Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Obstetrics and Gynaecology
versão On-line ISSN 2305-8862
versão impressa ISSN 0038-2329
SAJOG vol.29 no.1 Cape Town Mai. 2023
http://dx.doi.org/10.7196/SAJOG.2023.v291x.550
RESEARCH
Comparison of obstetric and perinatal outcomes in women with diabetes at Steve Biko Academic Hospital
N MalazaI, II; C PheifferIII, IV; S DiasV; S AdamVI, VII
IMSc (Med). Biomedical Research and Innovation Platform (BRIP), South African Medical Research Council, Tygerberg, South Africa. Department of Obstetrics and Gynaecology, School of Medicine, Faculty of Health Sciences, University of Pretoria, South Africa
IIMSc (Med). Department of Obstetrics and Gynaecology, School of Medicine, Faculty of Health Sciences, University of Pretoria, South Africa
IIIPhD (MPH). Biomedical Research and Innovation Platform (BRIP), South African Medical Research Council, Tygerberg, South Africa. Department of Obstetrics and Gynaecology, School of Medicine, Faculty of Health Sciences, University of Pretoria, South Africa
IVPhD (MPH). Department of Obstetrics and Gynaecology, School of Medicine, Faculty of Health Sciences, University of Pretoria, South Africa
VPhD. Biomedical Research and Innovation Platform (BRIP), South African Medical Research Council, Tygerberg, South Africa
VIMB ChB, FCOG (SA), MMed (O&G), PhD (O&G). Department of Obstetrics and Gynaecology, School of Medicine, Faculty of Health Sciences, University of Pretoria, South Africa
VIIMB ChB, FCOG (SA), MMed (O&G), PhD (O&G). Diabetes Research Centre, Faculty of Health Sciences, University of Pretoria, South Africa
ABSTRACT
BACKGROUND: Diabetes and obesity in pregnancy have been associated with increased rates of adverse maternal and neonatal outcomes compared with women with normoglycaemia and normal weight.
OBJECTIVE: To investigate the effect of diabetes and pre-pregnancy obesity on obstetric and perinatal outcomes.
METHODS: This study included women with pregestational diabetes types 1 (T1DM) and 2 (T2DM), gestational diabetes (GDM) and normoglycaemia, who received care at the Steve Biko Academic Hospital antenatal clinic between 2017 and 2022. The women were followed up until delivery. Data collected included obstetric history and care, diabetes, obstetric and perinatal outcomes.
RESULTS: A total of 183 women were recruited: 13 (7.1%) with T1DM, 65 (35.5%) with T2DM, 39 (21.3%) with GDM and 66 (36.1%) normoglycaemic controls. Women with T2DM and GDM were older (p<0.01) and more likely to have a history of chronic hypertension (p=0.025) compared with controls. Women with GDM were more likely to be obese than their T1DM counterparts (p=0.036). T1DM and T2DM were associated with higher rates of preterm delivery than controls (p=0.002). The frequency of GDM was significantly higher in women with obesity (p=0.039). The frequency of caesarean section before the onset of labour was higher in women with a weight >80 kg compared with women with a weight <80 kg (p=0.015).
CONCLUSION: Diabetes in pregnancy is associated with adverse obstetric and perinatal outcomes. Therefore, adequate glucose control should be accompanied by preconceptual weight optimisation to reduce adverse outcomes during pregnancy.
