Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Obstetrics and Gynaecology
versão On-line ISSN 2305-8862
versão impressa ISSN 0038-2329
SAJOG vol.29 no.1 Cape Town Mai. 2023
http://dx.doi.org/10.7196/SAJOG.2023.v29i1.2104
RESEARCH
The incidence of clinically impactful postoperative nausea and vomiting after spinal anaesthesia for caesarean section in an academic hospital
C OgunjioforI; H J MoutlanaII; LT LushikuIII; Ρ Motshabi ChakaneIV
IMBBS; Department of Anaesthesiology, Chris Hani Baragwanath Academic Hospital and School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIMMed (Anaes) ; Department of Anaesthesiology, Chris Hani Baragwanath Academic Hospital and School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIIMMed (Anaes); Department of Anaesthesiology, Chris Hani Baragwanath Academic Hospital and School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IVFCA (SA), PhD; Department of Anaesthesiology, Chris Hani Baragwanath Academic Hospital and School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
BACKGROUND. Postoperative nausea and vomiting (PONV) are common side-effects following administration of spinal anaesthesia for caesarean section (CS). PONV is reportedly perceived to be more distressing than pain by patients, which necessitates assessment of its incidence to ensure that it is not undertreated and that effective measures are undertaken to address it.
OBJECTIVES. To study the incidence of PONV and associated factors in patients delivering via CS.
METHODS. A total of 308 healthy parturients undergoing CS under spinal anaesthesia were recruited. This was a single-centre prospective observational study conducted at an academic hospital. The institution standard of practice for spinal anaesthesia for CS was employed and consisted of injecting 1.8 - 2.0 mL of hyperbaric bupivacaine 0.5% solution plus 10 μg fentanyl at the L3/L4 interspace after preloading the patient with intravenous fluid 10 - 15 mL/kg. Phenylephrine boluses were used in managing any spinal-induced hypotension according to standard protocol. The demographic data and patient characteristics were recorded. Complaints of PONV were recorded postoperatively in the recovery room and 3 hours after the procedure. The clinical significance of PONV was assessed using the PONV impact scale.
RESULTS. Of the 308 enrolled patients, 295 (95.8%) were black, 9 (2.9%) coloured and 4 (1.3%) white. The overall incidences of nausea and vomiting were 2.6% and 9.7%, respectively, with all episodes occurring during the first 3 hours after the procedure. The overall incidence of PONV was 10.1%. The odds of experiencing PONV for patients with increased birth weight, lowest blood pressure and Apfel score <3 were 1.0 (95% confidence interval (CI) 0.99 - 1.00), 1.01 (95% CI 0.98 - 1.04) and 0.35 (95% CI 0.11 - 1.12), respectively. There was no report of clinically significant PONV.
CONCLUSION. The overall incidence of PONV in this study was 10.1%, and there was no clinically significant PONV.
Postoperative nausea and vomiting (PONV) is a major and unwanted significant complication that occurs after general and regional anaesthesia. It is said to be more common in patients undergoing general anaesthesia.[1] Many studies have shown that the incidence of PONV after caesarean section (CS) ranges between 20% and 30%. Incidences as high as 80% have been reported in patients with severe risk factors,[2] including presence of Apfel's risk factors (Table 1), patient factors and surgical factors.[3-9]
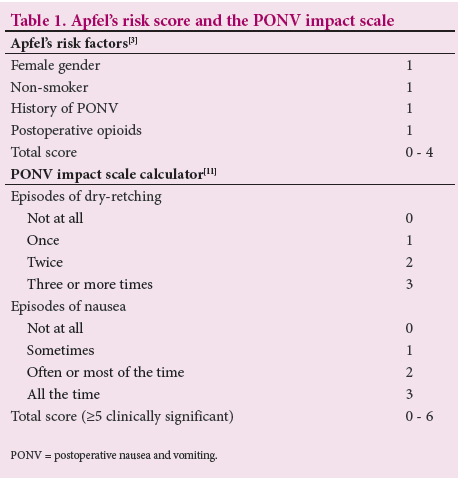
Clinically important PONV refers to PONV that becomes a significant postoperative complication.[10] A score to rate clinically important PONV from a patient's perspective was developed and validated by Wengritzsky et al.[10] and named the PONV intensity scale. The practicality of the PONV intensity scale led to the development and validation of a simplified score by Myles and Wengritzky,[11] named the PONV impact scale. It consists of two questions directed to the patient (Table 1). A score of >5 from the two questions defines clinically significant PONV.[11]
It has been reported that patients perceive PONV to be more distressing than pain, which necessitates assessment of its incidence to ensure that it is not undertreated and that effective measures are undertaken to address it.[12] A study by Magni et al.[13] reported an incidence of intraoperative nausea and vomiting (IONV) of 33% (95% confidence interval 28 - 40) in healthy term pregnant patients in South Africa (SA).
