Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Obstetrics and Gynaecology
On-line version ISSN 2305-8862
Print version ISSN 0038-2329
SAJOG vol.28 n.2 Cape Town Dec. 2022
http://dx.doi.org/10.7196/sajog.2022.v28i2.2017
RESEARCH
Screening for maternal and congenital syphilis with a chemiluminescence immunoassay in a South African private specialist healthcare sector setting
O A OnyangungaI; K MoodleyII; J MoodleyIII
IMB ChB, PhD; Women's Health and HIV Research Group, Department of Obstetrics and Gynaecology, Schoolof Clinical Medicine, University of KwaZulu-Natal, Durban, South Africa
IIMB ChB, FC Path (SA); Lancet Laboratory, Durban, South Africa
IIIMB ChB, FCOG (SA), FRCOG, MD; Women's Health and HIV Research Group, Department of Obstetrics and Gynaecology, Schoolof Clinical Medicine, University of KwaZulu-Natal, Durban, South Africa
ABSTRACT
BACKGROUND: Syphilis is a sexually transmitted infection that is most frequently found in lower socio-economic groups globally and is associated with significant maternal and fetal complications. In South Africa (SA), the last two to three decades have seen a rise in the number of people in the low and middle economic social groups seeking private specialist healthcare services
OBJECTIVE: To evaluate the prevalence rates of maternal and congenital syphilis in a private specialist healthcare setting
METHODS: The laboratory case records of women who had antenatal maternal syphilis (MS) screening using the automated chemiluminescence immunoassay (Architect Syphilis TP) in a private laboratory facility in Durban were reviewed
RESULTS: A total of 9 740 individual maternal serum samples were analysed and 256 were Architect Syphilis TP positive, resulting in a MS prevalence rate of 2.7%. Of the less than three-quarters of exposed neonates tested (71.1%; n=182/256), 38.5% (n=70/182) were Architect syphilis TP positive. Less than a tenth of exposed neonates (2.43%; n=6) had only rapid plasma reagin (RPR) titers test whereas 26.6% (n=68/256) did not have a syphilis screen test. Based on the 182 exposed neonates tested, the congenital syphilis (CS) prevalence from the laboratory records was 7.7%. The highest rate of MS was in the >35 years age group
CONCLUSION: The prevalence of MS in the private specialist healthcare sector in SA is relatively high and warrants continued maternal antenatal screening during early pregnancy across all socio-economic groups. The high rate of MS in the age group over 35 years warrants further investigations and explanation
Maternal syphilis (MS) is a chronic sexually transmitted infection caused by the spirochete Treponema pallidum (TP). In sub-Saharan Africa, the prevalence of MS varies between 2.5 and 17%.[1,2] More than 500 000 pregnant women are infected annually and if untreated or incompletely treated, potentially 50 - 80% of fetuses are exposed to this infection.[1,2] Furthermore, untreated MS results in adverse events including stillbirths, preterm births, low birthweight babies, neonatal deaths and infant morbidity such as bone malformations and neurological impairments owing to intra-uterine transmission of TP.[1-3] Because syphilis in its primary stage causes lesions in the cervix and vagina, the diagnosis may be missed. In addition, the disease may be asymptomatic. As a result, serological screening for MS in early pregnancy is strongly recommended to detect active infection and initiate treatment timeously to eliminate the associated maternal complications and congenital syphilis (CS).[4] However, screening for MS continues to be a challenge, particularly in developing countries because women access antenatal care at a late gestational period or only present at a healthcare facility at the time of childbirth.[5-7] Furthermore, it is known that social, behavioral and economic factors create considerable difficulties in implementing measures to decrease syphilis rates among males and females and in addition eliminate CS in developing countries.[8-10]
In developed countries such as the United Kingdom, even though the MS rate is <1%, the Infectious Diseases in Pregnancy Screening Program recommends that all pregnant women should be screened for syphilis. Indeed, despite the uptake of almost 98%, the treatment and follow-up of the partners remains a difficult task.[11] Given the increasing numbers of South Africans moving into the middle and upper socioeconomic groups,[12] and seeking private specialist healthcare, it would be interesting to establish the rates of MS and CS in socioeconomic groups attending private obstetricians for antenatal care and childbirth.[12-14]