Diabetes mellitus is a common pregnancy complication that poses a serious health threat to maternal and child health.111 Diabetes in pregnancy (DIP) can be classified as pregestational type 1 (T1DM) or 2 (T2DM) diabetes; T1DM or T2DM first diagnosed during pregnancy; or gestational diabetes mellitus (GDM), a milder form of carbohydrate intolerance that first develops during pregnancy, with glucose homeostasis usually restored within 6 weeks after delivery. Globally, ~16.7% (21.1 million) of live births are affected by DIP. Among these, pregestational T1DM and T2DM account for 10.6% of cases, T1DM and T2DM first detected in pregnancy account for 9.1% of cases and GDM accounts for 80.3% of cases.[1] South Africa (SA) is a low-to-middle-income country (LMIC) with high rates of DIP. Recent studies reported that the prevalence of GDM varied from 9.1% to 25.6%, depending on the diagnostic criteria..[2]
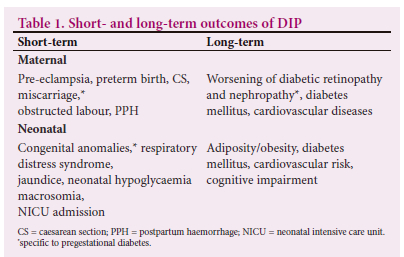
All types of DIP are associated with an increased risk of short-and long-term adverse outcomes for mother and child (Table 1), especially when glycaemic control is suboptimal. The severity and frequency of these adverse outcomes are higher in women with pregestational diabetes compared with GDM. Achieving adequate glycaemic control and appropriate gestational weight gain is critical to prevent pregnancy complications and adverse outcomes..[3]
Obesity is considered a major risk factor for DIP, with an increasing number of epidemiological studies supporting this association..[4] In addition, obesity has also been reported to independently increase the risk of maternal and fetal adverse outcomes..[5] In SA, the estimated prevalence of obesity in women of reproductive age is 35.2%,[6] highlighting the potential negative effects of obesity on both maternal and child health. Studies have shown that an increase in both maternal weight and body mass index (BMI) before and during pregnancy is associated with adverse pregnancy outcomes.[7-9] However, in resource-limited settings where measuring weight is more practical, the use of maternal weight instead of BMI to assess the risk of adverse outcomes related to weight during pregnancy might be a more viable option. This is substantiated by a study conducted by Wolfe et al.[10]
This study aimed to investigate the effect of DIP and obesity on obstetric and perinatal outcomes in women attending the diabetic antenatal clinic at a tertiary hospital in Tshwane, South Africa.
Methods
We conducted a prospective study including women with pregestational T1DM or T2DM, GDM and normoglycaemia (negative oral glucose tolerance test (OGTT)) who attended the high-risk antenatal clinic at Steve Biko Academic Hospital (SBAH), Tshwane, Pretoria, between May 2017 and March 2022. The study was approved by the University of Pretoria Health Science Research Ethics Committee (ref. no. 191/2016 and 41/2021). This study is part of a larger study investigating epigenetic mechanisms in women with DIP. At SBAH, the diabetes antenatal clinic manages referrals from local endocrine and internal medicine or antenatal clinics in the cluster. The referring clinics use the risk factor-based selective screening approach,[11] which includes screening for risk factors, such as family history of diabetes mellitus, previous GDM, advanced maternal age, obesity and previous adverse pregnancy outcome, including congenital abnormality, recurrent miscarriages, delivery of a stillborn child, delivery of a baby >4 000 g in a previous pregnancy or persistent glycosuria.[12]
Women were included in the study if they had singleton pregnancies, were aged between 18 - 40 years, were of black African ethnicity, were at <28 weeks' gestation and were HIV negative. DIP was categorised as TIDM if diagnosed prior to pregnancy or if first diagnosed in pregnancy and was confirmed by the presence of positive antibodies or the occurrence of diabetic ketoacidosis, which is determined in consultation with an endocrinologist. A T2DM diagnosis was made if it was identified prior to pregnancy or if overt diabetes was diagnosed during pregnancy (fasting plasma glucose level >7.0 mmol/L, random plasma glucose or 2-hour plasma glucose >11.1 mmol/L on the OGTT; or glycated haemoglobin (HbA1c) >6.5%). GDM was diagnosed if carbohydrate intolerance was first diagnosed during pregnancy according to the International Association of the Diabetes and Pregnancy Study Groups (IADPSG) criteria at 24 - 28 weeks' gestation (fasting plasma glucose level 5.1 -6.9 mmol/L or 1-hour plasma glucose >10 mmol/L or 2-hour plasma glucose 8.5 - 11.0 mmol/L after a 2-hour 75-g oral glucose tolerance test (OGTT)).