Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Obstetrics and Gynaecology
versão On-line ISSN 2305-8862
versão impressa ISSN 0038-2329
SAJOG vol.28 no.2 Cape Town Dez. 2022
http://dx.doi.org/10.7196/sajog.2022.v28i2.2069
RESEARCH
A proposed fetal risk scoring system for gestational diabetes to assist in optimising timing of delivery
I BhoratI; T ReddyII
IFCOG (SA), PhD; Department of Obstetrics and Gynaecology, Nelson Mandela School of Medicine, College of Health Sciences, University of KwaZulu-Natal, Durban, South Africa
IIMSc, PhD; Medical Research Council, Cape Town, South Africa
ABSTRACT
BACKGROUND: The pathophysiology of gestational diabetes, which is related to abnormal gluocose tolerance and hyperinsulinaemia, renders standard fetal monitoring models ineffective, insufficient and inappropriate, as these models revolve around detecting and prognosticating on placenta-mediated disease rather than increased metabolic rates due to hyperinsulinaemia, functional hypoxia and ischaemic trophoblastic thresholds. To improve perinatal morbidity and mortality in gestational diabetes, there is therefore a need to introduce new prognostic parameters and scoring systems
OBJECTIVES: A proposed risk scoring system has been developed, based on our previous studies, to risk-categorise patients with gestational diabetes in terms of fetal outcome in view of the fact that the pathophysiology of gestational diabetes is not recognised by standard monitoring models, which revolve around placental insufficiency rather than metabolic anomalies
METHODS: Patients with diabetes from four case-control studies were combined to form a total sample of 159 cases for validation of the risk scoring system. Univariate logistic regression analysis was used to assess the effect of individual risk factors with proposed cut-offs on adverse pregnancy outcome. The diagnostic accuracy of the total summative score was assessed by computing the area under the receiver operating characteristic (ROC) curve
RESULTS: Four potential parameters were identified to risk-categorise fetuses in a pregnancy complicated by gestational diabetes, i.e. the myocardial performance index (MPI), the E/A ratio (early diastolic filling/late diastolic filling, a marker of diastolic dysfunction), increasing fetal weight (macrosomia), and an increased amniotic fluid index. The total score, obtained by summation of the composite scores for these parameters, ranged from 0 to 11. The total score performed as an excellent predictor of adverse outcome, evidenced by an ROC area under the curve of 0.94. A cut-point of 6 on the score confers a sensitivity of 84.2% and specificity of 90.2% for predicting adverse outcome
CONCLUSION: To our knowledge, this is the first gestational diabetes scoring system proposed to predict an adverse outcome
Gestational diabetes starts with abnormal glucose tolerance in the mother causing maternal hyperglycaemia, which triggers a sequence of events resulting in fetal hyperglycaemia and subsequently fetal hyperinsulinaemia.[1,2] It is the hyperinsulinaemia in the fetus that forms the basis of the pathophysiology. Gestational diabetes is characterised by three main factors: macrosomia, an increased metabolic rate, and large vascular cross-sections. A critical and crucial finding in diabetic pregnancies is that significant acidaemia and hyperlacticaemia can occur in fetuses in the absence of hypoxaemia.[3,4] The so-called 'unexplained' stillbirths in diabetic pregnancies, especially in the third trimester, are probably due to fetal acidaemia as a consequence of an increased metabolic rate. The increased metabolic rate results in significant increases in oxidative metabolism, but this capacity is reduced in fetuses due to low pyruvate dehydrogenase activity, increasing the risk for acidosis. This pathophysiology is not recognised by standard monitoring models that revolve around placental insufficiency, which is not the problem in a pregnancy complicated by gestational diabetes. In fact, the most widely used antenatal surveillance technique in monitoring diabetic pregnancies is umbilical artery (UA) Doppler velocimetry,[5] which is a marker for placental insufficiency. Standard fetal monitoring models currently used in diabetic pregnancies are therefore inappropriate, inadequate and insufficient, as they do not answer the question of fetal compromise in gestational diabetes (in the absence of microvascular complications), rendering them ineffective.
Our research group has investigated cardiac Doppler, in particular the myocardial performance index (MPI) and E/A ratios (early diastolic filling/late diastolic filling, a marker of diastolic dysfunction), in a series of prospective studies in patients with poorly controlled diabetes, well-controlled diabetes and gestationally impaired glucose tolerance (GIGT), as well as biophysical parameters and their possible links to adverse outcomes,[6-9] after having first established gestational age-adjusted trends and reference ranges of the MPI in normal pregnancies.[10] Four parameters were identified in these studies that appeared to affect the diabetic pregnancy in terms of fetal outcome. These were elevated MPIs and decreasing E/A ratios (both cardiac Doppler parameters), macrosomia, and increasing amniotic fluid indices (AFIs). Using these data, a scoring system was proposed that could be used to risk-categorise patients with gestational diabetes in terms of fetal outcome and as a tool to guide clinicians in establishing optimal timing of delivery.