There are two current methods used to screen for MS and CS: the classical method using a non-treponemal test such as rapid plasma reagin (RPR) or a reverse treponemal screening test with Treponema pallidum haemagglutination (TPHA) or Treponema pallidum particle agglutination assay (TPPA). The high incidence of false negatives and positives has led to the use of the reverse screening test (Fig. 1).[15] Culture of TP is difficult and isolating the spirochete directly from exudates of a lesion is also technically challenging. Therefore, serological testing for TP antibodies is commonly used for the diagnosis and is a two-step process. Traditionally, the first step involves the use of non-treponemal antigens in tests such as the RPR or the venereal disease research laboratory (VDRL) test, and the second step is a confirmatory test used to directly screen for treponemal antibodies by utilising the fluorescent TP absorption test or TP agglutination test. The non-treponemal tests are particularly complex, associated with high sensitivity but a worrying false-positivity rate. Recently, the Centers for Disease Control in the USA recommended a reverse sequence algorithm process for the detection of MS, in which an automated treponemal test such as an enzyme immunoassay or a chemiluminescent microparticle immunoassay is the first step. If positive, a non-treponemal test such as the RPR is performed to determine titers if syphilis is active and to assess the effectiveness of treatment.[16] The aim of this present study therefore was to determine the prevalence of MS among women who consulted obstetricians in the private healthcare sector and had their screening tests in a private laboratory using the reverse algorithm with the chemiluminescence immune-assay as the diagnostic tool.
Methods
Regulatory and institutional authority permissions were obtained, following which the electronic laboratory data for pregnant women who had a screening syphilis test using the chemiluminescence immunoassay Architect Syphilis TP 8 D06' (Abbott Laboratories, Germany) in 2016 were reviewed (Fig. 1). The present study was done in the year 2019. All pregnant women were referred by five private obstetricians for routine antenatal serological investigations including syphilis screening in Durban, South Africa (SA). Results pertinent to the present study were obtained from the mother's and neonatal records. Syphilis testing was done routinely at the first antenatal visit. Pregnant women with positive serology were treated antenatally with Benzathine penicillin and their babies were managed following delivery by a paediatrician.
The Architect Syphilis TP is a two-step immunoassay for the qualitative detection of IgG and/or IgM to TP in human serum or plasma using chemiluminescent microparticle immunoassay technology. The Architect Syphilis TP test was performed on sera and considered positive or reactive for syphilis when the sample mean chemiluminescence signal/cut off (S/CO) value was >1 and negative with S/CO values <1. The RPR was performed according to the standard international procedures. The titers were analysed to determine whether the syphilis was still active or responded well to treatment. All mothers who were Architect Syphilis TP positive were treated for syphilis and most had RPR titers >1:8. The standard practice of clinical care followed the South African Sexually Transmitted Infections (STI) Guidelines.[17] The exposed babies whose mothers were fully treated would not have further investigations nor treatment, and those whose mothers had incomplete treatment or received the last injection <4 weeks before delivery needed treatment with a single dose of Benzathine penicillin (50 000 IU/kg intramuscularly). The symptomatic laboratory syphilis tests on the exposed neonates were analysed. Most of the exposed neonates had an Architect Syphilis TP test combined with the RPR titers test. HIV status of mothers was not available and was not considered for the analysis.
Statistical analysis
Statistical analysis was conducted using the Statistical Package for the Social Sciences (SPSS) software, version 25 (IBM Corp., USA). The data and all results are presented as frequencies and percentages.