This subject has not been explored at our institution. The objective of the present study was therefore to determine the incidence of PONV after spinal anaesthesia for CS. The study further aimed to assess the clinical significance of PONV by utilising the PONV impact scale (Table 1).
This subject has not been explored at our institution. The objective of the present study was therefore to determine the incidence of PONV after spinal anaesthesia for CS. The study further aimed to assess the clinical significance of PONV by utilising the PONV impact scale (Table 1).
Methods
A prospective observational study was performed at Chris Hani Baragwanath Academic Hospital (CHBAH), Johannesburg, SA. Ethics approval was obtained from the Human Research Ethics Committee of the University of the Witwatersrand (ref. no. M200549). A convenience sampling method was used in the selection of term parturients who were booked for either elective or emergency CS under spinal anaesthesia during the 3-month period of data collection.
Data were collected at two time intervals: in the recovery room (time zero) and at the end of the first 3 postoperative hours. Demographics, patient characteristics, and surgical and anaesthetic data were obtained and recorded. PONV events were recorded on the validated simplified PONV impact scale,[11] used with permission from the authors.
Eligibility
All parturients aged >18 years undergoing CS under spinal anaesthesia were considered eligible for the study. Written consent was obtained from all participants at the time of data collection.
Statistical analysis
Calculation of the sample size of 308 was based on a clinical estimate of an incidence of nausea and/or vomiting of 25% overall, with an absolute accuracy of ~6%. Categorical variables were summarised with frequency and percentage distributions. Continuous variables were summarised using means and standard deviations or medians and interquartile ranges (IQRs). Logistic regression analysis was used to determine the univariable association of independent fixed patient factors with the incidence of PONV as depicted in patient characteristics by PONV status. A multivariable logistic regression analysis was undertaken with factors that had a p-value >0.1 to determine unadjusted associations between patient characteristics and PONV; p-values <0.05 were accepted as significant.
Results
A total of 308 parturients were recruited (Table 2). The majority were young and overweight, with a median (IQR) age of 29 (24 - 34) years and a median body mass index of 30.4 (26.3 - 34.4).
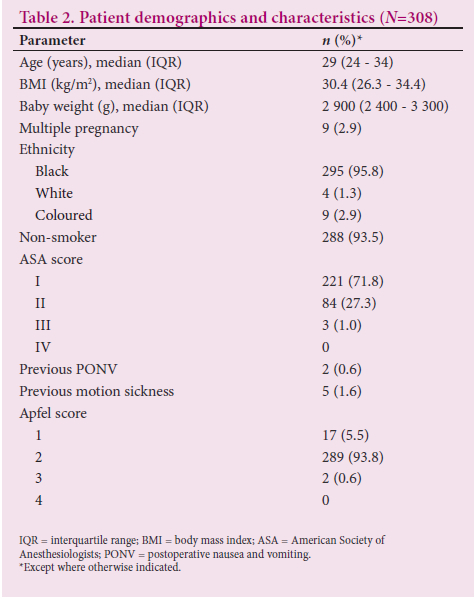
The median (IQR) preoperative fasting time was 16 (12 - 24) hours, with the majority of patients (n=303; 98.4%) having had no oral intake for 2 hours before CS (Table 3). All patients were given prophylactic antiemetic treatment. Only 2.9% were given antiemetics intraoperatively. The incidence of PONV was 10.1% (n=31). However, there was no report of clinically significant PONV as evidenced by the PONV impact score of >5 .
