Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Obstetrics and Gynaecology
versión On-line ISSN 2305-8862
versión impresa ISSN 0038-2329
SAJOG vol.28 no.2 Cape Town dic. 2022
http://dx.doi.org/10.7196/sajog.2022.v28i2.2075
RESEARCH
An assessment of mismatch repair deficiency in ovarian tumours at a public hospital in Johannesburg, South Africa
S R de KlerkI; R WadeeII
IBSc Hons; Department of Anatomical Pathology, National Health Laboratory Service, Johannesburg, South Africa
IIMMed (Anat Path), OrcID: 0000-0002-5981-4450; Department of Anatomical Pathology, School of Pathology, Faculty of Health Sciences, University of the Witwatersrand and National Health Laboratory Service, Johannesburg, South Africa
ABSTRACT
BACKGROUND: Epithelial ovarian carcinomas (EOCs) are lethal female genital tract malignancies with high-grade serous, low-grade serous, endometrioid, clear cell, mucinous and malignant Brenner subtypes. The lifetime risk for developing ovarian carcinoma (OC) is 15% in females who have mismatch repair deficiency (MMR-d). MMR-d is associated with Lynch syndrome, a cancer predisposition condition. Patients who have MMR-d may benefit from immunotherapy. To the best of the authors' knowledge, MMR-d testing of OCs in South Africa (SA) has not been undertaken to date
OBJECTIVES: To assess the clinicopathological characteristics and mismatch repair (MMR) status of non-serous EOCs at a single institution in SA
METHODS: Following ethical clearance and application of exclusion criteria, 19 cases of non-serous EOC from the Department of Anatomical Pathology at Charlotte Maxeke Johannesburg Academic Hospital were retrieved and assessed. Four immunohistochemical markers (MLH1, MSH2, MSH6 and PMS2) were used to evaluate MMR status
RESULTS: Most tumours were early-stage, unilateral, mucinous EOCs, without capsular breach or lymphovascular invasion (LVI). A single case of grade 1, stage I, unilateral, endometrioid EOC showed MMR-d for MLH1 and PMS2 MMR proteins. This patient had been diagnosed with endometrioid endometrial carcinoma 2 years prior to the diagnosis of OC
CONCLUSION: Our study documented a lower proportion of MMR-d OCs compared with international studies. However, our results are concordant with global studies regarding tumour subtype, laterality, grade, stage, LVI and capsular breach. Larger studies are required to estimate the true incidence of MMR-d OCs in SA and to direct effective treatment options globally
Globally, ovarian carcinoma (OC) is one of the most lethal female cancers.[1] In 2017, the age-standardised incidence rate of OC in South Africa (SA) was 2.2 per 100 000.[2] Ninety percent of OCs are epithelial OCs (EOCs), which comprise high-grade serous OC (HGSOC), low-grade serous OC (LGSOC), endometrioid OC (ENOC), clear cell OC (CCOC), mucinous OC (MOC) and malignant Brenner tumour.[3] EOC subtypes are traditionally classified into type I (HGSOC subtype) and type II tumours (LGSOC, ENOC, MOC, CCOC and malignant Brenner tumours).[4] Type I OCs are associated with early International Federation of Gynecology and Obstetrics (FIGO) stage, large tumour masses confined to one ovary, indolent behaviour and slow progression.[4] Type II OCs are aggressive, rapidly progressing tumours involving both ovaries, and are often of advanced tumour stage at presentation.[4] FIGO staging defines the extent of tumour spread and is a predictor of patient prognosis.[5]
The lifetime risk of developing OC increases from 2% in sporadic cases to 15% in women harbouring genetic or epigenetic mismatch repair (MMR) mutations.[6-9] The MMR system comprises major (mutL homolog 1/MLH1 and mutS homolog 2/MSH2) and minor (postmeiotic segregation increased 2/PMS2 and mutS homolog 6/ MSH6) heterodimeric complex partners.[9,10] MMR mutations result in genomic instability and protein dysfunction.[8,11] Subsequently, the MMR system is unable to detect and repair mutations.[8,11]
