Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
African Vision and Eye Health
On-line version ISSN 2410-1516
Print version ISSN 2413-3183
AVEH vol.82 n.1 Cape Town 2023
http://dx.doi.org/10.4102/aveh.v82i1.780
ORIGINAL RESEARCH
Keratoconic patient profile and management at public sector facilities in South Africa
Pheagane M.W. NkoanaI, II; Vanessa R. MoodleyI; Khathutshelo P. MashigeI
IDiscipline of Optometry, Faculty of Health Sciences, University of KwaZulu-Natal, Durban, South Africa
IIDepartment of Optometry, Faculty of Health Sciences, University of Limpopo, Polokwane, South Africa
ABSTRACT
BACKGROUND: Keratoconus (KC) is a condition marked by thinning and protrusion of the cornea resulting in high myopia and irregular astigmatism. Knowledge of KC patients' profiles and management approaches used can help to predict the needs of public hospitals to improve patient care.
AIM: This study aimed to describe the profiles and management of KC patients.
SETTING: Capricorn district, Limpopo Province, South Africa.
METHODS: Medical records of 188 KC patients attending public hospitals of Capricorn District from January 2017 to December 2020 were reviewed. Data on patient profile and their management were collected and analysed
RESULTS: The mean age of KC patients was 20.64 ± 6.82 years and the majority (56.9%) were males. Clinical findings were mean unaided visual acuity (UVA) of 0.19 ± 0.18, best corrected VA of 0.53 ± 0.24, spherical equivalence of -4.89 ± 9.17 dioptre (D), mean K of 57.37 ± 17 D and corneal astigmatism of -6.24 ± 4.27 D. A total of 54.5% of patients had severe KC. Bilateral KC was found in almost all patients (97.3%) and a mean K difference of 7.59 ± 6.08 D (p < 0.001) between the better and the worse eye. The study found no significant difference in KC severity by age (p = 0.451) and gender (p = 0.819). Patients fitted with scleral lenses had the highest VA improvement of 0.44 ± 0.17
CONCLUSION: Most patients presented with bilateral and severe KC. Scleral lenses provided higher VA improvement than other methods.
CONTRIBUTION: The study aimed to present the clinical profile and management of keratoconic patients attending public sector facilities. Knowledge of the patterns of KC presentation may assist in the development of intervention strategies and guidelines for best practice in the management of KC, especially in public sector facilities.
Keywords: keratoconus, demographic and clinical characteristics; keratoconus management; Capricorn District of Limpopo province; progression of keratoconus.
Introduction
Keratoconus (KC) is a corneal ectasia characterised by progressive thinning and protrusion of the cornea, commonly on the inferior-central aspect, resulting in reduced vision from high myopia and irregular astigmatism.1 The disease usually presents bilaterally although it is asymmetrical and, with progression, the cornea evolves to a conical shape.2,3 It is largely thought to be non-inflammatory,1 although there have been recent suggestions that it could be quasi-inflammatory (inflammatory-related) rather than non-inflammatory.2,3 Risk factors of KC include, among others, age, ethnicity, family history, atopy, eye rubbing, and exposure to sunlight.4 The disease usually presents at puberty and progresses to around the fourth decade of life.5 Although KC was historically thought to be more prevalent in males, two recent global review studies by Hashemi et al.6 and Santodomingo-Rubido et al.7 found no evidence of gender predilection. Keratoconus has been found to be more common in countries with hot and sunny climates and is associated with atopy and eye rubbing.4,6,8 Higher prevalence of KC has been found in Asian and Middle East populations and the authors suggested that this could be because of geographical locations and subsequent exposures to certain environments.4,7,9 However, higher prevalence of KC has also been found in populations of Asian origin residing in other geographical locations such as Europe,4,9 suggesting that ethnicity, rather than geographical location, could be responsible for the higher prevalence in these cases.
Based on the Amsler-Krumeich (AK) grading of KC, signs and symptoms may not appear at early stages of the disease but present as the condition progresses.5,10 Subclinical stages of KC are characterised by a scissor retinoscopy reflex with a clear cornea and no apparent signs.10 Fleischer's ring appears at mild stages of the disease with distorted retinoscopy reflex. Moderate stages present with more pronounced Fleischer's ring and Vogt's striae and corneal thinning.5,10 There is likely cloudy media, more pronounced Vogt's striae and Munson's sign with advanced KC.5,10 The cornea appears scarred at severe stages of the disease5,10 and corneal hydrops appear in advanced and severe cases of KC.5,11
The annual incidence of KC is estimated to be 50-230 cases in every 100 000 persons in the general population.4 Hashemi et al.6 reviewed 29 articles from 15 countries and estimated a global prevalence of KC to be 1.38 in 1000 persons in a population of about 50.4 million people.6 Studies found prevalence of KC to be less in colder environments estimated at 0.2 in 100 000 persons and more in warmer environments estimated at 4790 in 100 000 persons.7 Clinic or hospital-based studies have reported a higher prevalence of KC than population-based studies.7 Godefrooij et al.12 showed a likelihood of KC prevalence and incidence increasing rapidly as compared with earlier studies in the Netherlands. Recent review studies also confirm similar patterns of increasing KC prevalence worldwide.6,7 Authors believe that this is because of improved availability of more sophisticated technology such as corneal topography and ocular coherence tomography (OCT), enabling detection of sub-clinical KC earlier than what was possible before.13,14
Approaches to managing KC focus on restoring the patients' visual acuity to normality using spectacles and soft contact lenses in the early stages of KC,15 rigid gas permeable (RGP) lenses when there is irregular corneal astigmatism (CA)16 and scleral lenses in more advanced cases to maximise vision correction and patient comfort, as scleral lenses vault over the cornea.17 Contact lenses are used to reduce vision distortions.18 Corneal cross linking (CXL) is a procedure that is used to strengthen the stroma and halt the disease progression.16,19 Corneal transplants are necessary in cases of structural damage such as scarring, extreme thinning and their subsequent complications.20 The poor availability of donor corneas, limit the reliance on corneal transplants for management of KC. Although it is an effective option, it is made complex by its associated social, ethical and legal issues.20
Evidence from three South African studies suggests a possible high prevalence of KC in South Africa. Chetty and Rubin21 in a study spanning a 10-year duration (2007-2017), described the clinical characteristics of 206 patients seen at their university clinic. Two studies conducted at another university-based clinic, initially between 2010 and 201422 and thereafter between 2014 and 201723 reveal that the number of patients had almost tripled, from the first study (n = 106) to the second study (n = 293). This pattern of increasing KC is significant because these university clinics generally serve the communities in surrounding areas.21,22,23 There is also the possibility that other patients, seen by private optometrists in the area, may have been diagnosed with KC but not referred to these specific facilities. In addition, the majority (62.6%,22 56.6%23 and 61%21) of patients had severe KC when presenting at the respective facilities, possibly suggesting that mild cases are missed and only detected when they have progressed. Although there is no population-based prevalence study and those conducted were localised to only two cities (Johannesburg and Durban), their trends21,22,23 suggest that there is likely a rise in KC prevalence in South Africa.
