coefficient (L/g), R is the gas constant (8.314 Jmol-1K-1) and T is the temperature in Kelvin.
The data was further modeled using three classical adsorption isotherms, namely Langmuir, Freundlich and Dubinin-Radushkevich-Kaganer (D-R-K) isotherms. From the D-R-K isotherm, the mean adsorption energy Ea (kJ/mol), that provides insight on physisorption, and chemisorption mechanisms was calculated using:

Effect of adsorbent dosage
To 50 mL of 10 mg/L of the phthalate solutions, different weighed amounts of adsorbent (0.2, 0.4, 0.6, 0.8, 1.0, and 1.2 g) were dispersed at 298 K and agitated at 125 rpm until equilibration. All the other parameters were kept constant. The percentage of phthalate removed was calculated using:

RESULTS AND DISCUSSION
Elemental composition of WHB
The elemental percent composition of the WHB obtained by XRF analysis is presented in Table 3. The absence of toxic heavy metals indicates that the biochar is unlikely to cause secondary pollution during application through the leaching of toxic heavy metals into the aqueous solution.
Functional group analysis
The functional groups present and the variation in their vibrational frequencies after adsorption were inspected using FTIR spectroscopy. The IR spectra as shown are shown in the Figures 1 and 2.
The band 3464.28 cm-1, before adsorption, and 3506.15 cm-1, after adsorption represented the stretching vibrations of the OH group. This group was due to the water adsorbed on the biochar.32 The band centered at 2925.76 cm-1 observed after adsorption was assigned to C-H stretching aliphatic functional groups from the phthalates and provides evidence for adsorption.33 The bands between 1636.92 cm-1 and 1635.65 cm-1 for WHB before adsorption and after adsorption respectively, were found to be stretching vibration of OH deformation of water, and aromatic C=O stretching vibration of the carbonyl from the carboxyl group, respectively.33
The bands at 1401.13 cm-1 and 1461.51 cm-1 represent the COO-groups and C-H deformation vibrations, respectively. The band at 1384.91 cm-1 is attributed to C-H in-plane bending vibrations. The broad bands at 1034.13 cm-1 and 1033.02 cm-1 before and after adsorption, respectively, may be attributed to the C-O bending vibration or the band of the out-of-plane bending for carbonates (CO32-) or P-O bond of phosphate in the biochar. The minor shifts in the absorption bands indicate changes in the chemical environments of these functional groups and demonstrate weak interactions between the phthalates and the biochar functional groups. This provides circumstantial evidence for a physisorption mechanism.
Effect of contact time
The effect of contact time is essential in determination of the residence time of a water treatment process. In this work, equilibration was attained in 25 minutes beyond which there was no appreciable change in the amount adsorbed (mg/g) (Figure 3). The percent removal (%R) at equilibrium were 76.59%, 75.98% and 75.49% for DMP, BBP and BEHP, respectively.
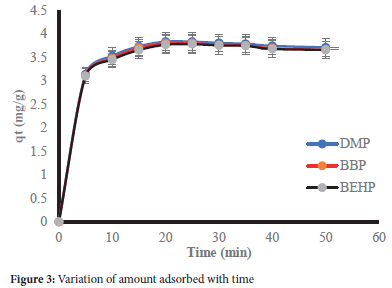
The data was further fitted to the quasi-first order (PFO), quasi-second order (PSO) and intra-particle diffusion (IPD) kinetic models to gain insight on adsorption rates and mechanisms involved. The kinetic parameters are summarized in Table 4.
From Table 4, the low coefficient of determination (R2) values and the wide variance between the experimental equilibrium adsorption capacity (qe(exp)) and the model-predicted values (qe(cal)) reveal that the PFO poorly fits the experimental data. The adsorption of phthalates onto WHB is therefore not a PFO reaction. On the other hand, the close agreement between the PSO-predicted values and the experimental values with R2 values closest to unity (Table 4) shows that the PSO kinetic model best described the adsorption data. The PSO model infers a chemisorption-mediated adsorption process in the rate-determining step. The adsorption rates, denoted by were independent of the molecular weight of the phthalates. This implies an intricate interplay between hydrophobicity, kinetic diameters, functional groups, and accessibility to active binding sites.