Microsatellites are short repeat DNA sequences that vary in base-pair length.[10] Owing to strand slippage, microsatellites are susceptible to error during DNA synthesis.[12,13] MMR deficiency (MMR-d) results in an accumulation of mutations in these microsatellite regions, resulting in microsatellite instability (MSI), which favours tumourigenesis.[12,13] Lynch syndrome (LS) is an autosomal dominant cancer predisposition syndrome caused by heterozygous germline MMR mutations.[8-10] LS is implicated in a range of tumours including endometrial, colorectal and ovarian tumours.[8-10] LS-associated tumours may manifest in a synchronous manner, where different primary malignancies develop <6 months apart, or metachronously, where different primary malignancies occur >6 months apart.[14] The presence of LS is suggested by screening tools such as immunohistochemical testing of MMR proteins or MSI polymerase chain reaction testing.[10] However, definitive diagnosis of LS requires germline mutational testing through sequencing.[10] MMR mutations are mainly located in MLH1, MSH2 and MSH6 proteins in OCs with MMR-d.[15,16] MMR-d is associated with non-serous EOC subtypes.[17]
The traditional management of OCs involves cytoreductive surgery followed by chemotherapy.[18] However, >80% of patients develop recurrent tumours.[18] Additionally, chemotherapy has been reported to further increase deficiency in patients with existing MMR-d systems.[19,20] As such, a potential targeted therapy that may benefit patients with MMR-d OC is an anti-PD-1 antibody such as pembrolizumab,[13,21] which targets and eliminates tumour cells through increased host T-cell activity.[10] Patients with MMR-d show increased immunogenicity resulting in accelerated elimination of tumour cells.[12,22,23] Determining the MMR status of OC patients can therefore direct treatment options for these patients. In addition, the identification of MMR-d may suggest possible LS in patients. To the best of the authors' knowledge, there has been no assessment of MMR status in OCs in SA to date. We aimed to assess the clinicopathological characteristics and the MMR status of non-serous EOCs at a single institution in SA.
Methods
Study design and sample collection
Following ethical clearance (ref. no. M200629), 19 cases were retrieved through a Systematised Nomenclature of Medicine (SNOMED) search of the National Health Laboratory Service (NHLS) database at Charlotte Maxeke Johannesburg Academic Hospital (CMJAH). We included microscopically confirmed primary non-serous EOCs in patients aged 18 - 99 years over a 6-year period. The period 2013 - 2019 was selected because our department had changed to an alternative laboratory information system, making it difficult to access cases prior to this time. MMR-d has been limited to non-serous EOCs, so serous subtypes were excluded.[8] Metastatic and borderline tumours were also excluded. The slides of each case were reviewed by an experienced histopathologist, in the absence of the pathology report. The findings were then compared with the patient's final histopathology report. Concordant results were noted in all cases. Patient histopathology reports were used to assess clinicopathological data.
Immunohistochemical staining
Immunohistochemical staining was carried out according to departmental standard operating procedures[24-27] using the following antibodies: MLH1 (Novocastra, UK), PMS2 (Agilent, Denmark), MSH2 (Cell Marque, USA) and MSH6 (Agilent, Denmark). Loss of staining in tumour nuclei while in the presence of appropriate internal controls (such as stromal cells, endothelial cells and lymphocytes) indicated protein expression loss. A case was considered MMR-d when at least one of the four MMR proteins showed loss of staining in the tumour nuclei.
Statistical analysis
Excel version 2016 (Microsoft, USA) was used to collect and analyse data. Descriptive statistics were used to investigate the relationship between MMR-d and the patient's age, tumour subtype, grade, stage, laterality, capsular breach and lymphovascular invasion (LVI).
Results
Clinicopathological data
The mean age of the patients in our cohort was 50 years (Table 1). The majority (78.9%) of tumours were unilateral, with 47.4% of tumours confined to the left ovary. Capsular breach was noted in 21.1%, while 15.8% showed LVI. In 3 cases with no ovarian capsular breach, the tumours were close to the ovarian capsule. Lymphadenectomy was performed in 4 cases, and only a single MOC case had nodal involvement by the tumour (Table 2). Over two-thirds (68.4%) of patients had stage I disease (Table 1). Non-malignant pathologies (such as salpingitis, peritonitis, endometritis, and endometriosis) were found in 12 cases (63.2%) (results not shown). In 7 patients, more than one pathological finding was present. Four patients had a history of previous cancer of the ovary, cervix or endometrium.