This study aimed to describe the demographic and clinical profile of KC patients, in the public hospitals in the Capricorn District of Limpopo province. Patterns of patient management in these facilities were also explored. The district is characterised by hot and sunny conditions,24 which is one of the risk factors for KC.4 This study, therefore, was necessary to describe patterns of KC in an area where conditions pose risks to the development of KC and to further determine how patients are cared for in view of suggesting improvements.
Methods
This quantitative, retrospective, and descriptive study was carried out in the seven public hospitals of the Capricorn district of Limpopo province, South Africa. Files of KC patients attending these public hospitals in the district between 01 January 2017 and December 2020 were selected and reviewed. Patients were examined by either optometrists, ophthalmic nurses or ophthalmologists, depending on the hospital at which they were seen. Central corneal curvatures were measured with either a keratometer, an Oculus 4 corneal topographer or an Oculus Pentacam. All patients' files without corneal curvature measure in at least one eye were excluded from the sample. There were very few files with measures of corneal thickness; hence, the measurement was excluded for analysis.
Data on the demography of the patients including age, gender and race were extracted from the patients' files. Clinical data extracted included signs and symptoms, unaided visual acuity (UVA) and best-corrected visual acuity (BCVA), central corneal curvature (k), refraction and clinical management of KC patients. Visual acuity was converted from the Snellen Acuity notation to a decimal notation to enable statistical analysis.25 Refraction results were also converted into the spherical equivalents (SEs) calculated by adding half the cylindrical power for each dioptre of a cylinder (SE = spherical power + ½ cylindrical power).25
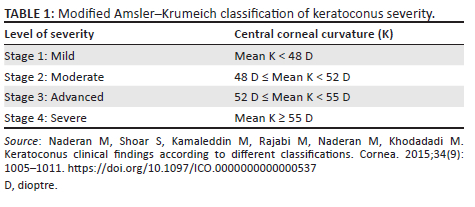
The modified AK grading system, adapted from Abdu et al.26 and Naderan et al.,27 was used to grade KC as the AK grading system is widely used.14,28,29 There are four stages of progression, which are mild, moderate, advanced and severe (Table 1). For comparison of age and gender, the mean K of the worst eye was the feature for comparison.
Data analysis
Data collected was captured on Microsoft Excel 2010 and analysed using Statistical Package for Social Sciences (SPSS) software, version 28.0. The means, standard deviations, and ranges of UVA, BCVA, mean SE, mean K and CA for all eyes were calculated to summarise and present data. Mean K was used for all comparisons on severity. Tests for normality of the distributions were conducted using Kolmogorov-Smirnov and the Shapiro-Wilk tests, which could not confirm normal distributions of data. Similarly, mean-median comparison and histogram curves could not confirm normal distributions of data. Comparisons of variables were then carried out using non-parametric tests. Mann-Whitney-Wilcoxon tests were conducted to confirm symmetry between the better and worse eyes and also to estimate the differences in findings between the UVA and BCVA. Spearman rank correlation was used to check association of variables with age. Kruskal-Wallis test was conducted to estimate the difference between males and females. The confidence level of all comparisons or measures was set at a 95% confidence level and significance level of p = 0.05.
Ethical considerations
Ethical clearance to conduct the study was obtained from the Biomedical Research Ethics Committee of the University of KwaZulu-Natal (UKZN) (BREC/000.01223/2020). Permission to conduct the study was also obtained from the Limpopo Province Department of Health (LP-202005-002).
Results
Demographics
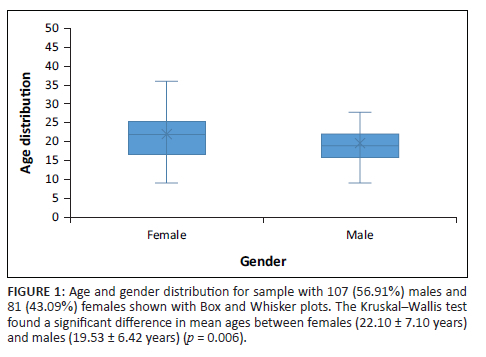
A total of 188 KC patient files were analysed of which 107 (56.9%) were males and 81 (43.1%) were females. The mean age of patients at their first presentation to the facilities was 20.64 ± 6.82 years. The mean age of males was 19.53 ± 6.42 years and 22.10 ± 7.10 years for females. The difference between the ages of males and females was statistically significant (p = 0.006). Figure 1 shows the age distribution between genders. The minimum age of patients was 9 years for both genders and the maximum age was 46 years.
Ethnicity
All patients were black Africans.