[13] Women were recruited as normoglycaemic controls if they had a negative OGTT. Women were followed up until delivery. Data collected included demographics, anthropometric measures, obstetric history and care, diabetes care and fetal outcomes, according to standard clinical care. Gestational age (GA) was determined using early ultrasound when available; otherwise, it was determined based on menstrual history or late ultrasound. Maternal weight at the first antenatal visit was recorded as pre-pregnancy weight was not available. Due to missing height measurements, maternal BMI data were limited. Consequently, both weight and BMI were collected. Obesity was defined as a BMI >30 kg/m2 as per Institute of Medicine guidelines.[14] Dietary counselling and education on diabetes were provided by a trained dietician. Post diagnosis, some women with GDM were initiated on metformin in consultation with specialists, while others were started on a low-carbohydrate diet (dependent on OGTT levels). Women were counselled on maintaining glycaemic targets, including fasting/pre-prandial glucose levels of <5.3 mmol/L and 2-hour post-prandial glucose levels of <6.7 mmol/L. All women with DIP monitored their glucose levels at home. Women were required to test their glucose with an On-Call Plus glucometer (On Call, Mexico) at least five times a day, at various times during the week: 30 minutes before each meal (fasting), 2 hours after each meal (post-prandial), at bedtime and 02h00. Poor glycaemic control was defined as having >25% of glucose values outside the recommended range based on home glucose monitoring.
Obstetric and perinatal outcomes included GA at delivery (weeks), onset of labour, route of delivery, birthweight (g), neonatal outcome and Apgar score at 5 minutes. GA at delivery was categorised into preterm (<37 weeks) and term delivery (>37 weeks). Fetal growth was classified as small for gestational age (SGA) if fetal growth <10th centile and large for gestational age (LGA) if fetal growth >90th centile. Birthweight was defined as low birthweight (501 - 2 500 g), normal weight (2 500 - 4 000 g) and macrosomia (>4 000 g).
Statistical analysis
Data were captured in Microsoft Office Excel 2010 (Microsoft Corp., US) and analysed using STATA 17 (Stata Corp., US). Baseline characteristics were summarised using descriptive statistics. A skewness-kurtosis test was performed to assess normality. Continuous variables are presented as the median and interquartile range (IQR), while categorical variables are expressed as counts and percentages. Continuous data were compared using the Kruskal-Wallis test, followed by Dunn's post hoc multiple comparisons test. Categorical data were compared using Pearson's chi-squared (χ2) test with the Bonferroni post hoc test. For counts less than 5, Fisher's exact test was used. Statistical significance was defined as p<0.05.
Results
The general characteristics of the population according to diabetes type are summarised in Table 2. A total of 183 women were recruited, including 13 (7.1%) with T1DM, 65 (35.5%) with T2DM, 39 (21.3%) with GDM and 66 (36.1%) who were classified as normoglycaemic. Women with T2DM and GDM were older (p<0.01), had higher BMI (p<0.05) and had a history of chronic hypertension (p=0.025) compared with the control group. Obesity results were based on 74.31% of BMI data. Women with GDM had a significantly higher frequency of obesity compared with women with T1DM (80.6% v. 36.4%) but were not different to women with T2DM and controls. Women with T1DM and T2DM had significantly higher glycated haemoglobin (HbA1c) compared with those with GDM (p<0.001). At enrollment, more women with T1DM were on insulin treatment compared with those with T2DM (76.9% v. 15.0%; p<0.01), while more women with T2DM were on metformin compared with women with GDM (53.3% v. 26.5%; p<0.05). At delivery, 67.2% of women with T2DM and 18.2% of women with T1DM were managed with a combination of metformin and insulin compared with 15.2% of women with GDM (p<0.001; Table 2).
Obstetric and perinatal outcomes were compared in the combined diabetic group (T1DM, T2DM, GDM) compared with controls. Overall, the frequency of preterm delivery was higher in the diabetic group compared with the controls (51.5% v. 17.1%; p<0.001). However, no between-group differences were noted in the other obstetric and perinatal outcomes. Next, the diabetic group was stratified into T1DM, T2DM and GDM and controls. Obstetric and perinatal outcomes in the sub-groups were compared with the control group (Table 3). The frequency of preterm birth was higher in women with T1DM (66.7% v. 17.1%; p<0.05) and T2DM (54.4% v. 17.1%; p<0.05). However, there was no significant difference between the GDM and control groups. No between-group differences were observed in the other obstetric and perinatal outcomes.