Epithelial OC subtypes
Overall, most malignancies were unilateral stage I tumours with no capsular breach or LVI (Table 2). MOC, ENOC, CCOC and malignant Brenner tumours were diagnosed at average ages of 47, 55, 47 and 58 years, respectively (Table 2). According to the 2020 World Health Organization classification of tumours, MOC, CCOC and malignant Brenner subtypes are not routinely pathologically graded.[28]
Mucinous OCs
MOCs accounted for 10/19 cases in our cohort (52.6%). Microscopically, 4 MOCs showed mucinous borderline tumour precursors while 2 showed mucinous cystadenoma precursors. The malignant glands were lined by mucin-rich gastrointestinal-type columnar epithelium with goblet cells (Fig. 1A). These glands were arranged as confluent masses or individually, with epithelial stratification, tufting and papillae formation. The tumour cells displayed marked cytological atypia with nuclear stratification. Areas of necrosis were evident, as was brisk mitotic activity. Overall, the expansile growth pattern was identified in MOCs, which is associated with back-to-back arrangement of glands and minimal stromal invasion.
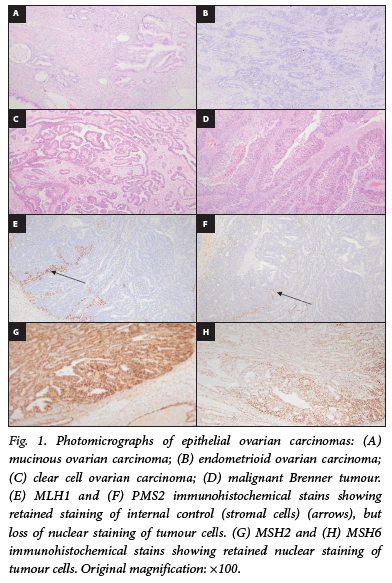
Endometrioid OCs
ENOCs accounted for 5/19 cases in our cohort (26.3%). Eighty percent of ENOCs were poorly differentiated (FIGO grade 3) tumours (Table 2). ENOCs had neoplastic glands that showed a complex architecture with back-to-back arrangement or round, oval, and tubular arrangements (Fig. 1B). The glands and papillae were lined by stratified non-mucin-containing tall columnar cells demonstrating marked cytological atypia and well-defined lumina. Areas of necrosis were evident, as was brisk mitotic activity.
Clear cell OCs
CCOCs accounted for 2/19 cases in our cohort (10.5%). CCOCs showed tubulocystic and papillary growth patterns (Fig. 1C). The papillae appeared broad, blunt and bulbous with cells displaying moderate to severe nuclear atypia. Endometriosis was observed in 1 CCOC case.
Malignant Brenner tumours
Malignant Brenner tumours accounted for 2/19 cases in our cohort (10.5%). The tumours were composed of cystic spaces lined by stratified transitional epithelium resembling invasive urothelial carcinoma (Fig. 1D). The tumour cells lining the cysts showed markedly pleomorphic cells. Areas of tumour necrosis were noted.
MMR status assessment
A single case in our cohort showed MMR-d for MLH1 and PMS2 antibodies (Fig. 1E and F), while MSH2 and MSH6 were immunohistochemically retained (Fig. 1G and H). The case was a unilateral, grade 1, stage I ENOC without capsular breach or LVI (Table 1). The ovarian laterality was not stated by the requesting clinician. This was a well-differentiated (FIGO grade 1) tumour (Table 2), and the patient had been diagnosed with an endometrioid endometrial carcinoma (EEC) 2 years prior to the diagnosis of OC.