Signs and symptoms
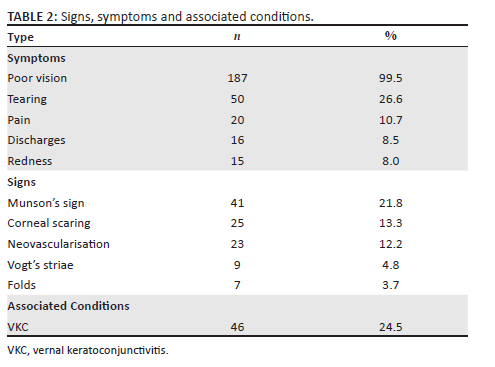
Reduced vision, distorted vision, and poor vision with or without spectacles were the primary reasons for 187 (99.5%) patients to seek consultation (Table 2). Forty (24.5%) patients were either under management for, or had a history of, vernal keratoconjunctivitis (VKC), 50 (26.6%) reported tearing, 20 (10.7%) pain, 16 (8.5%) discharges, and 15 (8.0%) redness. Other findings included Munson's sign recorded in 41 (21.8%), corneal scarring in 25 (13.3%), neovascularisation in 23 (12.2%) and Vogt's striae in 9 (4.8%) patients (Table 2).
General description
Table 3 shows a summary of measurements undertaken from the 376 eyes. The mean UVA was 0.19 ± 0.18, BCVA was 0.53 ± 0.24, mean SE was -4.89 ± 4.76 D, mean K was 57.37 ± 9.17 dioptre (D) and CA was -6.24 ± 4.27 D. Fifty (13.3%) eyes had mild KC and 205 (54.5%) of the eyes had severe KC.
Laterality
A total of 183 (97.34%) patients had bilateral KC and 5 (2.66%) patients had unilateral KC. Of the five patients with unilateral KC, one eye was classified to have mild KC, another had advanced KC and the remaining three had severe KC. Non-clinical or forme fruste KC was not reported.
Symmetry
As shown in Table 3, there were differences in UVA (p < 0.001), BCVA (p = 0.028), mean K (p = 0.000) and CA (p < 0.001) between the better eye and the worse eye. A total of 140 (73.9%) patients had severe KC in the worse eye and 65 (34.2%) in the better eye.
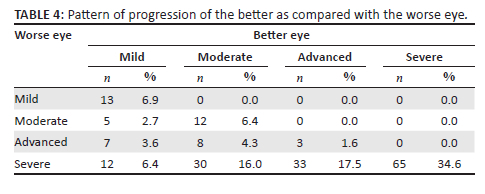
Table 4 shows the patterns of progression between the better eye and the worse eye comparing the severity of the worse eye with the best eye. For patients whose better eye had mild KC, 13 (6.9%) had mild KC, 5 (2.7%) had moderate KC, 7 (3.6%) had advanced KC and 12 (6.4%) had severe KC on the worse eye. For patients whose better eye had moderate KC, 12 (6.4%) had moderate KC, 8 (4.3%) had advanced KC and 30 (16.0%) had severe KC on the worse eye. For patients whose better eye had advanced KC, 3 (1.6%) had advanced KC and 33 (17.5%) had severe KC on the worse eye. For patients whose better eye had severe KC, 65 (34.6%) had severe KC on the worse eye.
Gender predilection
Table 3 shows that there were no statistical significant differences in UVA (0.552), BCVA (p = 0.555), CA (p = 0.099) and mean K (p = 0.948) between the two eyes except for that detected for SE (p = 0.021). Severe KC was found in 85 (79.4%) males and 63 (77.0%) females. Mild KC was found in 9 (8.4%) males and 3 (3.7%) females (p = 0.043).
Age predilection
Table 3 shows the correlation of age and the clinical data. Correlation coefficients and the levels of significance are indicated in brackets, respectively. Age correlated poorly with UVA (r = 0.36; p = 0.487), BCVA (r = 0.141; p = 0.019), SE (r = -0.70; p = 0.182), CA (r = 0.158; p = 0.021) mean K (r = -0.039; p = 0.457) although the correlations were not significant except that of the BCVA and CA.
Treatment and management
Table 5 shows that 132 (70.2%) patients were managed with optical devices distributed as follows: 89 (64.4%) scleral lenses, 41 (31.1%) spectacles and 2 (1.51%) RGP lenses. Of the eyes (worse eyes) that were fitted with scleral lenses, the mean K was 62 ± 10.52 D and a VA improvement of -0.44 ± 0.17 (p < 0.001) was achieved. In cases where RGP lenses were used on eyes with a mean of 52.38 ± 1.63 D, a VA improvement of 0.35 ± 0.07 (p = 0.090) was achieved. Visual acuity improvement of -0.21 ± 0.16 (p < 0.001) was achieved when spectacles were used for the correction of eyes with a mean K of 58.96 ± 7.75 D (p < 0.001). A total of (24.5%) persons were treated or managed for VKC and one was treated for corneal hydrops.
Discussion
The study describes the demographic and clinical profiles of keratoconic patients attending public sector facilities in the Capricorn District of South Africa. Clinical presentation and findings, laterality, symmetry between the two eyes, age and gender differences and also the optical devices used for the KC patient management in the facilities were included in the study. Although many such studies have been conducted in Western countries,23 KC has been poorly studied in Africa.30 A few university clinic-based studies conducted in South Africa, in Durban and Johannesburg, presented demographic and clinical profiles.21,22,23 These studies showed age variations in persons presenting with KC with those in the University of Johannesburg (UJ) study presenting earlier at 22.9 ± 7.46 years for females and 24.0 ± 8.52 years for males21 and those in University of KwaZulu-Natal presenting almost similar age patterns of 25.17 ± 11.42 years22 and 25.2 ± 9.6 years,23 respectively. There was a higher number of females in the UJ-based21 study even though there was no gender predisposition confirmed. Other studies did not present the gender profiles of KC patients. The findings of these studies are important for providing an overview of some aspects of KC epidemiology in South Africa, in the absence of nationwide prevalence studies. This study was the first to report on clinical profiles and also the management of KC patients in public hospitals in Limpopo Province.