Next, the effect of obesity and weight on obstetric and perinatal outcomes was investigated. The frequency of GDM was significantly higher in women with obesity compared with women without obesity (30.5% v. 11.5%; p=0.036) (Table 4). The frequency of caesarian section (CS) performed before the onset of labour was higher in women weighing >80 kg compared with women weighing <80 kg (45.6% v. 26.0%; p<0.05). The frequency of T1DM was lower in the >80 kg weight category compared with the <80 kg weight category (13.6% v. 3.6%; p<0.05), while the frequency of GDM was higher in the >80 kg compared with the <80 kg weight category (27.9% v. 12.1%; p<0.05) (Table 5). The frequency of spontaneous onset of labour was higher in the <80 kg weight category compared to the >80 kg and <120 kg weight category (52% v. 28.6; p<0.05). The rate of low Apgar scores at 5 minutes was significantly higher in the >120 kg group compared with the >80 and <120 kg groups (33.3% v. 4.2%; p<0.05) (Table 5).
Discussion
Literature has shown that diabetes and obesity in pregnancy are associated with adverse pregnancy outcomes for both the mother and child. Therefore, this study aimed to investigate the effect of diabetes and obesity in pregnancy on adverse obstetric and perinatal outcomes. The main findings of the study are i) higher rates of preterm delivery in women with T1DM and T2DM compared with the control group, ii) higher frequency of GDM in women with obesity compared with women without obesity, iii) higher risk of CS before the onset of labour in women who weighed more than 80 kg compared with women who weighed less than 80 kg and iv) lower rates of spontaneous onset of labour and higher rates of low Apgar scores in women who weighed more than 120 kg compared with women who weighed between 80 kg and 120 kg.
Our study showed that T1DM and T2DM were associated with higher rates of preterm delivery compared with the control group. These findings are consistent with those of previous studies that also reported elevated rates of preterm delivery in women with pregestational T1DM and T2DM compared with women with GDM and the control group.[15-17] In contrast, a systematic review reported deaths that showed higher or similar risks of preterm delivery in women with GDM compared with women with pregestational diabetes.[18] The optimal timing of delivery for women with DIP is contentious. Some recommendations suggest that in women with pregestational diabetes, especially those with vascular complications or suboptimal glycaemic control, early delivery (before 38.5 weeks' gestation) is the better option.[19] However, a 2018 Cochrane systematic review that aimed to determine the optimal timing of delivery for women with pregestational diabetes concluded that there were insufficient data to adequately determine the timing of delivery due to the lack of published trials.[20] Accordingly, the clinical decision regarding the timing of delivery in women with diabetes depends on several maternal and fetal factors, as well as the associated risk of adverse outcomes. Surprisingly, our study did not show differences in other obstetric and perinatal outcomes among the diabetes groups. This may be attributed to early delivery. Therefore, in our population, early delivery might be a better option to reduce adverse outcomes that may occur at term delivery.
The higher frequency of GDM in women with obesity, compared with their non-obese counterparts, is evidence that obesity is an independent risk factor for the development of GDM.[5] A meta-analysis including 20 studies reported that women who were overweight (2.1-fold), obese (3.6-fold) or severely obese (8.6-fold) had a significantly higher risk of developing diabetes compared with normal-weight pregnant women.[4] Furthermore, the Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) study reported that a high maternal BMI is associated with an increased risk of adverse outcomes, independent of glycaemic status.[21] Factors such as advanced maternal age, high rates of diabetes and obesity have contributed to increasing rates of GDM.[3] In 2021, the International Diabetes Federation (IDF) estimated that GDM affected 80.5% of live births, while the prevalence of GDM is estimated to be 14.1% in Africa.[1] Studies have shown that physical activity and weight loss prior to conception significantly reduced the risk of developing GDM.[22,23] This emphasises the importance of preconception health for women of reproductive age who are either overweight or obese. Initiatives to encourage weight loss prior to pregnancy and to maintain appropriate gestational weight gain to reduce the risk of developing GDM and subsequent adverse outcomes are recommended.