Discussion
MMR-d is implicated in the development of multiple tumour types.[9] Immunotherapeutic agents such as pembrolizumab have shown favourable results in MMR-d tumours.[13,21] Identification of MMR-d could therefore be used in the selection of patients who may be eligible for immunotherapy. MMR-d identification may also indicate underlying LS in patients, which would allow for surveillance of a range of LS-associated metachronous and synchronous tumours in the patient and immediate family members.[10]
Our cohort comprised 19 cases owing to exclusion of serous OCs. Although serous subtypes account for ~75% of all EOCs, they are not associated with MMR-d.[3,8,19] The overall patient ages and ovarian tumour subtypes in our study correspond to those in global studies.[6,19,28-32] Infrequent capsular breach (21.1%) and LVI (15.8%) were observed in our cohort, whereas a study on EOC in Japanese women showed ovarian capsular breach and LVI in 55.8% and 17.5% of cases, respectively.[33] Studies by Matsuo and colleagues[33,34] suggest that LVI and ovarian capsular breach in early-stage EOCs may be associated with recurrence and reduced survival. LVI increases the risk of early haematogenous and lymphatic tumour spread, resulting in accelerated disease progression.[33] Ovarian capsular breach results in spillage of malignant cells in areas surrounding the ovary, and accelerates disease progression.[34] Over a third of the patients in our cohort may therefore have had an increased risk of tumour recurrence and possible mortality.
The macroscopic features of the OC subtypes in our cohort were typical of malignant OCs.[28,35] Most of our cases were unilateral with stage I disease, without capsular breach or LVI, which is concordant with a study by Shahsiah et al.[19] Early-stage (FIGO stage I/II) ovarian tumours may involve one or both ovaries or fallopian tubes with extension below the pelvic brim or primary peritoneal cancer.[5] These early-stage OCs have a 5-year survival rate of 92%, which emphasises the importance of implementing screening and effective treatment options in early-stage disease to prevent development to advanced stages where patient survival decreases significantly.[3]
Twelve patients had non-malignant pathologies (such as endometriosis) that may be associated with OCs through shared risk factors such as inflammation, which may have exacerbated the risk of developing OC.[3] Four patients had previously been diagnosed with malignancies, suggesting that a history of previous gynaecological cancer increases the risk of developing OC, possibly owing to shared risk factors or field effect. In particular, one patient had recurrence of stage III ENOC less than a year after completion of seven cycles of adjuvant chemotherapy. Our findings are concordant with research indicating that EOCs are associated with a high recurrence rate and poor prognosis,[3,36] which highlights the importance of finding novel and effective therapeutic options for EOCs to improve patient prognosis.
Studies have shown that EOCs comprise 70% HGSOC, 5% LGSOC, 10% ENOC, 10% CCOC, 3% MOC and 1% malignant Brenner subtypes[3,4] MOCs were the predominant subtype (52.6%) in our cohort, while ENOC, CCOC and malignant Brenner subtypes accounted for 26.3%, 10.5% and 10.5% of cases, respectively. The discordant incidences of various subtypes between our study and global studies may be due to our small sample size, as serous subtypes were excluded from our cohort, while other studies included the serous subtype. In addition, genetic and environmental variations may result in discordant incidences of various subtypes between regions. The increased number of MOCs reported in our cohort suggests the importance of further research to aid in improved management strategies of this specific disease subtype.
Most of the MOCs in our study had precursor lesions (such as mucinous cystadenomas and borderline tumours), which supports the concept of stepwise progression of typical type I tumours from premalignant precursors to malignant tumours.[4] The expansile invasion pattern was identified in MOCs, which is associated with an improved prognosis compared with the infiltrative pattern.[37,38] No comment can be made on precursor lesions of the ENOC and malignant Brenner subtypes, as these were not identified in our cohort. The majority (80.0%) of ENOCs were poorly differentiated (FIGO grade 3) tumours. These are aggressive and spread faster than well-differentiated tumours,[39] which is evident in our study, as 2 ENOC cases showed grade 3 features with stage II and III disease. A single CCOC showed microscopic areas of endometriosis, which may have promoted tumorigenesis.[3] Our results suggest that each EOC histological subtype in our cohort is distinct regarding clinical presentation and molecular make-up, and therefore overall prognosis and possible therapeutic options.[37]
Immunohistochemical testing identified MMR-d in a single ENOC in our study. This patient had been diagnosed with a metachronous EEC 2 years before the OC diagnosis. However, the EEC was low grade, showed minimal myometrial invasion, and was associated with endometrial hyperplasia. There was no multinodular growth and no vascular or fallopian tube invasion by the EEC to suggest metastasis to the ovary. Furthermore, the ovarian tumour was confined to one ovary. The tumours were therefore interpreted as being two separate, independent primary metachronous malignancies. We did not perform immunohistochemical testing for MMR proteins on the endometrial tumour because our study's ethical clearance did not allow for testing of additional tumours from patients apart from their ovarian tumours. The metachronous manifestation of EEC and OC in this patient and MMR-d may suggest underlying LS. Immunohistochemical testing of MMR proteins merely serves as a screening tool for LS.[10] However, diagnostic confirmation of LS requires germline mutational testing following genetic counselling.[10] Confirmation of LS will allow for surveillance of the patient and family members, for identification of additional possible tumours.