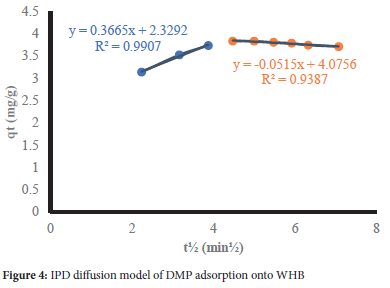
Figure 4 depicts the IPD model plot for DMP. The multi-linear plots show that several mechanisms are involved in the adsorption process with significant boundary layer effects.17 The C values are an index of the mass transfer across the boundary layer. The greater the magnitude of C, the greater the boundary layer effect.17 From Table 4, the non-zero interception implies that pore diffusion is not the sole operative adsorption mechanism. The interceptions obtained from all the adsorption cases are attributed to a wide range of pore sizes of the WHB.34
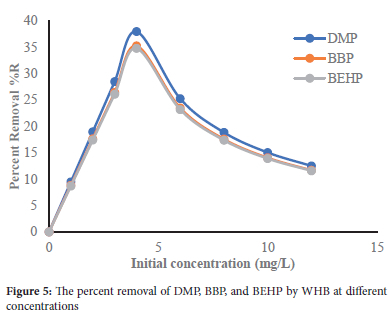
Adsorption isotherms
When the initial concentration was increased from 1 to 4 mg/L, the percent removal (%R) increased to a maximum of 37.99%, 35.28%, and 34.81% for DMP, BBP, and BEHP, respectively (Figure 5). This is attributable to the large number of vacant active sites and increased mass gradient between the bulk solution and the solid phase. However, beyond 4 mg/L the percent removal consistently decreased is beyond 4 mg/L. This decrease is due to the saturation of the limited number of active and energetically favorable adsorption sites of the WHBWBH and increased repulsion between the adsorbed phthalate molecules and those in the bulk solution.35
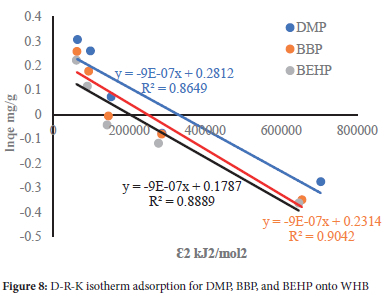
The data was further modeled using three classical adsorption isotherms, namely Langmuir, Freundlich and Dubinin-Radushkevich-Kaganer (D-R-K) isotherms, and their plots are shown in Figures 6, 7 and 8, respectively. The calculated constants are presented in Table 5.