Methods
Patients with gestational diabetes from four case-control studies by our research group[6-9] were combined to form a total sample of 159 cases for validation of the risk scoring system. Patient recruitment, categorisation of degree of gestational diabetes, methodology in calculating the MPI and patient management were consistent and standard across the studies and could be combined to form a total sample for validation of the risk scoring system. All the studies were prospective cross-sectional studies of the MPI in fetuses of pregnancies complicated by gestational diabetes, conducted at the tertiary referral fetal unit at Inkosi Albert Luthuli Central Hospital in Durban, South Africa. All the patients were in the third trimester of pregnancy.
The cases included patients with poorly or suboptimally controlled gestational diabetes, well-controlled gestational diabetics and GIGT. All patients in the study groups were categorised by a combination of an oral glucose tolerance test, blood glucose profiles and measurement of glycosylated haemoglobin (HbA1c) within 4 weeks of the echocardiographic assessment.
Poor or suboptimal control was defined by suboptimal blood glucose profiles and measurement of HbA1c within 4 weeks of the echocardiographic assessment revealing poor or suboptimal blood glucose control in the study patients, with an average HbAlc level >64 mmol/mol (8%) (National Institute for Health and Care Excellence (NICE) guidelines for gestational diabetes[10]) state that women with diabetes should aim to achieve an HbAlc level of 43.3 mmol/mol (6.1%) or lower). Two studies were performed in this category of gestational diabetes: in the first study, 29 consecutive women with poorly controlled gestational diabetes on insulin in the third trimester were recruited, matched with 29 normal controls,[6] and in the second study, 44 consecutive poorly controlled women on insulin in the third trimester were recruited, matched with 44 controls.[7]
Well-controlled gestational diabetes was defined: (i) according to the World Health Organization (WHO)'s criterion of a 2-hour blood glucose level >7.8 mmol/L and <11.0 mmol/L after a 75 g oral glucose tolerance test (OGTT) in the third trimester;[11] and (ii) on review of self-monitoring blood glucose levels achieving 2-hour post-prandial levels <7 mmol/L and pre-prandial levels <5.5 mmol/L. All patients in the group with well-controlled gestational diabetes had HbA1c results <6%, reflecting good control. The well-controlled group achieved good control on medication, either metformin or insulin. Fifty-four consecutive well-controlled women were recruited in the third trimester, with matched controls.
GIGT was defined: (i) according to the WHO's criterion of a 2-hour glucose level >7.8 mmol/L and <11.0 mmol/L after a 75 g OGTT in the third trimester;[11] and (ii) on review of self-monitoring blood glucose levels achieving 2-hour post-prandial levels <7 mmol/L and pre-prandial levels <5.5 mmol/L. All patients in the GIGT group had HbA1c results <6%, reflecting good control. In this group, control was achieved by means of diet alone. Thirty-two consecutive women defined as having GIGT were recruited in the third trimester, with matched controls.
The mean gestational age at which MPI was performed in all studies was 33 - 35 weeks. All the studies were prospective and cross-sectional.
All the different gestational diabetes categories had control groups of patients who were randomly selected from the antenatal clinic and were not diabetic as defined by the WHO criterion of a 2-hour glucose level <7.8 mmoL after a 75 g OGTT.[11] The controls were matched with the gestational diabetes patients in terms of gestational age, maternal age, parity, gravidity, body mass index and past obstetric history.
All the pregnancies in all the studies, in both the study and control groups, were spontaneously conceived.
Women with a history of pregestational diabetes were excluded from recruitment, to eliminate possible microvascular complications, which could be a confounding variable, and to give conformity to the study groups. Further exclusion criteria in all studies were multiple pregnancies, congenital malformations, evidence of placenta-mediated disease and abnormal fetal heart rates (either tachycardia or bradycardia). Placenta-mediated disease was defined as either the presence of growth restriction as reflected in the abdominal circumference or fetal weight <10th percentile for gestational age with an elevated UA resistance index (RI) >90th percentile for gestational age and/or the presence of pre-eclampsia as defined by a blood pressure >140/90 mmHg with proteinurea and/or systemic endothelial damage.[12]
Fetal echocardiography using either an E8 General Electric Voluson ultrasound system (GE Medical Systems, USA) or a Siemens Antares ultrasound system (Siemens Medical Systems, USA) was performed in each patient in all studies. The four-chamber view, outflow tract view, triple-vessel view, longitudinal view of the aortic arch and colour flow mapping were used to screen for cardiac malformations. The MPI in all studies was calculated in the fetal left ventricle.[13,14] Our previous study[13] established reference intervals and trends of the MPI in normal pregnancies, and the methodology of obtaining the MPI was described in detail in that article. A cross-sectional image of the fetal thorax at the level of the four-chamber view with an apical projection of the heart was obtained. The Doppler sample was opened to 3 mm and placed in the internal leaflet of the mitral valve (MV). In this location, owing to its closeness to the aortic valve (AV), the opening and closing AV clicks were registered. The angle of insonation was always <30o. The E/A waveform was always displayed as positive flow. The Doppler gain was lowered as far as possible to clearly visualise the echoes corresponding to the opening and closing clicks of the two valves at the beginning and at the end of the MV and AV waveforms. The peak of the valve clicks was used in the measurement of the time intervals rather than the base, as it is a clearer landmark, overcoming variations in valve click width and resulting in better reproducibility.[13,15,16] The Doppler sweep velocity was set at 5 cm/s and wall motion filter at 300 Hz. The three time periods were estimated as follows: isovolumetric contraction time (ICT) from beginning of MV closure to AV opening; ejection time (ET) from AV opening to closure; and isovolumetric relaxation time (IRT) from AV closure to MV opening. The modified MPI (Mod-MPI) = (ICT + IRT)/ ET. We have previously documented high levels of inter- and intra-observer variability agreement for the MPI and its components in our article establishing reference intervals of Mod-MPI in normal pregnancies.[13]
In addition to the echocardiographic data, sonographic data including estimated fetal weights and AFIs were determined and recorded. The UA RI and pulsatility index (PI), middle cerebral artery PI and ductus venosus PI were also determined in both groups. The cerebroplacental ratio was then determined.