Our study is concordant with previous global MMR studies which have demonstrated that MMR-d tumours show grade 1, stage I disease of the ENOC subtype without LVI or capsular breach.[16,19,29,30,40-43] While our sample size was small and we cannot extrapolate to the general population, our study suggests that MMR-d may be associated with improved prognosis, as the MMR-d ENOC was the only case among all the ENOC tumours that was a well-differentiated (FIGO grade 1) tumour.[39]
The ENOC subtype is predominantly associated with MMR-d in OCs, as documented in our study and international studies.[17,19,20,30,42,43] Leskela et al.[17] found that MMR-d predominantly corresponded with endometriosis-associated histological subtypes and was observed in 18% of ENOC and 2% of CCOC subtypes. This finding suggests that MMR-d-associated tumours may develop as a result of endometriosis-associated pathology. No endometriotic foci were identified in our MMR-d ENOC tumour. It is possible that if any endometriotic foci existed, these had been overrun by the tumour. The importance of MMR-d testing in women with concurrent endometriosis and EOC in MMR-d susceptible populations is thus highlighted. The patient with the MMR-d OC in our cohort was 56 years of age at the time of diagnosis. We cannot draw conclusions regarding correlations between age and MMR-d owing to our small sample size. However, it has been shown that MMR-d in patients with OC is associated with a lower age of onset.[15,40,44] In order to validate this, studies of larger sample sizes are required.
MMR-d was identified in MLH1 and PMS2 MMR proteins in our study. A loss of a major heterodimeric complex partner (MLH1) results in the loss of its minor partner (PMS2). Hence both MHL1 and PMS2 showed MMR-d. Our findings are consistent with global studies that have used both immunohistochemical stains and definitive mutational testing to demonstrate that MLH1 and MSH2 mutations account for the majority of MMR-d in OCs, while MSH6 and PMS2 mutations have not been commonly identified.[9,10,15,16] The identification of the specific mutated genes in tumorigenesis is vital, as this may assist in the development of targeted novel therapy in OCs.
Global studies have documented that MMR-d is identified in 2 - 29% of patients with OC.[9,13,20,42,43,45] However, most MMR studies in OCs have been performed on small sample sizes that included all EOC subtypes. The yield of 5.3% MMR-d in non-serous EOCs, as documented in our study, was in the lower spectrum of the range in comparison with global studies, which may be a result of our small sample size. The results of the present study suggest that MMR-d is uncommon among the non-serous EOCs diagnosed at CMJAH in SA.
Study limitations
Our cohort had a small sample size, partly owing to exclusion of serous EOC subtypes and the potential omission of cases with unassigned SNOMED codes. The small sample size and expected low proportion of MMR-d prevented the generation of p-values through statistical tests. Immunohistochemistry is a screening modality, and definitive mutational assessment could not be performed because we did not have ethical clearance or funding. Additionally, any genetic tests require appropriate counselling. Some of the patient histopathology reports lacked clinical data which were not stated by the requesting clinician. In some instances, the disease stage was not assigned because the patients did not undergo the surgery required for FIGO staging purposes.
Conclusion
Our cohort of cases were predominantly unilateral, early-stage tumours without capsular breach or LVI, which is concordant with international studies.[19,33] Approximately half of our cases were of the mucinous subtype, and MMR-d was identified in a single ENOC tumour. This case suggests possible LS due to MMR-d noted immunohistochemically, in addition to the patient having had a metachronous endometrial carcinoma diagnosed 2 years previously. However, mutational analysis is required for a definitive diagnosis of LS. Our study is a step towards correcting the absence of information on MMR status in EOCs in SA. The low proportion of MMR-d in our study suggests that MMR-d is not prevalent in the EOCs diagnosed in our population. We recommend that additional larger studies be undertaken to gain a better indication of the true incidence of MMR-d and possible LS in OCs in the SA state sector. Further research is required to assess ovarian molecular alterations to facilitate the development and administration of novel therapeutic options that will reduce the disease burden in the general population.