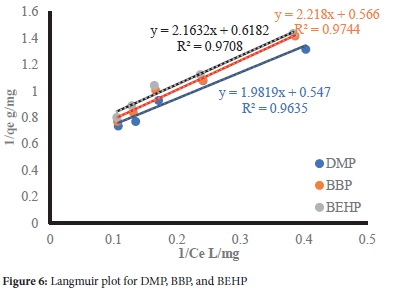
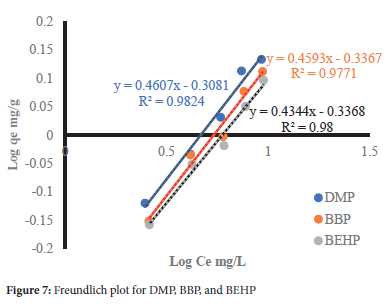
From the Langmuir isotherm, the maximum adsorption capacity (Qo) decreased in the order DMP>BBP>BEHP (Table 5) with increasing molecular weight and LeBas molar volume (Table 1). This shows that the adsorption capacity was controlled by the molecular weight and kinetic diameters of the phthalates. Furthermore, the adsorption capacities are comparable despite the wide variances in molecular weights and Lebas molar volume. This is due to the compensation effect of increasing hydrophobicity (log Kow) (Table 1), an indication that hydrophobic interactions were significant as the driving adsorption mechanism. This is further supported by the Freundlich affinity factors Kpthat were comparable across the phthalates. The 1/n values below unity denote a heterogeneous WHB surface with energetically different binding sites. Additionally, the low 1/n values signify weak adsorbate-adsorbent interactions consistent with the physisorption mechanisms from the FTIR study.36 The RL values (0<RL<1) show that the adsorption of the selected phthalates onto WHB was deemed favorable.37
The D-R isotherm did not show good linear regression with the adsorption of the selected phthalates, relative to the other models. Its low mean free energy (E = 0.01 kJ/mol) suggests a physisorption adsorption process.38
Thermodynamic Studies
The effect of temperature on phthalates adsorption onto WHB was examined in the 298-338 K temperature range. The percent removal decreased with increasing temperature, a characteristic of exothermic process. The maximum %R at 298 K was 73.03%, 72.24% and 71.46% for DMP, BBP, and BEHP, respectively (Figure 9). The fact that maximum percent removal was recorded at room temperature is significant for practical application since temperature adjustment is not required.
The decrease in %R with the temperature rise is probably due to increased solubility with an increase in temperature. The calculated thermodynamic functions are shown in Table 6.
The negative ΔΗ values (Table 8) confirm that the adsorption of the phthalates onto WHB is an exothermic reaction. According to Jemutai-Kimosop et al.,39 ΔΗ values below 40 kJ/mol denote a physisorption mechanism. In this work, all the enthalpy values were below 40 kJ/mol signifying that the adsorption of DMP, BBP, and BEHP onto WHB entails a physisorption process. This is further supported by the observations from the Freundlich and D-R-K isotherms and FTIR study. The negative ΔS values imply increased orderliness at the solid-liquid interface.
The negative ΔG values (Table 6) denote that the adsorption of the phthalates onto WHB was thermodynamically spontaneous. In general, ΔΟ values in the range -20 kJ/mol < ΔG < 0 kJ/mol imply physisorption whereas a chemisorption mechanism is implied for -400 kJ/mol < ΔG < -80 kJ/mol.40 The magnitudes of ΔΟ values (Table 6) correspond to a physical adsorption mechanism. This corroborates the earlier deductions. The thermodynamics data reveal that the adsorption of DMP, BBP, and BEHP by the WHB is an enthalpy-driven process
Effect of the Adsorbent Dosage
The effect of adsorbent dosage on adsorbent performance was studied in the range of 0.2g-1.2g of adsorbent in a 50 mL solution (Figure 10). The percent removal of all the phthalates increased when the adsorbent dosage increased from 0.2 to 0.8 g. This is attributed to the increase in the number of adsorption sites. The maximum %R at a WHB dosage of 0.8 g/50 mL was 73.07%, 68.97% and 67.87% for DMP, BBP and BEHP, respectively. However, beyond 0.8g/50mL dosage, there was no appreciable change in percent phthalate removal. This is due to agglomeration of the adsorption sites with an increase in the adsorbent particles.
Comparison with other adsorbents
The adsorption capacity of the water hyacinth roots derived biochar (WHB) was compared to other adsorbents reported in literature (Table 7). The differences are attributed to both precursor properties, synthesis conditions, identity of phthalate and experimental conditions. The suitability of the adsorbents should be evaluated also based on overall cost of synthesis, scalability, environmental impact and sustainability, especially for emerging economies.