All the patients in all the studies were delivered according to existing standard protocols for diabetic pregnant patients at our institution, which include non-reassuring fetal cardiotocogram findings, poor biophysical profiles, or persistent elevations of UA RI/ PI. If the findings on fetal monitoring were satisfactory, delivery was delayed until 39 -40 weeks, after which labour was induced or a caesarean section performed for obstetric reasons.
Pregnancy outcomes were recorded in both groups. An abnormal outcome in our studies was defined as any one of the following: stillbirth, neonatal death, tachypnoea with pulmonary oedema, neonatal hypoglycaemia, neonatal cord pH <7.2, 5-minute Apgar score <7, polycythaemia and nucleated red blood cells >10/100 white blood cell counts (markers for hypoxia), cardiomyopathy, and neonatal intensive care unit admission.
Cardiac Doppler data were not used by clinicians in the management of the patients with diabetes.
For details of the methodology of each study, please refer to the individual published articles.[6-9]
Statistical analysis
Univariate logistic regression analysis was used to assess the effect of individual risk factors with proposed cut-offs on adverse pregnancy outcome. The diagnostic accuracy of the total summative score was assessed by computing the area under the receiver operating characteristic (ROC) curve and by examining the sensitivity and specificity at key cut-points of interest.
Ethical considerations
Ethical approval for all studies was obtained from the Biomedical Research Ethics Committee at the University of KwaZulu-Natal (ref. no. BE 228/229). All studies were performed in accordance with the Declaration of Helsinki. All the participants were adults aged >18 years, and all provided written informed consent to participate.
Results
A total of 159 fetuses of women with gestational diabetes were studied, ranging from 31 to 38 weeks' gestation. Of these cases, 32 (20%), 73 (46%) and 54 (34%) were classified as GIGT, poorly controlled gestational diabetes and well-controlled gestational diabetes, respectively. For details of the results of each study, please refer to the individual published articles.[6-9] A summary of the distribution of key risk factors in the 159 fetuses is presented in Table 1. The variables listed above were further categorised into three or four category ordinal variables. E/A ratios <0.60 were observed in 8.8% of the cases. The majority of cases had E/A ratios >0.65. MPI values exceeding a cut-off of 0.67 were observed in 21 fetuses (13.2%). Of the fetuses, 65%, 24% and 12% had AFI measurements <20 cm, 20 - 25 cm and >25 cm, respectively.