The increased risk of CS before labour onset in women who weigh more than 80 kg compared with women who weigh less than 80 kg, and the reduced likelihood of spontaneous labour onset in women who weigh more than 120 kg, further demonstrates the negative impact of maternal weight on obstetric outcomes. Abdominal operative delivery in women with obesity is known to present significant problems such as anaesthetic difficulties, infections and greater blood loss, which pose a risk to both the mother and neonate.'[24] Brost et al.[8]reported that even after controlling for confounders, any increase in maternal weight and BMI before and during pregnancy was associated with an increased risk of CS. They reported that for each incremental BMI unit (1 kg/ m2) increase, there was an approximate 7.8% rise in the likelihood of CS. This complication is thought to be due to an increase in pelvic soft tissue, resulting in the narrowing of the birth canal, leading to difficulty in delivery.[24] A study conducted in Norway reported that European/Central Asian women who were overweight or obese were at an increased risk of elective CS compared with Norwegian women without overweight and obesity, while sub-Saharan African women who were overweight or obese had the highest risk for emergency CS compared with normal-weight women from Norway.[7] A study by Wolfe et al.[10]reported that calculating maternal BMI offers no advantage over simply using maternal weight in the initial risk assessment of outcomes related to maternal weight. This practice should be considered for risk assessment of pregnant women instead of BMI, especially in busy, resource-limited settings.
Increased rates of low Apgar scores in women weighing more than 120 kg compared with women weighing between 80 and 120 kg are consistent with studies that showed negative effects of higher maternal weight and BMI on neonatal outcomes. There is evidence that the 5-minute Apgar score is a good predictor and indicator of infant survival and low Apgar scores at either 1, 5 or 10 minutes are associated with long-term neurological disabilities in infants.[25] A study conducted in Pakistan reported that increasing maternal BMI was strongly associated with low Apgar scores at birth and NICU admissions. [9] Another study conducted in Germany found that women with obesity had a higher percentage of giving birth to neonates with a low Apgar score at 1 minute; however, no differences in Apgar scores were observed at 5 and 10 minutes among different BMI groups.[26] Since evidence has shown that Apgar scores are crucial indicators of neonatal and subsequent infant outcomes, knowledge of risk factors, especially modifiable risk factors such as maternal weight, that are associated with a low Apgar score, is important in reducing associated neonatal adverse outcomes.
The relationship between obesity and diabetes and their effect on pregnancy outcomes has been established. Globally, non-communicable diseases such as diabetes and obesity are negatively associated with maternal and perinatal health. A study by Rosenberg et al.[27]suggested that diabetes and excess maternal weight can adversely affect maternal and delivery outcomes through two different pathways. The first pathway involves the contribution of diabetes and excess weight to the development of pre-eclampsia, which can trigger preterm delivery and CS. The second pathway pertains to the increased risk of macrosomia in neonates born to women with either diabetes, obesity or both. Babies with macrosomia often contribute to labour dystocia, which can result in an increased prompt for CS 4delivery.
The strength of our study lies in its ability to demonstrate the negative effect of maternal diabetes and obesity on obstetric and perinatal outcomes.
The limitations of the study include a small sample size and restriction to a specific ethnic group, namely black African ethnicity, which restrict the generalisability of our findings. A larger study that includes multiple ethnicities is needed to further validate our results. Also, the study had limited maternal BMI data due to missing height measurements. Therefore, we reported on both BMI and weight, as weight is easily obtained. Nevertheless, despite the low number of BMI measurements, we still observed the effects of both BMI and weight on obstetric and perinatal outcomes. Lastly, hypertension was not categorised into chronic, gestational or preeclampsia.