Declaration. The research for this study was in partial fulfilment of SRdK's medical scientist internship.
Acknowledgements. Mr Maanda Mudau is thanked for his assistance with data analysis.
Author contributions. SRdK performed the immunohistochemical staining and data collection and wrote the manuscript. RW reviewed slides. RW conceived the study, assisted with the write-up, and critically assessed the manuscript.
Funding. This research was funded by the NHLS Research Trust.
Conflicts of interest. None.
References
1. Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer, 2020. http://gco.iarc.fr/today/ (accessed 4 May 2021). [ Links ]
2. South African National Cancer Registry, National Health Laboratory Service. Summary statistics of cancer diagnosed histologically in 2017: Female - all population groups combined. National Institute for Communicable Diseases, 2018. https://www.nicd.ac.za/wp-content/uploads/2020/12/NCR_2017_Final_02dec2020.pdf (accessed 13 May 2021). [ Links ]
3. Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: A review. Cancer Biol Med 2017;14(1):9-32. https://doi.org/10.20892/j.issn.2095-3941.2016.0084 [ Links ]
4. Seidman J. Advances in sub-classification of ovarian carcinomas by cell type: An update. Diagn Histopathol 2014;20(9):351-356. https://doi.org/10.1016/j.mpdhp.2014.07.005 [ Links ]
5. Prat J; FIGO Committee on Gynecologic Oncology. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynecol Obstet 2014;124(1):1-5. https://doi.org/10.1016/j.ijgo.2013.10.001 [ Links ]
6. Chui MH, Gilks CB, Cooper K, Clarke BA. Identifying Lynch syndrome in patients with ovarian carcinoma: The significance of tumor subtype. Adv Anat Pathol 2013;20(6):378-386. https://doi.org/10.1097/pap.0b013e3182a92cf8 [ Links ]
7. Strafford JC. Genetic testing for Lynch syndrome, an inherited cancer of the bowel, endometrium, and ovary. Rev Obstet Gynecol 2012;5(1):42-49. [ Links ]
8. Pal T, Permuth-Wey J, Sellers TA. A review of the clinical relevance of mismatch-repair deficiency in ovarian cancer. Cancer 2008;113(4):733-742. https://doi.org/10.1002/cncr.23601 [ Links ]
9. Richman S. Deficient mismatch repair: Read all about it (Review). Int J Oncol 2015;47(4):1189-1202. https://doi.org/10.3892/ijo.2015.3119 [ Links ]
10. Wadee R, Grayson W A potpourri of pathogenetic pathways in endometrial carcinoma with a focus on Lynch syndrome. Ann Diagn Pathol 2019;39:92-104. https://doi.org/10.1016/j.anndiagpath.2019.02.003 [ Links ]
11. Barrow E, Hill J, Evans DG. Cancer risk in Lynch syndrome. Fam Cancer 2013;12(2):229-240. https://doi.org/10.1007/s10689-013-9615-1 [ Links ]
12. Lee V, Murphy A, Le DT, Diaz LA. Mismatch repair deficiency and response to immune checkpoint blockade. Oncologist 2016;21(10):1200-1211. https://doi.org/10.1634/theoncologist.2016-0046 [ Links ]
13. Haunschild CE, Tewari KS. The current landscape of molecular profiling in the treatment of epithelial ovarian cancer. Gynecol Oncol 2021;160(1):333-345. https://doi.org/10.1016/j.ygyno.2020.09.043 [ Links ]
14. Torina TB, Hudspeth EL, Chun JM, Zaloga W, Alderink C, Abdeen Y. An unusual occurrence of multiple metachronous and synchronous primary cancers in a female patient. Case Rep Oncol Med 2020;2020:5691732. https://doi.org/10.1155/2020/5691732 [ Links ]
15. Grindedal EM, Renkonen-Sinisalo L, Vasen H, et al. Survival in women with MMR mutations and ovarian cancer: A multicentre study in Lynch syndrome kindreds. J Med Genet 2010;47(2):99-102. https://doi.org/10.1136/jmg.2009.068130 [ Links ]