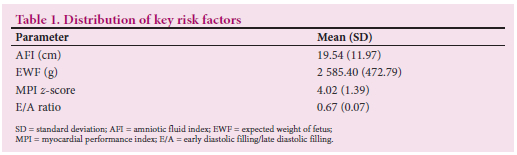
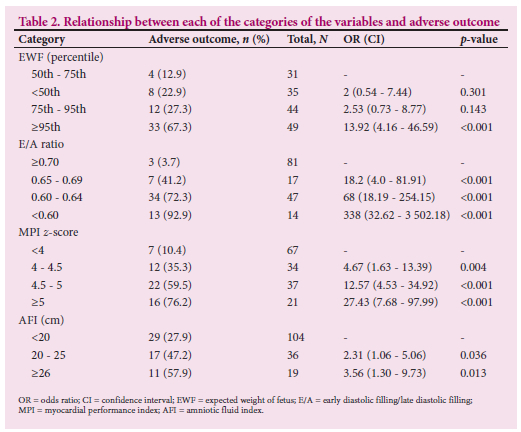
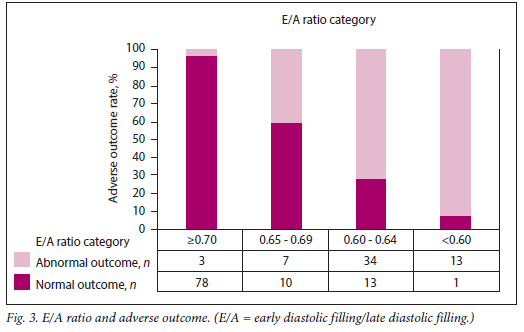
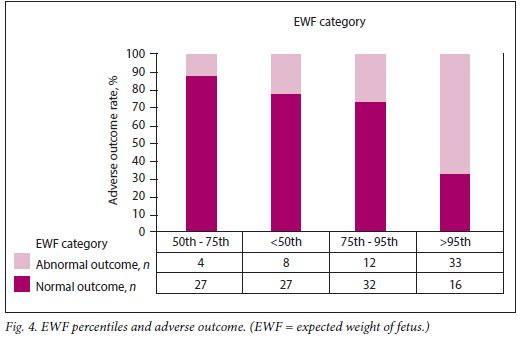
The relationship between each of the categories of these variables and adverse outcome is presented in Table 2 and Figs 1 - 4. Fetuses with an expected weight of fetus (EWF) >95th percentile had 14 times higher odds of adverse outcome compared with those with an EWF in the 50th -75th percentile range. In all E/A ratio and MPI categories of higher severity, highly statistically significant increases in the odds of adverse outcome were observed. Fetuses with an AFI between 20 cm and 25 cm were more than twice as likely to experience adverse outcomes (p=0.026) compared with fetuses with an AFI <20 cm. This risk was significantly higher for fetuses with an AFI >25 cm, where the odds of an adverse outcome were approximately three times higher than for those with an AFI <20 cm (p=0.013).
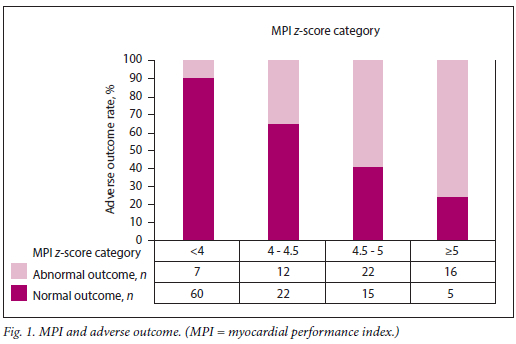
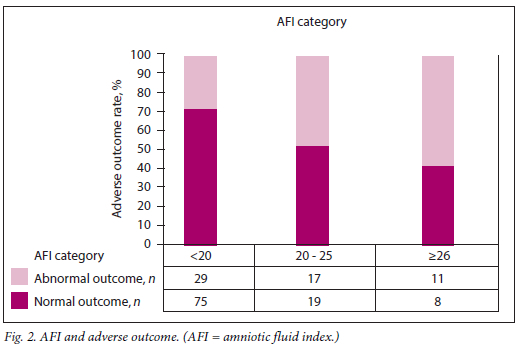
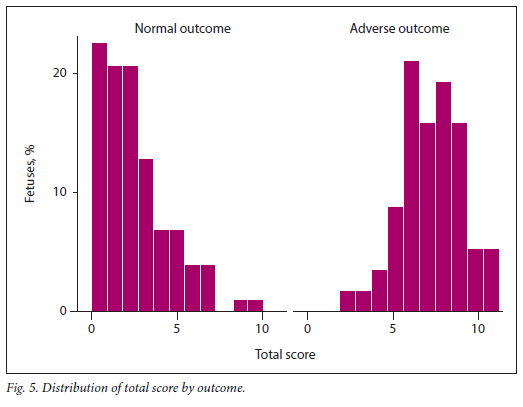
The total score, obtained by summation of the composite scores for MPI, E/A ratio, EWF and AFI, ranged from 0 to 11. The distribution of this score is presented by outcome in Fig. 5. It is clear that the majority of fetuses with normal outcomes had risk scores <5.
The total score performed as an excellent predictor of adverse outcome, evidenced by the ROC area under the curve of 0.94 (Fig. 6 and Table 3). A cut-point of 6 on the score confers a sensitivity of 84.2% and specificity of 90.2% for prediction of adverse outcome.