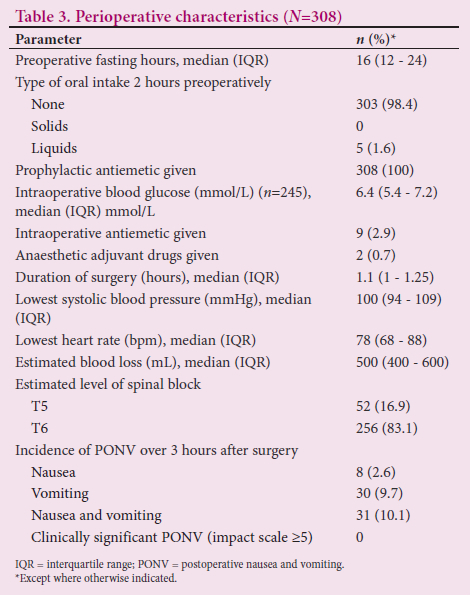
Two hundred and seventy-eight of the study population (90.3%) experienced no impactful PONV postoperatively, despite 265 parturients having an Apfel score of 2 (Table 4). The remaining patients (~10%) recorded between 1 and 3 on the Apfel score and PONV impact scale. The majority of characteristics of parturients who experienced PONV and those who did not were comparable (Table 5). Previous motion sickness and previous PONV predisposed patients to PONV. The proportions of white and coloured patients who experienced PONV were higher than the proportions who did not (n=2/31; 6.5% and n=2/277; 0.7%, and n=4/31; 12.9% and n=5/277;1.8%, respectively). The proportion of smokers who experienced PONV was four times higher than that of smokers who did not. There was no relationship between perioperative factors and PONV (Table 6).
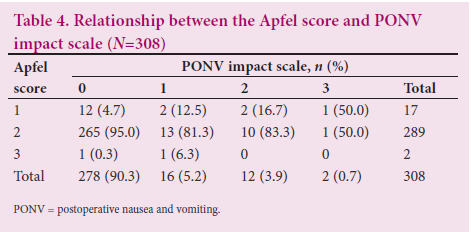
Smoking history, previous motion sickness and coloured ethnicity were univariably associated with PONV (Table 7). In a multivariable regression model, ethnicity (white and coloured) was independently associated with PONV.
Discussion
This study showed an overall PONV incidence of ~10% after spinal anaesthesia for CS at CHBAH in a population that was predominantly black (95.8%). There were also significantly lower incidences of nausea and vomiting of 2.6% and 9.7%, respectively. Although we had a 10% incidence of PONV, there was no report of clinically significant PONV using the adapted validated PONV impact scale. In 2016, in SA, Magni et al.m reported a 33% incidence of IONV after spinal anaesthesia for CS. Previous reports have suggested that the incidence of PONV is low among blacks. In the present study, black parturients had a low incidence of PONV (8.5%), which is in keeping with reports of black Africans having a low incidence of IONV and PONV compared with non-black African groups.[12,13] A study by Alli et al.[14] in a similar population in Johannesburg also concluded that blacks were at lower risk of PONV than other ethnic groups.
A history of previous PONV was not identified as a risk factor for PONV in the present study, and this could be due to the low proportion (0.6%) of parturients who had such a history. However, parturients with previous motion sickness had an increased risk of developing PONV. Previous studies identified a relationship between a history of motion sickness/PONV and the risk of PONV.[3] Susceptibility to PONV in individuals with motion sickness was also attributed to potential genetic markers that have been shown in first-degree relatives of those with a history of PONV.[15]
The present study identified higher intraoperative blood glucose levels in the group that did not experience PONV compared with the group that did, with no statistical significance. Glucose levels remained within the normal range in spite of the fasting guidelines of no oral intake for 2 hours prior to surgery. Rajan et al.[16] and Hausel et al.[17] reported an increased incidence of PONV in patients who did not receive a carbohydrate load 2 hours before surgery. They further reported that preoperative fasting with a non-particulate carbohydrate load 2 hours preoperatively reduced insulin resistance and surgical stress. There are reports that administration of intravenous dextrose may prevent PONV because it reduces insulin resistance and decreases gastric acid secretion, which would have contributed to PONV.[18,19]
In the present study, in contrast to Apfel et al.,[20] smoking was significantly associated with PONV. The association of smoking with PONV is attributed to its lowering effect on oestrogen levels, which in parturients causes smooth-muscle relaxation, increased gastrin secretion, decreased gastrointestinal motility and lower oesophageal sphincter tone,[21] and its influence on the activity of hepatocellular enzymes, resulting in identification and increased breakdown of emetogenic substances.[22]
Although all the parturients in the present study received antiemetic prophylaxis, a few (2.9%) nevertheless experienced intraoperative vomiting and were managed with a further antiemetic. The intraoperative vomiting could be due to unavoidable manipulation or exteriorisation of the uterus,[8] as notable haemodynamic risk factors such as hypotension and bradycardia secondary to sympathectomy and increased vagal tone from spinal anaesthesia were adequately managed.