More males (56.9%) attended the facilities than females (43.1%). This is an interesting finding given that the district has a higher population of females than males and studies suggested that both had a similar likelihood to use the public eyecare service.24,31 The finding is consistent with the KC prevalence and incidence in Africa.31,32 Male preponderance is associated with the environmental factors under the assumption that males spend more time outdoors than females and hence are more exposed to factors such as pollen, dust, animal fur, and high sunlight exposure.6,33 Contrary to the findings on male preponderance, one of the studies in South Africa, reported a female gender preponderance.21 Female preponderance is reportedly mostly associated with hormonal effects, especially sex hormones where females show more signs of KC progression during their gestational period although there are limited studies about this.26 Gender and KC is a contentious phenomenon given that some studies confirm a male preponderance,6,28,34 some confirm a female preponderance,21,35 while others are inconclusive.27,36,37
The mean age of patients was 20.64 ± 6.82 years with a range between 9 and 46 years. It should be acknowledged that this was the mean age at consultation and not the age of onset, known to be much earlier in pubescent years. Considering the asymmetrical nature of KC, there is a likelihood that it develops in one eye without detection for a period of time. Patients become aware only when the other eye also experiences a deterioration in vision as highlighted by Chetty and Rubin who state that the age of onset and that of detection may be disparate and that KC is usually detected when the better eye deteriorates. This might be the reason that many patients are detected very late, usually at severe stages in many studies.9,21,23,32 Usually, at this time, the worse eye would have reached advanced or severe stages of progression.
Keratoconus was diagnosed in much younger patients in this study as compared with other studies conducted worldwide.7,21,38 Isolated cases were, however, reported where patients were detected in early ages of 4 years,39 5 years37 and 6 years,13,23,40 and there was also presence of KC in much older persons of 57 years21 and 67 years.41 Keratoconus spans through pubescent years to the fourth decade of life but it has been observed to be common in patients between 20 and 30 years.7 The first and third quartiles age spread in the Box and Whisker plot (Figure 1), which fell between 16 and 26 years suggest that many persons were younger. The youngest age in which KC was detected in this study was 9 years in both males and females, although males were generally about 2.56 years (p < 0.006) younger than females. This finding suggests that KC in Capricorn district progressed faster and earlier than elsewhere. With the availability and use of an OCT, more severe cases may have been detected at much younger ages. The Box and Whisker plot showed that male patients had a lower median age than females suggesting that males were generally younger. Men are assumed to spend more time outdoors than females, hence are exposed to ultraviolet light, pollen, dust, and physical damage though rubbing. They are also more likely to receive greater attention to seek a vision consultation as compared with females from their parents.42 The patriarchal inclination of many African households may influence the preference of males over females to get healthcare as females are assumed to be subordinates in terms of access to resources and socio-economic position and power.42
All patients were black South Africans. Public health facilities are mostly accessed by black Africans because of their higher prevalence in the district (97%), proximity to the hospitals and low socio-economic status.24,31 The patients attending these facilities cannot afford medical insurance31 or out of pocket payments, making private healthcare inaccessible and unaffordable. Most of the study facilities are also remote from the cities and towns, where a proportion of people of other race groups, other than black Africans, predominantly reside.24,31
Presenting signs and symptoms are important features for consideration in the diagnosis, grading and management of KC.26 Most of these features do not appear at early stages of KC but become more pronounced as the condition progresses.5 Poor vision results from high myopia and astigmatism induced by KC43 was reported in almost all patients (99.5%) whose files were reviewed in this study. Keratoconus is strongly associated with atopy and allergy, which exacerbate rubbing, resulting in physical trauma to the ocular structures.6 In this study, common symptoms of trauma and auto-immunological reactions including tearing (26.6%), discharges (10.7%), pain (8.5%) and redness (8.0%) were less reported. The majority of patients in this study presented with severe cases of KC, which are commonly associated with the symptoms presented in the latter. Therefore, lower frequencies than expected were reported.9,10,44 Patients may not voluntarily provide such information, for fear that it may be irrelevant to the poor vision experienced, so it is necessary for practitioners to specifically enquire about possible risk factors and presenting symptoms as part of the case history. Practitioners could design a clinical record form that lists KC risk factors to facilitate this enquiry during consultations.
The presenting signs of KC were less reported than the case of symptoms. From the AK grading, Fleischer's ring appears in mild stages and becomes more visible in later stages, Vogt's striae appear in moderate stages, Munson's sign is common in advanced KC, and corneal hydrops and corneal scarring are common in severe stages.9,10,44 Severe KC was common in most patients especially in the worse eye (73.9%), hence a higher rate of occurrence of the signs was expected. Folds were reported although their source or causative factors were not stipulated. It can be conceded that signs, symptoms and other vision distortions were under-reported in the study and this is inconsistent with their usual presentation in other studies.9,10,44 There is a likelihood that there was no standard protocol followed in the screening and examination of the patients across all facilities included in the study. In addition, the possibility exists that signs may not have been identified and not recorded, all of which potentially compromise patient diagnosis. Facilitators are advised to develop protocols to enhance screening, examination, diagnosis, grading of KC and accurate recording to better manage the disease.
Vernal keratoconjunctivitis was diagnosed in some patients (24.5%) in this study. This is a disease presenting commonly in children, especially males, and is characterised by excessive rubbing.9 Higher associations between VKC and KC have been reported with some authors referring to KC as a complication of VKC.9,45,46,47 In addition, a study by Mohale48 in one of the facilities found that of the 2012 patients VKC was prevalent in 22.6% patients, and the majority of patients (81.8%) who were diagnosed with KC were initially managed for VKC. Therefore, there is a likelihood that many KC patients, among the VKC patient population at these facilities, are undiagnosed. This may be attributed to patients with VKC being managed according to presenting symptoms and that clinical procedures such as keratometry, retinoscopy and corneal thickness measurements are not performed. This practice could be because of the absence of standard clinical guidelines for VKC and KC patient management or that the hospitals do not have equipment adequate to detect KC much earlier. The occurrence of both VKC and KC in this study may have an association with the sunny and hot weather patterns of the Tropic of Capricorn latitude, which passes through some areas where the hospitals are located.