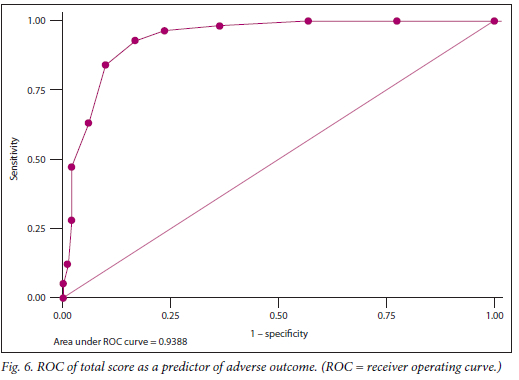
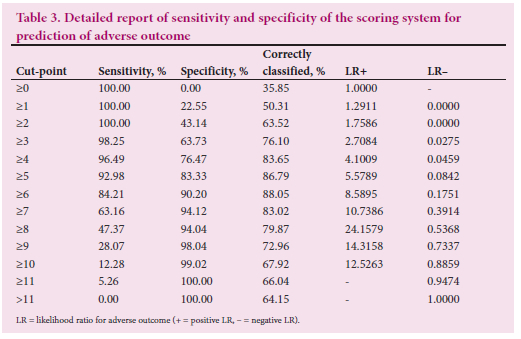
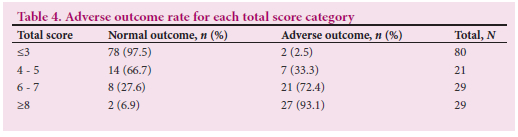
Fig. 6 demonstrates the ROC curve of the total score as a predictor of adverse outcome. The adverse outcome rates in fetuses with scores of <3, 4 - 5, 6 - 7 and >8 were 2.5%, 33.3%, 72.4% and 93.1%, respectively (Table 4).
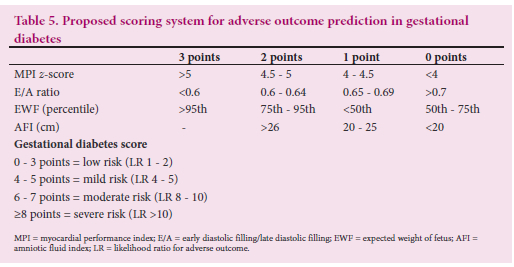
The complete scoring system with risk categorisation is outlined in Table 5.
Discussion
In a series of prospective studies,[6-9] we identified four parameters that could potentially be used in a scoring system to risk-categorise patients with gestational diabetes in terms of fetal outcome and serve as a guide to clinicians managing these patients to optimise timing of delivery. Our score is based on the MPI, the E/A ratio (a marker of diastolic dysfunction), increasing fetal weight (macrosomia), and an increased AFI. Based on these four parameters, a gestational diabetes score to predict adverse fetal outcome is proposed, risk-categorising the fetal condition into four groups from low risk to high risk. These four parameters probably reflect different pathophysiological mechanisms inherent in a fetus in a pregnancy complicated by gestational diabetes (without microvascular complications), and as a combination should give a more or less holistic view of the fetal condition. The total score, obtained by summation of the composite scores for MPI, E/A ratio, EWF and AFI, ranges from 0 to 11. The total score performed as an excellent predictor of adverse outcome, as evidenced by the ROC area under the curve of 0.94. A cut-point of 6 on the score confers a sensitivity of 84.2% and specificity of 90.2% for predicting adverse outcome.
Our first study in this area of investigation showed that the MPI was significantly increased and the E/A ratio significantly lower in fetuses of women with poorly controlled diabetes compared with controls.[6] A total of 17 out of 25 fetuses with an elevated MPI had abnormal outcomes. Of significance in this study was that adverse outcomes appeared to be related to the severity of an abnormal MPI. All control births had a normal outcome. This finding was corroborated in a follow-up study investigating 44 women with poorly controlled gestational diabetes. [7] In this study, adverse outcomes were observed in 20 of the 44 patients in the group with diabetes, which corresponded to an adverse outcome rate of 45% with a similar correlation between increasing MPI values and adverse outcome. All control births had a normal outcome. We also showed that even in milder forms of gestational diabetes, there is a proportion of cases in which the fetus is sensitive to the impaired glucose tolerance, and these adverse events can occur in these pregnancies despite the clinical label of mild disease, resulting in a higher than expected rate of adverse outcome in the GIGT group of 25%,[8] as well as in the well-controlled gestational diabetes group, with an adverse outcome rate of 22%.[9] Macrosomia and polyhydramnios were also noted to be associated with a significantly increased risk of adverse outcome in these studies.[6-9]
Judging from the link between abnormal cardiac function and adverse outcomes in gestational diabetic pregnancies, as well as the demonstration of 'hypoxic' markers, viz. polycythaemia and increased nucleated red blood cells (reflecting evidence of tissue hypoxia), in worsening cardiac function,[7] it is reasonable to hypothesise that fetal cardiac dysfunction appears to predict abnormal metabolic mileus (and possible metabolic shifts) in diabetic pregnancies, and that direct myocardial depression from the functional hypoxia leads to global cardiac dysfunction, as reflected by the increased MPI. The concept of a 'hypoxic' state in gestational diabetes is based on the fact that hyperinsulinaemia results in an increased metabolic rate that causes increased glucose oxidation and oxygen consumption, but the capacity for oxidative metabolism is reduced in fetuses owing to low pyruvate dehydrogenase activity, and the risk of anaerobic metabolism increases irrespective of the prevailing partial pressure of oxygen in the fetal circulation, thus increasing the risk of fetal acidosis. The hypoxia in gestational diabetes is therefore a functional and not an absolute hypoxia. Macrosomia reflects
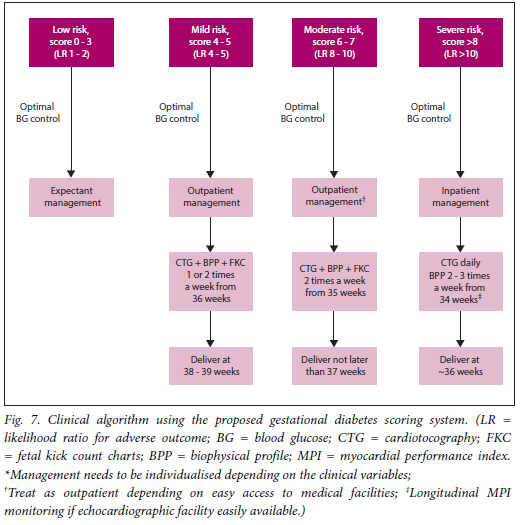
the degree of fetal hyperinsulinaemia, and the polyhydramnios reflects fetal osmotic diuresis and is indicative of suboptimal control. A gestational diabetes score of 0 - 3 conferred a low-risk status (likelihood ratio (LR) of 1 - 2 for an adverse outcome), a score of 4 - 5 a mild risk status (LR of 4-5 for an adverse outcome), a score of 6 - 7 a moderate risk status (LR of 8 - 10 for an adverse outcome), and a score of >8 a severe risk status (LR of >10 for an adverse outcome). The majority of fetuses with normal outcomes had risk scores <5, and the majority of fetuses with scores >8 had adverse outcomes. A suggested clinical way of using the scoring system based on our clinical experience, notwithstanding that each case has its own set of clinical variables that need to be accounted for, would be the following:
• Low risk. Expectant management, with a focus on optimal blood glucose control.
• Mild risk. Delivery at 38 - 39 weeks with close fetomaternal monitoring from 36 weeks, with cardiotocography and biophysical profiles once or twice a week and fetal kick count charts. The patient can be managed as an outpatient, focusing on optimal blood glucose control.
• Moderate risk. Delivery not later than 37 weeks with close fetomaternal monitoring from 35 weeks, with cardiotocography and biophysical profiles twice a week, fetal kick count charts and a focus on optimal blood glucose control. The patient can be managed as an outpatient if medical facilities and care are easily accessible.
• Severe risk. These patients should be monitored as inpatients from 34 weeks, with cardiotocography daily, biophysical profiles every third day, optimal blood glucose control, and delivery not later than 36 weeks. Longitudinal MPI monitoring can be performed if there is access to echocardiographic facilities, to time delivery better.
An algorithm describing the suggested clinical use of the scoring system is presented in Fig. 7.
The proposed scoring system could also allow contingent scoring. In other words, where fetal cardiac Doppler assessment expertise is not readily available, contingent scoring could be undertaken, for example initially scoring on EWF and the AFI (which are standard measurements), and if the patient scores high on this biophysical assessment, referring her to a fetal unit for detailed cardiac Doppler assessment to complete the full scoring system. This scoring system could evolve with time and could be adapted depending on circumstances.
There are a number of reasons for the association of an elevated MPI and lower E/A ratios with adverse outcome. Impaired cardiac function and ventricular compliance, especially diastolic dysfunction, in fetuses of diabetic pregnancies is well documented.[17-19] Even in well-controlled uncomplicated diabetic pregnancies, ventricular septal thickness of up to 5 mm was observed at 35 weeks, which provides some evidence that the level of metabolic control commonly achieved during pregnancy does not prevent progressive fetal myocardial thickening in a number of affected cases.[20] This is certainly true for so-called well-controlled or milder forms of gestational diabetes. Independent of the degree of glycaemic control, it has been demonstrated that hypertrophic cardiomyopathy with impaired cardiac function can complicate maternal diabetes,[21,22] which reiterates the point. There may already be development of septal thickening before 20 weeks' gestation. An increased preload index has been shown in the inferior vena cava of these fetuses that may be associated with an increased haematocrit at birth, increased neonatal morbidity and lower UA blood pH.[23] In a recent study investigating stillbirths in diabetic pregnancies, it has been reported that women with diabetes have a 14 times higher risk of fetal cardiomyopathy identified at fetal autopsy compared with women without diabetes.[24] This fact re-emphasises the importance of cardiac function determination in fetuses of gestational diabetic pregnancies as a proxy for adverse outcome. One of the main mechanisms inducing fetal compromise in gestational diabetes could therefore be the development of myocardial dysfunction.
A diastolic dysfunction score in diabetic pregnancies was proposed by Zielinsky et al.l25] using the septum primum excursion index, left atrial fractional shortening, mitral E/A ratio, pulmonary vein PI, ductus venosus PI, foramen ovale PI, aortic isthmus flow index and myocardial hypertrophy. This score still needs to be validated, but its practicability in a clinical setting is questionable, given the extent of the parameters that need to be investigated. Huhta et al.l26] also proposed a cardiovascular score of 10 using venous Doppler, heart size, four-valve filling, assessing regurgitation and patterns of filling, fractional shortening on the ventricle and UA Doppler using positive flow, absent flow and reversed flow as parameters in the evaluation of heart failure in the fetus with and without hydrops. The Huhta score is useful in sick fetuses but probably not useful in gestational diabetes, where the main pathophysiology is metabolic anomalies rather than placental insufficiency, so the UA Doppler component would not be useful, and probably not useful as a warning or predictive parameter of severity, as the score seems more useful in an already established severe clinical state such as hydrops.