CONCLUSION
In this work, water hyacinth biochar (WHB) was used as a low-cost, eco-conscious adsorbent for the removal of three phthalates, DMP, BBP, and BEHP, from synthetic wastewater in single solute solutions. The maximum monolayer adsorption capacity was 1.83, 1.77, and 1.62 mg/g for DMP, BBP and BEHP, respectively. The adsorption of the phthalates was inhibited by the molecular weight and kinetic diameters/bulkiness of the molecules but compensated by their hydrophobicity. The kinetic data was best predicted by the quasi-second order model (PSO) with an equilibrium time of 25 min. The equilibrium adsorption data was predicted by adsorption isotherm models in the order Freundlich>Langmuir>D-R-K. The thermodynamics functions showed that the adsorption of the phthalates onto WHB was spontaneous, exothermic, physical, enthalpically-driven, and energetically favorable. Therefore, WHB is a potential next-generation low-cost adsorbent for the removal of DMP, BBP, and BEHP from water.
STATEMENT OF COMPETING INTEREST
The authors declare no competing interest
DECLARATION OF GENERATIVE AI AND AI-ASSISTED TECHNOLOGIES
During the preparation of this work, the author(s) used Grammarly to check and proofread grammatical mistakes and punctuation. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
ACKNOWLEDGMENTS
We gratefully acknowledge the financial support from the National Research Fund (NRF - Kenya)
ORCID IDS
Elkanah N. Ogora: https://orcid.org/0000-0003-3562-6372
Zachary M. Getenga: https://orcid.org/0000-0003-0570-7403
Victor O. Shikuku: https://orcid.org/0000-0002-2295-293X
REFERENCES
1. Jackson J, Sutton R. Sources of Endocrine-Disrupting Chemicals in Urban Wastewater, Oakland, CA. Sci Total Environ. 2008;405(1-3):153-160. https://doi.org/10.1016/j.scitotenv.2008.06.033. [ Links ]
2. Iñigo-Nuñez S, Herreros MA, Encinas T, Gonzalez-Bulnes A. Estimated Daily Intake of Pesticides and Xenoestrogenic Exposure by Fruit Consumption in the Female Population from a Mediterranean Country (Spain). Food Control. 2010;21(4):471-477. https://doi.org/10.1016/j.foodcont.2009.07.009. [ Links ]
3. Annamalai J, Namasivayam V. Endocrine Disrupting Chemicals in the Atmosphere: Their Effects on Humans and Wildlife. Environ Int. 2015;76:78-97. https://doi.org/10.1016/j.envint.2014.12.006. [ Links ]
4. Ngeno E, Ongulu R, Orata F, Matovu H, Shikuku V, Onchiri R, Mayaka A, Majanga E, Getenga Z, Gichumbi J, et al. Endocrine Disrupting Chemicals in Wastewater Treatment Plants in Kenya, East Africa: Concentrations, Removal Efficiency, Mass Loading Rates and Ecological Impacts. Environ Res. 2023;237:117076. https://doi.org/10.1016/j.envres.2023.117076. [ Links ]
5. Diamanti-Kandarakis E, Bourguignon J-P, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement. Endocr Rev. 2009;30(4):293-342. https://doi.org/10.1210/er.2009-0002. [ Links ]
6. Shikuku VO, Ngeno EC, Njewa JB, Ssebugere P. Pharmaceutical and Personal Care Products (PPCPs) and per- and Polyfluoroalkyl Substances (PFAS) in East African Water Resources: Progress, Challenges, and Future. Basic Sci. Sustain. Dev. 2023;2:21-38. https://doi.org/10.1515/9783111071206-002. [ Links ]