Table 3 shows some of the major clinical findings. Patients had UVA of 0.19 ± 0.18 D, BCVA of 0.53 ± 0.24 D, mean SE of -4.89 ± 4.76 D, mean K of 57.37 ± 9.17 and CA of -6.25 ± 9.13. Of the total number of eyes investigated, over half (54.4%) of patients had severe KC. Further investigation conducted for the worse eyes only, found that 73.9% of patients had severe KC and only 7.1% had mild KC. This is a cause for concern with implications that the condition is either detected late or intervention is implemented late, reducing the chances of success to get satisfactory vision with correction. Elsewhere in South Africa, similar patterns of severe KC were observed in two studies, with one study reporting 61%21 severe KC and the other 56.6%.23
A pattern of severe KC has been observed mostly in hospital and clinic-based populations such as in this study.21,23 As a result of poor socio-economic status and high costs of services and corrective devices, patients may not seek clinical care until such time that they are unable to perform any daily activities with ease. In some instances, patients are inappropriately managed with spectacles for the myopia and astigmatism, with no investigation for possible KC, which may be attributed to limited practitioner skills or non-adherence to KC screening and examination protocols. Rupnarain et al.23 argued that most patients sampled in their study were initially managed elsewhere and KC was only detected when spectacles were unable to correct refractive errors at the stages where KC would have progressed to severe stages. This, therefore, compromises patient care and hence providing practitioner education through upskilling programmes may enhance KC patients' care.
A large proportion of patients (97.3%) in this study had bilateral KC with a mean K difference (or CA) of 7.59 ± 6.08 D (p = 0.002), which is much higher than that revealed in many studies in Malaysian49 and South African populations21,23 and almost similar to that in others in the Iranian37 and Kenyan50 populations. Keratoconus is a bilateral but asymmetrical condition and this finding is similar to those in other studies.1,6,37 According to Eppig et al.,51 the level of asymmetry increases as the level of severity of KC increases as found in this study where at consultation, more than half of the patients (53.1%) had progressed to advanced or severe stages in the better eye compared with the progression of the worse eye in 83.7% of patients. The use of equipment such as OCT and pachymeters enables the detection of KC at early stages before it advances.
From Table 3, mean K had poor negative correlation (r = -0.017) with age although findings were not statistically significant (p = 0.819). In addition, there was no difference in mean K between males and females although this finding was also not statistically significant (p = 0.948). These findings suggest that the progression or severity patterns of KC were inconclusive or their difference in terms of age and gender could not be confirmed. This finding is similar to previous reports.25,52 The symmetry of KC and age at diagnosis in a resource constrained environment such as most public facilities in South Africa is dependent on socio-economic factors. Patients seek a consultation only when the visual acuity in their better eye has deteriorated,21 and accordingly, their motive will be affected by access, availability and affordability of the service. Socio-economic factors, as opposed to onset, determine when the patient is likely to consult for an eye test and the subsequent diagnosis of KC.
Of all the KC patients who attended the public service hospitals, most (70.2%) had vision correction. Scleral lenses were used in 64.4% of patients, 31.06% with spectacles and 1.5% with RGP lenses. About one-quarter of these patients (24.5%) were managed with medication to relieve the symptoms associated with VKC. Progression and severity of KC are determinants of procedures and devices used for its management.15,16 Patients fitted with scleral contact lenses had the most improvement in visual acuity (0.44 ± 0.17) as compared with those who were fitted with RGP lenses (0.35 ± 0.07) and also those fitted with spectacles (0.21 ± 0.16). Although patients fitted with corneal RGP lenses had better vision improvement than those fitted with spectacles, the rate of use of RGP lenses as corrective devices was the least (1.5%).
Despite the cited superior comfort with improved vision provided by scleral lenses as compared with corneal RGP lenses,53 the corneal lenses are more cost effective than scleral lenses54 within the context of an extremely resource constrained public health sector in South Africa and are additionally easier to maintain by patients. Yego et al.32 highlighted that in patients with poor socio-economic status, especially in low- to middle-income countries, scleral lenses are usually beyond the financial reach of patients and hence corneal RGP lenses are the most affordable option. Some public hospitals provide optical devices at subsidised rates for patients. In such cases, opting for corneal RGP lenses may be more affordable for indigent patients and enable facilities to cover more patients with their limited funds, ultimately attaining a higher impact on managing KC patients.
With the increasing number of children being diagnosed, with their small palpebral aperture sizes, they may find it easier to use smaller diameter RGP lenses than scleral lenses.25 Anecdotal reports from practitioners indicate that fitting corneal RGP lenses on the highly irregular, advanced KC cornea requires very good fitting skills and, in many cases, influence the lens option fitted. However, optometrists could attend upskilling workshops as part of their continuous professional development to improve their lens fitting skills. In cases where corneal RGP lenses are not or no longer effective, hybrid or scleral lenses remain an option to be provided.15,16,53
The pattern of use of devices in this study was not consistent with that suggested in the literature.15,16,53 Spectacle users had mean K of 58.96 ± 7.75 D (worse eye) as compared with 52.38 ± 1.63 D (worse eye) of corneal RGP lens users and suggesting that most of the spectacle users could have equally benefited from RGP contact lenses. In addition, the UVA of spectacles users could have been better improved with RGP and scleral lenses. From the public health perspective, affordability, availability of resources and challenges with eyecare policy could be determinants of this outcome.55,56 Upon diagnosis of KC, spectacles may have been used because of their prompt availability and affordability and not on the clinical merit of the cases. Another possibility is that most of these spectacle users could have been put on a waiting list such as to be fitted with rigid contact lenses when possible.
It is commendable that other forms of patient management, using medication for managing VKC and hypertonic solution for managing hydrops by ophthalmologists, were implemented although there is a need to explore other forms such as corneal strengthening CXL. Corneal cross linking and corneal grafts were not observed in the reviewed files. Corneal cross linking may not have been performed because of the absence of equipment for the early detection of KC at most of the facilities. For patients to undergo CXL, they need to be detected and diagnosed before their cornea thins beyond 400 µm to avoid UV damage on the endothelium and they should have notable progression of the UVA, BCVA, and corneal shape as the procedure aims to halt progression.57,58 The cornea should have a good healing process and fair tear film quality and should be free from infections and autoimmune conditions.57,58 Performing CXL, however, will limit or delay the progression of KC to advanced and severe stages in either eye and should be considered as a management option in the district.