In this study, we have presented a statistically validated scoring system that risk-categorises a fetus in a pregnancy complicated by gestational diabetes. Further work, which requires a larger number of cases, includes a regression coefficient-weighted scoring system derived from multivariate analysis.
Strengths of the scoring system are that it is based entirely on prospective studies, and owing to consistency in recruitment, definition, methodology in assessing fetal cardiac function and management, it was possible to combine the studies in a validation study of a proposed risk scoring system. A limitation of the scoring system is that cardiac Doppler, i.e. the MPI and E/A ratio, requires experience and training to obtain a reliable result. However, this parameter shows very good reproducibility when its evaluation is performed using specific settings with valve clicks as landmarks, as we demonstrated in our study establishing reference intervals of the MPI in normal pregnancy.[13] A challenge would be to transfer this assessment to more general units and to be sure that reliable results are being obtained. With commitment, practice and focus, the MPI can be learned. The E/A ratio is an easier test to perform and also performs well independently in predicting an adverse outcome. Fetal weight estimation and AFI determination are standard measurements in ultrasound assessments.
Conclusion
To our knowledge, this is the first statistically validated scoring system using cardiac Doppler and biophysical parameters to be proposed to predict an adverse outcome in gestational diabetes. It could potentially serve as a guide for clinicians to optimise timing of delivery in gestational diabetes, as indicated above. Importantly, the scoring system contributes to a new, more scientific way of assessing a fetus in a pregnancy complicated by gestational diabetes, taking into account the pathophysiology inherent in these fetuses and moving away from standard monitoring models that are inappropriate and ineffective because they mainly revolve around placental insufficiency rather than metabolic anomalies, which is the core pathophysiological problem in gestational diabetes.
Declaration. None.
Acknowledgements. None.
Author contributions. IB: protocol/project development, data collection and management, data analysis, manuscript writing/editing. TR: data analysis, manuscript writing/editing.
Funding. None.
Conflicts of interest. None.
References
1. Salvesen DR, Brudenell JM, Proudler A, Crook D, Nicolaides KH. Fetal pancreatic betacell function in pregnancies complicated by maternal diabetes mellitus. Am J Obstet Gynecol 1993;168(5):1363-1369. https://doi.org/10.1016/s0002-9378(11)90766-2 [ Links ]
2. Pedersen J. The Pregnant Diabetic and her Newborn. 2nd ed. Baltimore: Williams & Wilkins, 1977:211-220. [ Links ]
3. Bradley RJ, Brudenell JM, Nicolaides KH. Fetal acidosis and hyperlacticaemia diagnosed by cordocentesis in pregnancies complicated by maternal diabetes mellitus. Diabet Med 1991;8(5):464-468. https://doi.org/10.1111/j.1464-5491.199Ltb01633.x [ Links ]
4. Salvesen DR, Brudenell JM, Nicolaides KH. Fetal polycythaemia and thrombocytopenia in pregnancies complicated by maternal diabetes mellitus. Am J Obstet Gynecol 1992;166(4):1287-1293. https://doi.org/10.1016/s0002-9378(11)90623-1 [ Links ]
5. Wong SF, Chan FY, Cincotta RB. Use of umbilical artery Doppler velocimetry in the monitoring of pregnancy in women with pre-existing diabetes. Aust N Z J Obstet Gynaecol 2003;43(4):302-306. https://doi.org/10.1046/j.0004-8666.2003.00094.x [ Links ]
6. Bhorat IE, Bagratee JS, Pillay M, Reddy T. Use of the myocardial performance index as a prognostic indicator of adverse fetal outcome in poorly controlled gestational diabetic pregnancies. Prenat Diagn 2014;34(13):1301-1306. https://doi.org/10.1002/pd.4471 [ Links ]
7. Bhorat I, Foolchand S, Reddy T. Cardiac Doppler in poorly controlled gestational diabetics and its link to markers of hypoxia and adverse outcome. J Obstet Gynaecol 2019;41(1):66-72. https://doi.org/10.1080/01443615.2019.1710480 [ Links ]
8. Bhorat I, Pillay M, Reddy T. Determination of the fetal myocardial performance index in women with gestational impaired glucose tolerance and to assess whether this parameter is a possible prognostic indicator of adverse fetal outcome. J Matern Fetal Neonatal Med 2018;31(15):2019-2026. https://doi.org/10.1080/14767058.2017.1334047 [ Links ]