7. Casajuana N, Lacorte S. Presence and Release of Phthalic Esters and Other Endocrine Disrupting Compounds in Drinking Water. Chromatographia. 2003;57(9-10):649-655. https://doi.org/10.1007/BF02491744. [ Links ]
8. Esfandian H, Yousefi E, Sharifzadeh BM. Removal of Dimethyl Phthalate from Aqueous Solution by Synthetic Modified Nano Zeolite Using Cu2O Nanoparticles. Int. J. Eng. Trans. A Basics. 2016,29(9):1198-1207. https://doi.org/10.5829/idosi.ije.2016.29.09c.03. [ Links ]
9. Tapia-Orozco N, Ibarra-Cabrera R, Tecante A, Gimeno M, Parra R, Garcia-Arrazola R. Removal Strategies for Endocrine Disrupting Chemicals Using Cellulose-Based Materials as Adsorbents: A Review. J Environ Chem Eng. 2016;4(3):3122-3142. https://doi.org/10.1016/j.jece.2016.06.025. [ Links ]
10. Fang ZQ, Huang HJ. Adsorption of Di-n-Butyl Phthalate onto Nutshell-Based Activated Carbon. Equilibrium, Kinetics and Thermodynamics. Adsorpt Sci Technol. 2009;27(7):685-700. https://doi.org/10.1260/0263-6174.27.7.685. [ Links ]
11. Xu Z, Zhang W, Pan B, Lv L, Jiang Z. Treatment of Aqueous Diethyl Phthalate by Adsorption Using a Functional Polymer Resin. Environ Technol. 2011;32(2):145-153. https://doi.org/10.1080/09593330.2010.490854. [ Links ]
12. Den W, Liu H-C, Chan S-F, Kin KT, Huang C. Adsorption of Phthalate Esters with Multiwalled Carbon Nanotubes and Its Application. Adsorption of phthalate esters with multiwalled carbon nanotubes and its applications. J Environ Econ Manage. 2006;16(4):275-282. [ Links ]
13. Chen C, Chen C, Chung Y. Removal of phthalate esters by α-cyclodextrin-linked chitosan bead. Bioresour Technol. 2007;98(13):2578-2583. https://doi.org/10.1016/j.biortech.2006.09.009. [ Links ]
14. Chan HW, Lau TC, Ang PO, Wu M, Wong PK. Biosorption of Di(2-Ethylhexyl)Phthalate by Seaweed Biomass. J Appl Phycol. 2004;16(4):263-274. https://doi.org/10.1023/B:JAPH.0000047778.93467.af. [ Links ]
15. Ngeno EC, Mbuci KE, Necibi MC, Shikuku VO, Olisah C, Ongulu R, Matovu H, Ssebugere P, Abushaban A, Sillanpâá M. Sustainable Re-Utilization of Waste Materials as Adsorbents for Water and Wastewater Treatment in Africa: Recent Studies, Research Gaps, and Way Forward for Emerging Economies. Environ Adv. 2022;9(August):100282. https://doi.org/10.1016/j.envadv.2022.100282. [ Links ]
16. Wang H, Hua H, Zhang L, Wen S. On the Resistance-Harary Index of Graphs Given Cut Edges. J Chem. 2017;2017:1-7. https://doi.org/10.1155/2017/3531746. [ Links ]
17. Orata F, Ngeno EC, Baraza LD, Shikuku VO, Kimosop SJ. Adsorption of Caffeine and Ciprofloxacin onto Pyrolitically Derived Water Hyacinth Biochar: Isothermal, Kinetic and Thermodynamic Studies. J Chem Chem Eng. 2016;10(4). https://doi.org/10.17265/1934-7375/2016.04.006. [ Links ]
18. Hashem MA, Hasan M, Momen MA, Payel S, Nur-A-Tomal MS. Water Hyacinth Biochar for Trivalent Chromium Adsorption from Tannery Wastewater. Environ. Sustain. Indic. 2020;5:100022. https://doi.org/10.1016/j.indic.2020.100022. [ Links ]