16. Backes FJ, Cohn DE. Lynch syndrome. Clin Obstet Gynecol 2011;54(2):199-214. https://doi.org/10.1097/GRF.0b013e3182185a41 [ Links ]
17. Leskela S, Romero I, Cristobal E, et al. Mismatch repair deficiency in ovarian carcinoma: Frequency, causes, and consequences. Am J Surg Pathol 2020;44(5):649-656. https://doi.org/10.1097/pas.0000000000001432 [ Links ]
18. Romero I, Bast RC. Minireview: Human ovarian cancer: Biology, current management, and paths to personalizing therapy. Endocrinology 2012;153(4):1593-1602. https://doi.org/10.1210/en.2011-2123 [ Links ]
19. Shahsiah R, Salarvand S, Miri R, Ghalehtaki R, Rakhshani N. The prevalence and associated factors of microsatellite instability in ovarian epithelial cancers detected by molecular genetic studies in a sample of Iranian women. Int J Cancer Manag 2017;10(12):e11599. https://doi.org/10.5812/ijcm.11599 [ Links ]
20. Murphy MA, Wentzensen N. Frequency of mismatch repair deficiency in ovarian cancer: A systematic review: This article is a US Government work and, as such, is in the public domain of the United States of America. Int J Cancer 2011;129(8):1914-1922. https://doi.org/10.1002/ijc.25835 [ Links ]
21. Lokadasan R, James FV, Naranayan G, Prabhakaran PK. Targeted agents in epithelial ovarian cancer: Review on emerging therapies and future developments. Ecancermedicalscience 2016;10:626. https://doi.org/10.3332/ecancer.2016.626 [ Links ]
22. Lheureux S, Braunstein M, Oza AM. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA Cancer J Clin 2019;69(4):280-304. https://doi.org/10.3322/caac.21559 [ Links ]
23. Zhu J, Ke G, Bi R, Wu X. Clinicopathological and survival characteristic of mismatch repair status in ovarian clear cell carcinoma. J Surg Oncol 2020;122(3):538-546. https://doi.org/10.1002/jso.25965 [ Links ]
24. Lobandji A. Antigen retrieval on formalin-fixed paraffin-embedded tissue. Standard Operating Procedure, document no. ANA0008, version 3. Johannesburg: National Health Laboratory Service, active 23 January 2017, last review 18 January 2019. [ Links ]
25. Lobandji A. Immunohistochemistry - standard protocol for making adhesive slides. Standard Operating Procedure, document no. ANA0005, version 1. Johannesburg: National Health Laboratory Service, active 24 March 2006, last review 18 January 2019. [ Links ]
26. Lobandji A. Making antibody dilutions. Standard Operating Procedure, document no. ANA0590, version 1. Johannesburg: National Health Laboratory Service, active 22 May 2008, last review 30 April 2019. [ Links ]
27. Lobandji A. Optimisation of primary antibodies. Standard Operating Procedure, document no. ANA0010, version 1. Johannesburg: National Health Laboratory Service, active 24 March 2006, last review 18 January 2019. [ Links ]
28. World Health Organization Classification of Tumours Editorial Board. Female Genital Tumours. WHO Classification of Tumours Series, 5th ed., vol. 4. Lyon, France: International Agency for Research on Cancer, 2020. https://publications.iarc.fr/592 (accessed 4 May 2021). [ Links ]
29. Akbari MR, Zhang S, Cragun D, et al. Correlation between germline mutations in MMR genes and microsatellite instability in ovarian cancer specimens. Fam Cancer 2017;16(3):351-355. https://doi.org/10.1007/s10689-017-9973-1 [ Links ]
30. Tajima Y, Eguchi H, Chika N, et al. Prevalence and molecular characteristics of defective mismatch repair epithelial ovarian cancer in a Japanese hospital-based population. Jpn J Clin Oncol 2018;48(8):728-735. https://doi.org/10.1093/jjco/hyy081 [ Links ]