Of concern is that at the time of review, almost a third (29.8%) of patients were not under any form of KC care and it was not indicated if any treatment was planned. Noting the negative impact that KC has on the quality of life of the mostly young population, it is recommended that the respective public health facilities ensure that every patient is fully managed with care processes and progress documented and patients monitored.
Conclusion
This study presented the demographic and clinical characteristics as well as management protocols used among 188 KC patients in the Capricorn District of Limpopo province, South Africa. There was a preponderance of males in the study and most patients were diagnosed with KC at severe stages. Almost all patients had bilateral and asymmetrical KC with no significant difference in clinical features by age and gender. Patients fitted with scleral lenses had the highest improvement in vision as compared with those who were fitted with spectacles and those who were fitted with RGP lenses. Challenges for early diagnoses remain, hence there is a need to raise awareness about KC and its presentation patterns in South Africa among all affected, including patients, practitioners, and policymakers.
Furthermore, the findings suggest a likelihood of a higher prevalence of KC as patients remain underdiagnosed or misdiagnosed, either because of the non-availability of technologically advanced equipment and the skills gap of practitioners. There is a dire need to improve the skill level of practitioners to upgrade and enhance KC patient care. The findings on patient records and management patterns suggest a lack of clinical protocols that are consistent with those in literature to effectively and efficiently detect and manage KC. Public health facilities, therefore, need to develop such protocols and practitioners need to strictly adhere to these protocols when providing care to patients. Demographic, socio-economic and other relevant factors need to be borne in mind while planning so that decisions are not only informed by clinical factors but also on the micro and macro-environmental factors.
Acknowledgements
The authors would like to acknowledge invaluable contribution and selflessness of the optometrists and eyecare managers in public hospitals in the Capricorn District of Limpopo, South Africa. Special acknowledgement goes to Dr S. Ntuli who performed the data analysis and interpretation of results.
Competing interests
The authors declare that this article was written in the interest of knowledge contribution and not influenced by any financial or personal relationships.
Authors' contributions
The article was developed from the PhD thesis of the study undertaken by P.M.W.N. under the supervision of V.R.M. and K.P.M. as supervisor and co-supervisor, respectively.
Funding information
This research work received a grant from the College of Health Sciences at the University of KwaZulu-Natal to cover data collection costs.
Data availability
Data used to support the findings of the study are available from the corresponding author, P.M.W.N., upon request.
Disclaimer
The authors declare that views expressed in this article are those of their own and are not necessarily views, position or policy of their affiliated agencies.
References
1.Tur V, MacGregor C, Jayaswal R, O'Brart D, Maycock N. A review of keratoconus: Diagnosis, pathophysiology, and genetics. Surv Ophthalmol. 2017;62(6):770-783. https://doi.org/10.1016/j.survophthal.2017.06.009 [ Links ]
2.Loh IP, Sherwin T. Is keratoconus an inflammatory disease? The implication of inflammatory pathways. Ocul Immunol Inflamm. 2022;30(1):246-55. https://doi.org/10.1080/09273948.2020.1780271 [ Links ]
3.McMonnies CW. Inflammation and keratoconus. Optom Vis Sci. 2015;92(2):e35-e41. https://doi.org/10.1097/OPX.0000000000000455 [ Links ]
4.Omer K. Epidemiology of keratoconus worldwide. Open Ophthalmol J. 2018;12(1):289-299. https://doi.org/10.2174/1874364101812010289 [ Links ]
5.Romero-Jiménez M, Santodomingo-Rubido J, Wolffsohn JS. Keratoconus: A review. Contact Lens Anterior Eye. 2010;33(4):157-166. https://doi.org/10.1016/j.clae.2010.04.006 [ Links ]
6.Hashemi H, Heydarian S, Hooshmand E, et al. The prevalence and risk factors for keratoconus: A systematic review and meta-analysis. Cornea. 2020;39(2):263-270. https://doi.org/10.1097/ICO.0000000000002150 [ Links ]
7.Santodomingo-Rubido J, Carracedo G, Suzaki A, Villa-Collar C, Vincent SJ, Wolffsohn JS. Keratoconus: An updated review. Contact Lens Anterior Eye. 2022;45(3):101559-101577. https://doi.org/10.1016/j.clae.2021.101559 [ Links ]
8.Weed K, MacEwen C, Giles T, Low J, McGhee C. The Dundee University Scottish Keratoconus study: Demographics, corneal signs, associated diseases, and eye rubbing. Eye. 2008;22(4):534-541. https://doi.org/10.1038/sj.eye.6702692 [ Links ]
9.Rahman A, Rahman S, Faridi J, Salam A, Zafrullah TA, Sadia S. Keratoconus: Early onset, the worst prognosis, eye rubbing and hand-dominance. Adv Ophthalmol Vis Syst. 2020;10(4):79-84. https://doi.org/10.15406/aovs.2020.10.00390 [ Links ]