We found no statistically significant difference in the duration of surgery in the PONV and no-PONV groups, which could be due to the overall short duration of CS. However, it has been reported that longer duration of surgery has been identified as an important predictor of PONV.[7]
Using the PONV impact scale,[11] the present study showed no case of clinically significant PONV. Importantly, in this study there was routine use of preoperative antiemetic agents in a low-risk group of patients. The patient population was at low risk for PONV according to previous studies.
Study limitations
A limitation of the study is that because of the location of the hospital, most patients were black Africans, so the findings may not be applicable to centres that manage a more balanced mixture of races. Also, because the study was limited to the first 3 hours after surgery, we may not have been able to measure the overall incidence of PONV after spinal anaesthesia for CS. An extension of the postoperative hours would be of interest in a future study of PONV after spinal anaesthesia for CS.
Conclusion
The overall incidence of PONV in this study was 10%, and there was no clinically significant PONV. This relatively low incidence could be due to recent advances in medical treatment and anaesthetic technique, which have played huge roles in the study outcome.
Declaration. The research for this study was done in partial fulfilment of the requirements for CO's MMed (Anaes) degree at the University of the Witwatersrand.
Acknowledgements. The authors acknowledge the immense contribution of Prof. P Motshabi Chakane in analysing the study data.
Author contributions. All authors contributed equally to the manuscript.
Funding. None.
Conflicts of interest. None.
References
1. Borgeat A, Ekatodramis G, Schenker CA. Postoperative nausea and vomiting in regional anesthesia: A review. Anesthesiology 2003;98(2):530-547. https://doi.org/10.0000542-200302000-00036 [ Links ]
2. Zhang D, Shen Z, You J, Zhu X, Tang QF. Effect of ondansetron in preventing postoperative nausea and vomiting under different conditions of general anesthesia: A preliminary, randomised, controlled study. Ups J Med Sci 2013;118(2):87-90. https://doi.org/10.3109/03009734.2013.768315 [ Links ]
3. Apfel CC, Greim CA, Haubitz I, et al. A risk score to predict the probability ofpostoperative vomiting in adults. Acta Anaesthesiol Scand 1998;42(5):495-501. https://doi.org/10.1111/j.1399-6576.1998.tb05157.x [ Links ]
4. Sinclair DR, Chung F, Mezei G. Can postoperative nausea and vomiting be predicted? Anesthesiology 1999;91(1):109-118. https://doi.org/10.1097/00000542-199907000-00018 [ Links ]
5. Standl T, Eckert S, Schulteam Esch J. Postoperative complaints after spinal and thiopentone-isoflurane anaesthesia in patients undergoing orthopaedic surgery: Spinal versus general anaesthesia. Acta Anaesthesiol Scand 1996;40(2):222-226. https://doi.org/10.1111/j.1399-6576.1996.tb04423.x [ Links ]
6. Kalso E. Effects of intrathecal morphine, injected with bupivacaine, on pain after orthopaedic surgery. Br J Anaesth 1983;55(5):415-422. https://doi.org/10.1093/bja/55.5.415 [ Links ]
7. Koivuranta M, Laara E, Snare L, Alahuhta S. A survey of postoperative nausea and vomiting. Anaesthesia 1997;52(5):443-449. https://doi.org/10.1111/j.1365-2044.1997.117-az0113.x [ Links ]
8. Jelting Y, Klein C, Harlander T, Eberhart L, Roewer N, Kranke P. Preventing nausea and vomiting in women undergoing regional anesthesia for cesarean section: Challenges and solutions. Local Reg Anesth 2017;10:83-90. https://doi.org/10.2147/lra.S111459 [ Links ]