19. Liu Y, Gao Z, Ji X, Wang Y, Zhang Y, Sun H, Li W, Wang L, Duan J. Efficient Adsorption of Tebuconazole in Aqueous Solution by Calcium Modified Water Hyacinth-Based Biochar: Adsorption Kinetics, Mechanism, and Feasibility. Molecules. 2023;28(8):3478. https://doi.org/10.3390/molecules28083478. [ Links ]
20. Bian P, Shao Q. Performance and Mechanism of Functionalized Water Hyacinth Biochar for Adsorption and Removal of Benzotriazole and Lead in Water. Int J Mol Sci. 2023;24(10):8936. https://doi.org/10.3390/ijms24108936. [ Links ]
21. Onchiri R, Mayaka A, Majanga A, Ongulu R, Orata F, Getenga Z, Gichumbi J, Ogora E. Phthalate Levels in Wastewater Treatment Plants of Lake Victoria Basin. Appl. Ecol. Environ. Sci. 2025;9(12):1011-1017. https://doi.org/10.12691/aees-9-12-4. [ Links ]
22. Cousins IT, Mackay D, Parkerton TF. Physical-Chemical Properties and Evaluative Fate Modelling of Phthalate Esters. Berlin Heidelberg: Springer-Verlag; 2003. pp. 57-84. https://doi.org/10.1007/b11463. [ Links ]
23. Shikuku VO, Jemutai-Kimosop S. Efficient Removal of Sulfamethoxazole onto Sugarcane Bagasse-Derived Biochar: Two and Three-Parameter Isotherms, Kinetics and Thermodynamics. S Afr J Chem. 2020;73(1):111-119. https://doi.org/10.17159/0379-4350/2020/v73a16. [ Links ]
24. Ho YS, McKay G. Sorption of Dye from Aqueous Solution by Peat. Chem Eng J. 1998;70(2):115-124. https://doi.org/10.1016/S0923-0467(98)00076-1. [ Links ]
25. Ho Y, Ofomaja A. Pseudo-second-order model for lead ion sorption from aqueous solutions onto palm kernel fiber. J Hazard Mater. 2006;129(1-3):137-142. https://doi.org/10.1016/j.jhazmat.2005.08.020. [ Links ]
26. Weber WJ Jr, Rumer RR Jr. Intraparticle Transport of Sulfonated Alkylbenzenes in a Porous Solid: Diffusion with Nonlinear Adsorption. Water Resour Res. 1965;1(3):361-373. https://doi.org/10.1029/WR001i003p00361. [ Links ]
27. Langmuir I. THE ADSORPTION OF GASES ON PLANE SURFACES OF GLASS, MICA AND PLATINUM. J Am Chem Soc. 1918;40(9):1361-1403. https://doi.org/10.1021/ja02242a004. [ Links ]
28. Hall KR, Eagleton LC, Acrivos A, Vermeulen T. Pore- and Solid-Diffusion Kinetics in Fixed-Bed Adsorption under Constant-Pattern Conditions. Ind Eng Chem Fundam. 1966;5(2):212-223. https://doi.org/10.1021/i160018a011. [ Links ]
29. Freundlich H. Über Die Adsorption in Lösungen. Z Phys Chem. 1907;57U(1):385-470. https://doi.org/10.1515/zpch-1907-5723. [ Links ]
30. Cerofolini GF. A Model Which Allows for the Freundlich and the Dubinin-Radushkevich Adsorption Isotherms. Surf Sci. 1975;51(1):333-335. https://doi.org/10.1016/0039-6028(75)90260-5. [ Links ]
31. Galhetas M, Mestre AS, Pinto ML, Gulyurtlu I, Lopes H, Carvalho AP. Carbon-Based Materials Prepared from Pine Gasification Residues for Acetaminophen Adsorption. Chem Eng J. 2014;240:344-351. https://doi.org/10.1016/j.cej.2013.11.067. [ Links ]
32. Mboka JM, Tamaguelon HD, Shikuku V, Tome S, Deugueu VF, Othman H, Janiak C, Dika MM, Etoh MA, Dina DJD. Novel Superadsorbent from Pozzolan-Charcoal Based Geopolymer Composite for the Efficient Removal of Aqueous Crystal Violet. Water Air Soil Pollut. 2024;235(7):430. https://doi.org/10.1007/s11270-024-07257-4. [ Links ]