Conclusion
This study showed that pregestational diabetes is associated with high rates of preterm birth and obesity is associated with the development of GDM, high rates of CS and low Apgar scores at 5 minutes. Adequate glycaemic control and weight loss prior to pregnancy, as well as appropriate gestational weight gain, have been shown to reduce the risk of adverse pregnancy outcomes. Therefore, clinicians should prioritise pre-pregnancy glycaemic control and weight optimisation. Additionally, pregnant women with DIP should be advised about the importance of glycaemic control to reduce adverse pregnancy outcomes. Pregnant women with obesity should be counselled on the importance of appropriate gestational weight gain to prevent the development of GDM. Good antenatal care and education are essential to reduce adverse pregnancy outcomes for mothers with diabetes and obesity.
Declaration. None
Acknowledgements. None
Author contributions. None
Funding. None
Conflicts of interest. None.
References
1. International Diabetes Federation. IDF Diabetes Atlas 2021 | IDF Diabetes Atlas 'Internet]. Available from: https://diabetesatlas.org/atlas/tenth-edition (accessed 14 March 2022). [ Links ]
2. Dias S, Pheiffer C, Rheeder P, Adam S. Screening and diagnosis of gestational diabetes mellitus in South Africa: What we know so far. S Afr Med J 2019;109(7):457-462. https://hdl.handle.net/10520/EJC-16b9469d5d [ Links ]
3. Schaefer-Graf U, Napoli A, Nolan CJ. Diabetes in pregnancy: a new decade of challenges ahead. Diabetologia 2018;61(5):1012-1021. https://doi.org/10.1007/s00125-018-4545-y [ Links ]
4. Chu SY, Callaghan WM, Kim SY, et al. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care 2007;30(8):2070-2076. https://doi.org/10.2337/dc06-2559a [ Links ]
5. Ehrenberg HM, Durnwald CP, Catalano P, Mercer BM. The influence of obesity and diabetes on the risk of cesarean delivery. Am J Obstet Gynecol 2004;191(3):969-974. https://doi.org/10.1016/j.ajog.2004.06.057 [ Links ]
6. Nglazi MD, Ataguba JEO. Overweight and obesity in non-pregnant women of childbearing age in South Africa: subgroup regression analyses of survey data from 1998 to 2017. BMC Public Health 2022;22(1):395. https://doi.org/10.1186/s12889-022-12601-6 [ Links ]
7. Jatta F, Sundby J, Vangen S, Lindskog BV, S0rbye IK, Owe KM. Association between Maternal Origin, Pre-Pregnancy Body Mass Index and Caesarean Section: A Nation-Wide Registry Study. Int J Environ Res Public Health 2021;18(11):5938. https://doi.org/10.3390/ijerph18115938 [ Links ]
8. Brost BC, Goldenberg RL, Mercer BM, et al. The Preterm Prediction Study: Association of cesarean delivery with increases in maternal weight and body mass index. Am J Obstet Gynecol 1997;177(2):333-341. https://doi.org/10.1016/S0002-9378(97)70195-9 [ Links ]
9. Parveen T, Hameed F, Kahn J. The impact of maternal obesity on neonatal Apgar score and neonatal intensive care unit admissions in a tertiary care hospital. Pak J Surj 2018;34(2):161-165. [ Links ]
10. Wolfe HM, Zador IE, Gross TL, Martier SS, Sokol RJ. The clinical utility of maternal body mass index in pregnancy. Am J Obstet Gynecol 1991;164(5, Part 1):1306-1310. https://doi.org/10.1016/0002-9378(91)90705-V [ Links ]
11. The 2012 SEMDSA Guideline for the Management of Type 2 Diabetes (Revised). JEMDSA 2012;17(2):S1-95. https://hdl.handle.net/10520/EJC119949 [ Links ]
12. Benhalima K, Crombrugge PV, Moyson C, et al. Risk factor screening for gestational diabetes mellitus based on the 2013 WHO criteria. Eur J Endocrinol 2019;180(6):353-363. https://doi.org/10.1530/EJE-19-0117 [ Links ]
13. International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010;33(3):676-682. [ Links ]