10.Asimellis G, Kaufman EJ. Keratoconus. Treasure Island (FL): StatPearls Publishing; January, 2019. [ Links ]
11.Najmi H, Mobarki Y, Mania K, et al. The correlation between keratoconus and eye rubbing: A review. Int J Ophthalmol. 2019;12(11):1775. https://doi.org/10.18240/ijo.2019.11.17 [ Links ]
12.Godefrooij DA, De Wit GA, Uiterwaal CS, Imhof SM, Wisse RPL. Age-specific incidence and prevalence of keratoconus: A nationwide registration study. Am J Ophthalmol. 2017;175:169-172. https://doi.org/10.1016/j.ajo.2016.12.015 [ Links ]
13.Netto EAT, Al-Otaibi WM, Hafezi NL, et al. Prevalence of keratoconus in paediatric patients in Riyadh, Saudi Arabia. Br J Ophthalmol. 2018;102(10):1436-1441. https://doi.org/10.1136/bjophthalmol-2017-311391 [ Links ]
14.Gomes JAP, Tan D, Rapuano CJ, et al. Global consensus on keratoconus and ectatic diseases. Cornea. 2015;34(4):359-369. https://doi.org/10.1097/ICO.0000000000000408 [ Links ]
15.Rico-Del-Viejo L, Garcia-Montero M, Hernández-Verdejo JL, García-Lázaro S, Gómez-Sanz FJ, Lorente-Velázquez A. Nonsurgical procedures for keratoconus management. J Ophthalmol. 2017;2017:1-17. https://doi.org/10.1155/2017/9707650 [ Links ]
16.Mohammadpour M, Heidari Z, Hashemi H. Updates on managements for keratoconus. J Curr Ophthalmol. 2018;30(2):110. https://doi.org/10.1016/j.joco.2017.11.002 [ Links ]
17.Otchere H, Jones L, Sorbara L. The impact of scleral contact lens vault on visual acuity and comfort. Eye Contact Lens. 2018;44:S54-S59. https://doi.org/10.1097/ICL.0000000000000427 [ Links ]
18.Ambekar R, Toussaint KC, Wagoner Johnson A. The effect of keratoconus on the structural, mechanical, and optical properties of the cornea. J Mech Behav Biomed Mater. 2011;4(3):223-236. https://doi.org/10.1016/j.jmbbm.2010.09.014 [ Links ]
19.Olivo-Payne A, Abdala-Figuerola A, Hernandez-Bogantes E, Pedro-Aguilar L, Chan E, Godefrooij D. Optimal management of pediatric keratoconus: challenges and solutions. Clin Ophthalmol. 2019;2019(13):1183. https://doi.org/10.2147/OPTH.S183347 [ Links ]
20.Arnalich-Montiel F, Alió del Barrio JL, Alió JL. Corneal surgery in keratoconus: Which type, which technique, which outcomes? Eye Vis. 2016;3(1):1-14. https://doi.org/10.1186/s40662-016-0033-y [ Links ]
21.Chetty E, Rubin A. Preliminary demographics for patients with keratoconus attending a university-based clinic in Johannesburg, South Africa. Afr Vision Eye Health. 2019;78(1):a472. https://doi.org/10.4102/aveh.v78i1.472 [ Links ]
22.Munsamy A, Moodley V, Naidoo P, et al. A frequency analysis of cone characteristics for the different stages of keratoconus. Afr Vision Eye Health. 2015;74(1):6. https://doi.org/10.4102/aveh.v74i1.302 [ Links ]
23.Rupnarain S, Madlala N, Memela N, et al. Clinical characteristics of keratoconus patients at the University of KwaZulu-Natal eye clinic. Afr Vision Eye Health. 2020;79(1):a528. https://doi.org/10.4102/aveh.v79i1.528 [ Links ]
24.Department of Cooperative Governance and Traditional Affairs. District Development Model, Capricorn District Municipality, Limpopo Province - Profiles and analysis. Pretoria; 2020. [ Links ]
25.Abdu M, Binnawi K, Hassan R. Clinical profile of keratoconus patients in Sudan. Sudan J Ophthalmol. 2016;8(1):20. https://doi.org/10.4103/1858-540X.184235 [ Links ]
26.Sharif R, Bak-Nielsen S, Hjortdal J, Karamichos D. Pathogenesis of keratoconus: The intriguing therapeutic potential of Prolactin-inducible protein. Prog Retin Eye Res. 2018;67:150. https://doi.org/10.1016/j.preteyeres.2018.05.002 [ Links ]
27.Hashemi H, Heydarian S, Yekta A, et al. High prevalence and familial aggregation of keratoconus in an Iranian rural population: A population-based study. Ophthalmic Physiol Opt. 2018;38(4):447-455. https://doi.org/10.1111/opo.12448 [ Links ]
28.Belin M, Augenheilkunde JDM für, 2016 undefined. Keratoconus: The ABCD grading system. thieme-connect.com. 2016;233(6):701-707. https://doi.org/10.1055/s-0042-100626 [ Links ]
29.Belin M, Kundu G, Shetty N, Gupta K, Mullick R, Thakur P. ABCD: A new classification for keratoconus. Indian J Ophthalmol. 2020;68(12):2831. https://doi.org/10.4103/ijo.IJO_2078_20 [ Links ]
30.Akowuah PK, Kobia-Acquah E, Donkor R, Adjei-Anang J, Ankamah-Lomotey S. Keratoconus in Africa: A systematic review and meta-analysis. Ophthalmic Physiol Opt. 2021;41(4):736-47. https://doi.org/10.1111/opo.12825 [ Links ]
31.Ntsoane MD, Oduntan OA, Mpolokeng BL. Utilisation of public eye care services by the rural community residents in the Capricorn district, Limpopo Province, South Africa. Afr J Prim Health Care Fam Med. 2012;4(1):1-7. https://doi.org/10.4102/phcfm.v4i1.412 [ Links ]
32.Yego WK, Moodley VR. Visual acuity and refractive error improvement in keratoconic patients: A low-income context management perspective. Clin Optom. 2020;12:113-122. https://doi.org/10.2147/OPTO.S258905 [ Links ]
33.Gordon-Shaag A, Millodot M, Shneor E, Liu Y. The genetic and environmental factors for keratoconus. Biomed Res Int. 2015;2015:795738. https://doi.org/10.1155/2015/795738 [ Links ]
34.Millodot M, Shneor E, Albou S, Atlani E, Gordon-Shaag A. Prevalence and associated factors of keratoconus in Jerusalem: A cross-sectional study. Ophthalmic Epidemiol. 2011;18(2):91-97. https://doi.org/10.3109/09286586.2011.560747 [ Links ]