9. Bhorat I, Pillay M, Reddy T. Assessment of the fetal myocardial performance index in well-controlled gestational diabetics and to determine whether it is predictive of adverse perinatal outcomes. Paediatr Cardiol 2019;40(7):1460-1467. https://doi.org/10.1007/s00246-019-02158-4 [ Links ]
10. National Institute for Health and Care Excellence (NICE). Diabetes in pregnancy: Management from preconception to the postnatal period. Last updated 16 December 2020. https://www.nice.org.uk/guidance/ng3 (accessed 31 October 2022). [ Links ]
11. Alberti KG, Zimmett PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet Med 1998;15(7):539-553. https://doi.org/10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S [ Links ]
12. Figueras F, Gratacós E. Update on the diagnosis and classification of fetal growth restriction and proposal of a stage based management protocol. Fetal Diagn Ther 2014;36(2):86-98. https://doi.org/10.1159/000357592 [ Links ]
13. Bhorat IE, Bagratee J, Reddy T. Gestational age-adjusted trends and reference intervals of the myocardial performance index (Mod-MPI) with its interpretation in the context of established cardiac physiological principles. Prenat Diagn 2014;34(11):1031-1036. https://doi.org/10.1002/pd.4414 [ Links ]
14. Hernandez-Andrade E, Figuero-Diesel H, Kottman C, et al. Gestational-age adjusted reference values for the modified myocardial performance index for evaluation of left fetal cardiac function. Ultrasound Obstet Gynecol 2007;29(3):321-325. https://doi.org/10.1002/uog.3947 [ Links ]
15. Cruz-Martinez R, Figueras F, Bennasar M, et al. Normal reference ranges from 11 to 14 weeks' gestation of fetal left modified myocardial performance index by conventional Doppler with the use of stringent criteria for delimitation of time periods. Fetal Diagn Ther 2012;32(1-2):79-86. https://doi.org/10.1159/000330798 [ Links ]
16. Meriki N, Izurieta A, Welsh AW. Fetal left modified myocardial performance index: Technical refinements in obtaining pulsed-Doppler waveforms. Ultrasound Obstet Gynecol 2012;39(4):421-429. https://doi.org/10.1002/uog.9090 [ Links ]
17. Rizzo G, Arduini D, Romanini C. Cardiac function in fetuses of type 1 diabetic mothers. Am J Obstet Gynecol 1991;164(3):837-843. https://doi.org/10.1016/0002-9378(91)90526-w [ Links ]
18. Rizzo G, Pietropolli A, Capponi A, Cacciatore C, Arduini D, Romanini C. Analysis of factors influencing ventricular filling patterns in fetuses of type 1 diabetic mothers. J Perinat Med 1994;22(2):149-157. https://doi.org/10.1515/jpme.1994.22.2.149 [ Links ]
19. Weiner Z, Zloczower M, Lerner A, Zimmer E, Itskovitz-Eldor J. Cardiac compliance in fetuses of diabetic women. Obstet Gynecol 1999;93(6):948-951. https://doi.org/10.1016/s0029-7844(99)00003-4 [ Links ]
20. Jaeggi ET, Fouron JC, Proulx F. Fetal cardiac performance in uncomplicated and well-controlled maternal type 1 diabetes. Ultrasound Obstet Gynecol 2001;17(4):311-315. https://doi.org/10.1046/j.1469-0705.2001.00365.x [ Links ]
21. Weber HS, Copel JA, Reece EA, Green J, Kleinman CS. Cardiac growth in foetuses of diabetic mothers with good metabolic control. J Pediatr 1991;118(1):103-107. https://doi.org/10.1016/s0022-3476(05)81858-x [ Links ]
22. Gandhi JA, Zhang XY, Maidman JE. Fetal cardiac hypertrophy and cardiac function in diabetic pregnancies. Am J Obstet Gynecol 1995;173(4):1132-1136. https://doi.org/10.1016/0002-9378(95)91339-4 [ Links ]
23. Nicolaides KH, Rizzo G, Hecher K. Placental and Fetal Doppler. London: Parthenon Publishing Group, 2000:128-129. [ Links ]
24. Lynch TA, Westen E, Li D, Katzman PJ, Malshe A, Drennan K. Stillbirth in women with diabetes: A retrospective analysis of fetal autopsy reports. J Matern Fetal Neonatal Med 2022;35(11):2091-2098. https://doi.org/10.1080.14767058.202017719213 [ Links ]
25. Zielinsky P, Luiz Piiccoli A. Myocardial hypertrophy and dysfunction in maternal diabetes. Early Hum Dev 2012;88(5):273-238. https://doi.org/10.1016/j.earlhumdev.2012.02.006 [ Links ]
26. Huhta JC. Guidelines for the evaluation of heart failure in the fetus with or without hydrops. Pediatr Cardiol 2004;25(3):274-286. https://doi.org/10.1007/s00246-003-0591-3 [ Links ]
 Correspondence:
Correspondence:
I Bhorat
ismail@iebhorat.co.za
Accepted 13 August 2022