33. Ngeno E, Ongulu R, Shikuku V, Ssentongo D, Otieno B, Ssebugere P, Orata F. Response Surface Methodology Directed Modeling of the Biosorption of Progesterone onto Acid Activated Moringa Oleifera Seed Biomass: parameters and Mechanisms. Chemosphere. 2024;360:142457. https://doi.org/10.1016/j.chemosphere.2024.142457. [ Links ]
34. Xu Y, Liu Y, Liu S, Tan X, Zeng G, Zeng W, Ding Y, Cao W, Zheng B. Enhanced Adsorption of Methylene Blue by Citric Acid Modification of Biochar Derived from Water Hyacinth (Eichornia Crassipes). Environ Sci Pollut Res Int. 2016;23(23):23606-23618. https://doi.org/10.1007/s11356-016-7572-6. [ Links ]
35. Jedynak K, Wide! D, Oszczudlowski J. Removal of Selected Phthalates from Aqueous Solution by Mesoporous-Ordered Carbon Adsorbent. Adsorpt Sci Technol. 2017;35(7-8):744-750. https://doi.org/10.1177/0263617417708675. [ Links ]
36. Achieng G, Shikuku V. Adsorption of Copper Ions from Water onto Fish Scales Derived Biochar: isothermal Perspectives. J Mater Environ Sci. 2020;2020(11):1816-1827. [ Links ]
37. Krupadam RJ, Sridevi P, Sakunthala S. Removal of Endocrine Disrupting Chemicals from Contaminated Industrial Groundwater Using Chitin as a Biosorbent. J Chem Technol Biotechnol. 2011;86(3):367-374. https://doi.org/10.1002/jctb.2525. [ Links ]
38. Dada AO, Olalekan AP, Olatunya AM, Dada O. Langmuir, Freundlich, Temkin and Dubinin-Radushkevich Isotherms Studies of Equilibrium Sorption of Zn2+ Unto Phosphoric Acid Modified Rice Husk. lOSR J Appl Chem. 2012;3(1):38-45. https://doi.org/10.9790/5736-0313845. [ Links ]
39. Jemutai-Kimosop S, Okello VA, Shikuku VO, Orata F, Getenga ZM. Synthesis of Mesoporous Akaganeite Functionalized Maize Cob Biochar for Adsorptive Abatement of Carbamazepine: Kinetics, Isotherms, and Thermodynamics. Clean Mater. 2022;5(June):100104. https://doi.org/10.1016/j.clema.2022.100104. [ Links ]
40. Dzoujo HT, Shikuku VO, Tome S, Akiri S, Kengne NM, Abdpour S, Janiak C, Etoh MA, Dina D. Synthesis of Pozzolan and Sugarcane Bagasse Derived Geopolymer-Biochar Composites for Methylene Blue Sequestration from Aqueous Medium. J Environ Manage. 2022;318(March):115533. https://doi.org/10.1016/j.jenvman.2022.115533. [ Links ]
41. Abdoul ASI, Islam MS, Chen Y, Weng L, Sun Y, Chang X, Zhou B, Ma J, Li Y. Competitive adsorption of Dibutyl phthalate (DBP) and Di(2-ethylhexyl) phthalate (DEHP) onto fresh and oxidized corncob biochar. Chemosphere. 2021;280:130639. https://doi.org/10.1016/j.chemosphere.2021.130639. [ Links ]
42. Falahrodbari S. Adsorption of Benzene Butyl Phthalate with Mullti-walled Carbon Nanotubes/Ag nanoparticles and its Applications. Orient J Chem 2017;33(2):330241. http://dx.doi.org/10.13005/ojc/330241. [ Links ]
43. Liu Q, Ye J, Han Y, Wang P, Fei Z, Chen X, Zhang Z, Tang J, Cui M, Qiao X. Defective UiO-67 for enhanced adsorption of dimethyl phthalate and phthalic acid. J. Mol. Liq. 2020;321:114477. https://doi.org/10.1016/j.molliq.2020.114477. [ Links ]
Received 1 October 2024
Revised 11 November 2024
Accepted 13 March 2025
* To whom correspondence should be addressed Email: vshikuku@kafu.ac.ke