35.Hashemi H, Beiranvand A, Khabazkhoob M, et al. Prevalence of keratoconus in a population-based study in Shahroud. Cornea. 2013;32(11):1441-1445. https://doi.org/10.1097/ICO.0b013e3182a0d014 [ Links ]
36.Jonas JB, Nangia V, Matin A, Kulkarni M, Bhojwani K. Prevalence and associations of keratoconus in rural maharashtra in central India: The central India eye and medical study. Am J Ophthalmol. 2009;148(5):760-765. https://doi.org/10.1016/j.ajo.2009.06.024 [ Links ]
37.Naderan M, Shoar S, Kamaleddin M, Rajabi M, Naderan M, Khodadadi M. Keratoconus clinical findings according to different classifications. Cornea. 2015;34(9):1005-1011. https://doi.org/10.1097/ICO.0000000000000537 [ Links ]
38.Alzahrani K, Al-Rashah A, Al-Salem S, et al. Keratoconus epidemiology presentations at Najran Province, Saudi Arabia. Clin Optom. 2021;13:175-179. https://doi.org/10.2147/OPTO.S309651 [ Links ]
39.Sabti S, Tappeiner C, Frueh BE. Corneal cross-linking in a 4-year-old child with keratoconus and down syndrome. Cornea. 2015;34(9):1157-1160. https://doi.org/10.1097/ICO.0000000000000491 [ Links ]
40.Dimacali V, Balidis M, Adamopoulou A, Kozei A, Kozeis N. A case of early keratoconus associated with eye rubbing in a young child. Ophthalmol Ther. 2020;9(3):667-676. https://doi.org/10.1007/s40123-020-00264-8 [ Links ]
41.Millodot M, Ortenberg I, Lahav-Yacouel K, Behrman S. Effect of ageing on keratoconic corneas. J Optom. 2016;9(2):72. https://doi.org/10.1016/j.optom.2015.05.001 [ Links ]
42.Azad AD, Charles AG, Ding Q, Trickey AW, Wren SM. The gender gap and healthcare: Associations between gender roles and factors affecting healthcare access in Central Malawi, June-August 2017. Arch Public Health. 2020;78(1):1-11. https://doi.org/10.1186/s13690-020-00497-w [ Links ]
43.Nagib Omar IA. Keratoconus screening among myopic children. Clin Ophthalmol. 2019;13:1909. https://doi.org/10.2147/OPTH.S225326 [ Links ]
44.Christy J, Tagare S. Classical signs of Keratoconus. Delhi J Ophthalmol. 2020;31(1):87-89. https://doi.org/10.7869/djo.580 [ Links ]
45.Wajnsztajn D, Solomon A. Vernal keratoconjunctivitis and keratoconus. Curr Opin Allergy Clin Immunol. 2021;21(5):507-514. https://doi.org/10.1097/ACI.0000000000000765 [ Links ]
46.Al-Akily SA, Bamashmus MA. Ocular complications of severe vernal keratoconjunctivitis (VKC) in Yemen. Saudi J Ophthalmol. 2011;25(3):291-294. https://doi.org/10.1016/j.sjopt.2011.02.001 [ Links ]
47.Sharma N, Rao K, Maharana PK, Vajpayee RB. Ocular allergy and keratoconus. Indian J Ophthalmol. 2013;61(8):407-409. https://doi.org/10.4103/0301-4738.116063 [ Links ]
48.Mohale V. Association of VKC and KC in patients attending Mankweng Hospital in Limpopo Province, South Africa [Masters' dissertation]. Durban: University of KwaZulu- Natal, 2021; 22p. [ Links ]
49.Mohd-Ali B, Abdu M, Yaw CY, Mohidin N. Clinical characteristics of keratoconus patients in Malaysia: A review from a cornea specialist centre. J Optom. 2012;5(1):38-42. https://doi.org/10.1016/j.optom.2012.01.002 [ Links ]
50.Rashid ZA, Millodot M, Evans KSE. Characteristics of keratoconic patients attending a specialist contact lens clinic in Kenya. Middle East Afr J Ophthalmol. 2016;23(4):283. https://doi.org/10.4103/0974-9233.194074 [ Links ]
51.Eppig T, Spira-Eppig C, Goebels S, Seitz B, El-Husseiny M, Lenhart M, et al. Asymmetry between left and right eyes in keratoconus patients increases with the severity of the worse eye. Curr Eye Res. 2018;43(7):848-855. https://doi.org/10.1080/02713683.2018.1451545 [ Links ]
52.Rana RS, Bajracharya L, Gurung R. Clinical profile on keratoconus presenting at a Tertiary Eye Care Centre - Tilganga Institute of Ophthalmology. Nepal J Ophthalmol. 2019;11(2):138-144. https://doi.org/10.3126/nepjoph.v11i2.27818 [ Links ]
53.Singh R, Gupta N, Vanathi M, Tandon R. Corneal transplantation in the modern era. Indian J Med Res. 2019;150(1):7. https://doi.org/10.4103/ijmr.IJMR_141_19 [ Links ]
54.Contact Lenses Laboratory of South Africa. Price list - Scleral and GP Lens fitting sets. Johannesburg; 2020. [ Links ]
55.Van Staden D, Buthelezi L. Integrating eye health into policy: Evidence for health systems strengthening in KwaZulu-Natal. Afr Vision Eye Health. 2020;79(1):1-10. https://doi.org/10.4102/aveh.v79i1.549 [ Links ]
56.Bakkar MM, Haddad MF, Qadire M Al. Patient-related barriers to Rigid Gas Permeable (RGP) lens wear among keratoconus patients in Jordan. Cont Lens Anterior Eye. 2018;41(3):267-272. https://doi.org/10.1016/j.clae.2017.12.007 [ Links ]
57.Sorkin N, Varssano D. Corneal collagen crosslinking: A systematic review. Ophthalmologica. 2014;232(1):10-27. https://doi.org/10.1159/000357979 [ Links ]
58.Galvis V, Tello A, Ortiz AI, Escaf LC. Patient selection for corneal collagen cross-linking: An updated review. Clin Ophthalmol. 2017;2017(11):657. https://doi.org/10.2147/OPTH.S101386 [ Links ]
 Correspondence:
Correspondence:
Pheagane Nkoana
pheagane.nkoana@ul.ac.za
Received: 02 June 2022
Accepted: 16 Jan. 2023
Published: 20 June 2023