31. Lee J-H, Cragun D, Thompson Z, et al. Association between IHC and MSI testing to identify mismatch repair-deficient patients with ovarian cancer. Genet Test Mol Biomarkers 2014;18(4):229-235. https://doi.org/10.1089/gtmb.2013.0393 [ Links ]
32. Peres LC, Cushing-Haugen KL, Köbel M, et al. Invasive epithelial ovarian cancer survival by histotype and disease stage. J Natl Cancer Inst 2019;111(1):60-68. https://doi.org/10.1093/jnci/djy071 [ Links ]
33. Matsuo K, Yoshino K, Hiramatsu K, et al. Effect of lymphovascular space invasion on survival of stage I epithelial ovarian cancer. Obstet Gynecol 2014;123(5):957-965. https://doi.org/10.1097/aog.0000000000000240 [ Links ]
34. Matsuo K, Machida H, Yamagami W, et al. Intraoperative capsule rupture, postoperative chemotherapy, and survival of women with stage I epithelial ovarian cancer. Obstet Gynecol 2019;134(5):1017-1026. https://doi.org/10.1097/AOG.0000000000003507 [ Links ]
35. Garg S, Kaur A, Mohi JK, Sibia PK, Kaur N. Evaluation of IOTA simple ultrasound rules to distinguish benign and malignant ovarian tumours. J Clin Diagn Res 2017;11(8):TC06-TC09. https://doi.org/10.1097/10.7860/JCDR/2017/26790.10353 [ Links ]
36. Lheureux S, Gourley C, Vergote I, Oza AM. Epithelial ovarian cancer. Lancet 2019;393(10177):1240-1253. https://doi.org/10.1016/S0140-6736(18)32552-2 [ Links ]
37. Prat J. New insights into ovarian cancer pathology. Ann Oncol 2012;23 (Suppl 10):x111-x117. https://doi.org/10.1093/annonc/mds300 [ Links ]
38. Busca A, Nofech-Mozes S, Olkhov-Mitsel E, et al. Histological grading of ovarian mucinous carcinoma - an outcome-based analysis of traditional and novel systems. Histopathology 2020;77(1):26-34. https://doi.org/10.1111/his.14039 [ Links ]
39. Vang R, Shih I-M, Kurman RJ. Ovarian low-grade and high-grade serous carcinoma: Pathogenesis, clinicopathologic and molecular biologic features, and diagnostic problems. Adv Anat Pathol 2009;16(5):267-282. https://doi.org/10.1097/pap.0b013e3181b4fffa [ Links ]
40. Ryan NAJ, Evans DG, Green K, Crosbie EJ. Pathological features and clinical behavior of Lynch syndrome-associated ovarian cancer. Gynecol Oncol 2017;144(3):491-495. https://doi.org/10.1016/j.ygyno.2017.01.005 [ Links ]
41. Fraune C, Rosebrock J, Simon R, et al. High homogeneity of MMR deficiency in ovarian cancer. Gynecol Oncol 2020;156(3):669-675. https://doi.org/10.1016/j.ygyno.2019.12.031 [ Links ]
42. Hodan R, Kingham K, Cotter K, et al. Prevalence of Lynch syndrome in women with mismatch repair-deficient ovarian cancer. Cancer Med 2021;10(3):1012-1017. https://doi.org/10.1002/cam4.3688 [ Links ]
43. Crosbie EJ, Ryan NAJ, McVey RJ, et al. Assessment of mismatch repair deficiency in ovarian cancer. J Med Genet 2021;58(10):687-691. https://doi.org/10.1136/jmedgenet-2020-107270 [ Links ]
44. Nakamura K, Banno K, Yanokura M, et al. Features of ovarian cancer in Lynch syndrome (Review). Mol Clin Oncol 2014;2(6):909-9016. https://doi.org/10.3892/mco.2014.397 [ Links ]
45. Kim SR, Tone A, Kim RH, et al. Performance characteristics of screening strategies to identify Lynch syndrome in women with ovarian cancer. Cancer 2020;126(22):4886-4894. https://doi.org/10.1002/cncr.33144 [ Links ]
 Correspondence:
Correspondence:
S R de Klerk
sheodk@gmail.com
R Wadee
reubinawadee@gmail.com
Accepted 3 June 2022