9. Bellville JW, Bross ID, Howland WS. Postoperative nausea and vomiting. IV. Factors related to postoperative nausea and vomiting. Anesthesiology 1960;21:186-193. https://doi.org/10.1097/00000542-196003000-00009 [ Links ]
10. Wengritzky R, Mettho T, Myles PS, Burke J, Kakos A. Development and validation of a postoperative nausea and vomiting intensity scale. Br J Anaesth 2010;104(2):158-166. https://doi.org/10.1093/bja/aep370 [ Links ]
11. Myles PS, Wengritzky R. Simplified postoperative nausea and vomiting impact scale for audit and post-discharge review. Br J Anaesth 2012;108(3):423-429. https://doi.org/10.1093/bja/aer505 [ Links ]
12. Rodseth RN, Gopalan PD, Cassimjee HM, Goga S. Reduced incidence of postoperative nausea and vomiting in black South Africans and its utility for a modified risk scoring system. Anesth Analg 2010;110(6):1591-1594. https://doi.org/10.1213/ANE.0b013e3181da9005 [ Links ]
13. Magni BJ, Dyer RA, van Dyk D, van Nugteren J. Incidence of intraoperative nausea and vomiting during spinal anaesthesia for caesarean section in two Cape Town state hospitals. South Afr J Anaesth Analg 2016;22(5):131-134. https://doi.org/10.1080/22201181.2016.1215784 [ Links ]
14. Alli A, Omar S, Tsang S, Naik BI. The effect of ethnicity on the incidence of postoperative nausea and vomiting in moderate to high risk patients undergoing general anaesthesia in South Africa: A controlled observational study. Middle East J Anaesthesiol 2017;24(2):119-129. [ Links ]
15. Apfel CC, Heidrich FM, et al. Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br J Anaesth 2012;109(5):742-753. https://doi.org/10.1093/bja/aes276 [ Links ]
16. Rajan S, Rahman A, Kumar L. Preoperative oral carbohydrate loading: Effects on intraoperative blood glucose levels, post-operative nausea and vomiting, and intensive care unit stay. J Anaesthesiol Clin Pharmacol 2021;37(4):622-627. https://doi.org/10.4103/joacp.JOACP_382_19 [ Links ]
17. Hausel J, Nygren J, Thorell A, Lagerkranser M, Ljungqvist O. Randomised clinical trial of the effects of oral preoperative carbohydrates on postoperative nausea and vomiting after laparoscopic cholecystectomy. Br J Surg 2005;92(4):415-421. https://doi.org/10.1002/bjs.4901 [ Links ]
18. Yokoyama C, Mihara T, Kashiwagi S, Koga M, Goto T. Effects of intravenous dextrose on preventing postoperative nausea and vomiting: A systematic review and meta-analysis with trial sequential analysis. PLoS ONE 2020;15(4):e0231958. https://doi.org/10.1371/journal.pone.0231958 [ Links ]
19. Perrone F, Da-Silva-Filho AC, Adôrno IF, et al. Effects of preoperative feeding with a whey protein plus carbohydrate drink on the acute phase response and insulin resistance: A randomised trial. Nutr J 2011;10:66. https://doi.org/10.1186/1475-2891-10-66 [ Links ]
20. Apfel CC, Laara E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: Conclusions from cross-validations between two centers. Anesthesiology 1999;91(3):693-700. https://doi.org/10.1097/00000542-199909000-00022 [ Links ]
21. Jabalameli M, Honarmand A, Safavi M, Chitsaz M. Treatment of postoperative nausea and vomiting after spinal anesthesia for cesarean delivery: A randomised, double-blinded comparison of midazolam, ondansetron, and a combination. Adv Biomed Res 2012;1:2. https://doi.org/10.4103/2277-9175.94424 [ Links ]
22. Jacobs NF, Veronese LR, Okano S, Hurst C, Dyer RA. The incidence of postoperative nausea and vomiting after caesarean section in patients with hyperemesis gravidarum: A retrospective cohort study. Int J Obstet Anesth 2020;44:81-89. https://doi.org/10.1016/j.ijoa.2020.07.003 [ Links ]
 Correspondence:
Correspondence:
C Ogunjiofor
asinobi2002@yahoo.com
Accepted 13 March 2023