Results
MS prevalence
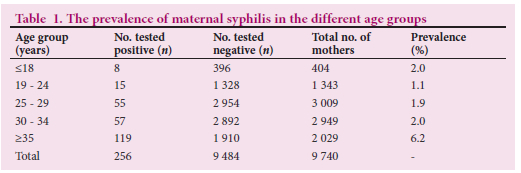
Among the 9 740 women screened with Architect Syphilis TP, 256 tested positive, with a prevalence rate of 2.62%. From the 256 Architect Syphilis-positive mothers, 247 had RPR tests done and 89.6% (n=228) of these mothers had a positive RPR at > 1:8 titers and 7.42% (n=19) had a positive RPR at <1:4 titers. The prevalence of MS using Architect Syphilis TP followed by RPR was calculated at 2.34%. No data on further investigations were available (Fig. 2).
Age distribution of those who tested positive for syphilis
The MS prevalence rate was highest in women aged >35 years compared with other age groups (Table 1).
Neonatal testing
Of the 256 mothers who tested positive for syphilis, laboratory records were available for 71.1% (n=182) exposed babies who had at least one follow-up visit. The exposed babies were tested with the Architect Syphilis TP chemiluminescence test within the first year of life. More than a third of exposed babies (38.5%; n=70) tested positive. The RPR was done on 182 exposed neonates at different ages (Table 2). Less than a tenth of exposed babies (7.7%; n=14) had RPR titers >1:8 and 92.3% (n=168) had titers <1:4. Six RPR tests not associated with Architect Syphilis TP were done with titers <1:8. The overall CS prevalence calculated from 182 exposed babies with both Architect Syphilis TP and RPR was 7.7%.
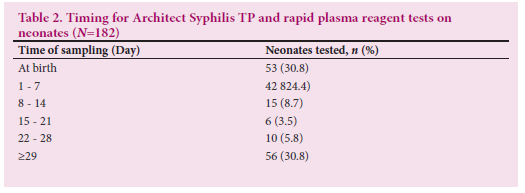
Table 2 shows that 182 babies had a treponemal test followed by a non-treponemal test. More than half of babies (52%) had been tested with both Architect Syphilis TP and RPR during their first week of life and 25.3% (n=46) of babies were first tested after 4 weeks. Ten babies were re-tested only with RPR after 28 weeks and six others had only a RPR test done.
Discussion
This present study found that the prevalence rates of MS using a reverse sequence algorithm testing method (Architect Syphilis TP) alone and followed with RPR test in women attending a private specialist healthcare setting in SA was 2.34%. In addition, women >35 years formed a large proportion of those who tested positive for MS, accounting for 119 of the 256 women in this age group (Table 1). Furthermore, the incidence of CS as assessed by chemiluminescence serological testing/RPR was 7.7% of those exposed and tested for syphilis. The reverse sequence algorithm used in the laboratory was not systematically used in all mothers and neonates and might have interfered in the prevalence value of MS and CS.
The finding that women >35 years of age are in the group at greatest risk of MS is difficult to explain. Similar findings were reported by the 2015 National Antenatal HIV and Syphilis Sentinel Survey (NAHSSS) carried out by the National Department of Health, which found that women >40 years had high prevalence rates of TP infections.[18] Syphilis like HIV and other STIs is strongly related to socio-cultural and sexual behavior patterns of a population.[7,9,19,20] Socio-cultural and sexual behaviours where older married male partners engage sexually with younger women has been suggested to contribute to the finding of high rates of HIV and syphilis in women aged >35 years.[7,919,20] This finding obviously needs further in-depth investigation so that appropriate strategies are implemented to control the spread of syphilis and HIV in all socioeconomic communities.