14. ACOG. Weight Gain During Pregnancy. Available from: https://www.acog.org/en/clinical/clinical-guidance/committee-opinion/articles/2013/01/weight-gain-during-pregnancy (accessed 2 August 2022). [ Links ]
15. Van Zyl H, Levitt NS. Pregnancy outcome in patients with pregestational and gestational diabetes attending Groote Schuur Hospital, Cape Town, South Africa. S Afr Med J 2018;108(9):772-776. https://hdl.handle.net/10520/EJC-109b8c04e6 [ Links ]
16. Gualdani E, Di Cianni G, Seghieri M, Francesconi P, Seghieri G. Pregnancy outcomes and maternal characteristics in women with pregestational and gestational diabetes: a retrospective study on 206,917 singleton live births. Acta Diabetologica 2021;1-8. https://doi.org/10.1007/s00592-021-01710-0 [ Links ]
17. Peticca P, Keely EJ, Walker MC, Yang Q, Bottomley J. Pregnancy outcomes in diabetes subtypes: How do they compare? A Province-based Study of Ontario, 2005-2006. J Obstet Gynaecol Can 2009;31(6):487-496. https://doi.org/10.1016/S1701-2163(16)34210-4 [ Links ]
18. Malaza N, Masete M, Adam S, Dias S, Nyawo T, Pheiffer C. A systematic review to compare adverse pregnancy outcomes in women with pregestational diabetes and gestational diabetes. Int J Environ Res Public Health 2022;19(17):10846. https://doi.org/10.3390/ijerph191710846 [ Links ]
19. Graves CR. Antepartum fetal surveillance and timing of delivery in the pregnancy complicated by diabetes mellitus. Clin Obstet Gynecol 2007;50(4):1007-1013. doi:10.1097/GRF.0b013e31815a63cc [ Links ]
20. Biesty LM, Egan AM, Dunne F, et al. Planned birth at or near term for improving health outcomes for pregnant women with pre-existing diabetes and their infants. Cochrane Database of Systematic Reviews 2018. Available from: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD012948/full (accessed 28 June 2022). [ Links ]
21. Group HSCR. Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) Study: associations with maternal body mass index. BJOG 2010;117(5):575-584. https://doi.org/10.1111/j.1471-0528.2009.02486.x [ Links ]
22. Schummers L, Hutcheon JA, Bodnar LM, Lieberman E, Himes KP. Risk of adverse pregnancy outcomes by prepregnancy body mass index: a population-based study to inform prepregnancy weight loss counseling. Obstetrics and Gynecology 2015;125(1):133. doi: 10.1097/AOG.0000000000000591 [ Links ]
23. Tobias DK, Zhang C, Van Dam RM, Bowers K, Hu FB. Physical activity before and during pregnancy and risk of gestational diabetes mellitus: a meta-analysis. Diabetes Care 2011;34(1):223-229. https://doi.org/10.2337/dc10-1368 [ Links ]
24. Creanga AA, Catalano PM, Bateman BT. Obesity in Pregnancy. N Engl J Med 2022;387(3):248-259. https://doi.org/10.1056/NEJMra1801040 [ Links ]
25. Straube S, Voigt M, Jorch G, Hallier E, Briese V, Borchardt U. Investigation of the association of Apgar score with maternal socio-economic and biological factors: an analysis of German perinatal statistics. Arch Gynecol Obstet 2010;282(2):135-141. https://doi.org/10.1007/s00404-009-1217-7 [ Links ]
26. Stepan H, Scheithauer S, Dornhöfer N, Krämer T, Faber R. Obesity as an obstetric risk factor: Does it matter in a perinatal center? Obesity 2006;14(5):770-773. https://doi.org/10.1038/oby.2006.88 [ Links ]
27. Rosenberg TJ, Garbers S, Lipkind H, Chiasson MA. Maternal obesity and diabetes as risk factors for adverse pregnancy outcomes: Differences among 4 racial/ethnic groups. Am J Public Health 2005;95(9):1545-1551. https://doi.org/10.2105/AJPH.2005.065680 [ Links ]
 Correspondence:
Correspondence:
S Adam
sumaiya.adam@up.ac.za
Accepted 29 September 2023