The MS prevalence rate of 2.7% is slightly higher than that of the 2015 NAHSSS.[18] It suggests that syphilis rates are increasing across all socioeconomic groups. Furthermore, the rate of 2.6% is slightly higher than the 2.3% for the province of KwaZulu-Natal reported by the NAHSSS.[18] Increases in prevalence rates of syphilis have also been reported in the USA, where the increasing rates have been found across all racial and socioeconomic groups, may be driven by men who have sex with men and by 'tight' social groups in which many clients report having been treated for syphilis previously.[21-23] MS and CS rates in the USA have also concomitantly increased and there are reports of CS in late antenatal bookers.l21-23] In SA, there has been an irregular supply/shortage of Benzathine penicillin, which may have contributed to increasing rates of syphilis. The SA public health authorities are investigating ways to increase the availability of penicillin.
As far as we are aware, this is the first study to evaluate rates of MS and CS in a private specialist healthcare setting in SA. There is, however, a report by Schneider et al.,[24] who performed a structured telephone interview study to establish the quality of clinical management provided by generalist medical practitioners to men and women presenting with urethral and vaginal discharges, either in their private practices or at workplace health services. These authors reported that clinical management provided was poor and may have been due to poor knowledge of the management of STIs or a misunderstanding of the syndromic management of STIs. In addition, the GPs may have been tailoring their clinical management according to whether the client was a cash-paying individual or on medical aid. Obstetricians in specialist private practice may also have a similar approach and it was not clear from the laboratory data whether investigations were done to assess the effect of treatment on syphilis using RPR quantitative titers to evaluate cure and there was doubt as to whether the specialists were repeating syphilis testing at birth in patients at high risk of syphilis. Furthermore, it is of concern that the exposed neonates were only tested for syphilis and that this testing was done between birth and one year. It is possible that the specialist paediatricians did not have a tracking system and many mothers did not return on the follow-up dates. It also should be noted that only 188 of the 256 exposed babies returned for a check-up. These factors may be related to payments and that the medical aid funders place limits on the number of return visits. Private health institutions in SA are expensive, and they provide healthcare for cash-paying patients or those subsidised by a collective medical scheme.
The detection rates of the reverse sequence algorithm were like those reported by the NAHSSS for KwaZulu-Natal Province, which uses the RPR as a confirmatory test.[18] The chemiluminescence test is an automated process that is relatively cheap and has a high detection rate for those who have been exposed to TP. Despite its high sensitivity, syphilis TP has limitations on its specificity with the possibility of false positives requiring the use of another treponemal antibody test such as the TP haemagglutination test.[25] Positive results are combined with a confirmation test such as RPR and a fall in titers can be used to evaluate cure. Discordant results and results that are borderline do occur and, in such cases, a different TP antibody should be used. It appears from the laboratory data that this may not have been done mainly for financial reasons or a misunderstanding of the laboratory's comments on the results, leaving it to the obstetrician to decide on borderline results and the need for further testing. Again, it appears that finances play an important role in such circumstances. In our view, specialist obstetricians must fully inform patients/clients of the options to overcome potential medical litigation. Most guidelines recommend retesting of discordant and borderline results. The development of automated tests with greater specificity may overcome this problem.118,20,26] In this present study, the rate of congenital syphilis was found to be low. However, almost 26.5% of exposed neonates did not have a serological follow-up test.[26] The fact that neonates were probably not followed up and treated has a negative impact on both financial aspects for the healthcare system and on disability for the affected individual[27] Therefore, it is mandatory to have a strict follow-up for the exposed neonates. The prevalence of CS might suggest that the mother was treated late or incorrectly.
Study limitations
The limitation of our study was that because it evaluated laboratory data, it could not explore all demographic socio-economic factors and comorbidities such as HIV It does, however point to a need for a prospective and more holistic study on syphilis in pregnancy in both the private and public healthcare sectors in SA involving all socioeconomic groups. Further, there is a need to compare in detail the reverse sequence algorithm approach with the traditional approach.
Conclusion
Strong recommendations must be made to the public and private healthcare sectors to ensure that screening for syphilis in pregnancy is done at the first antenatal visit, to repeat MS testing at 28 - 32 weeks and to ensure that all exposed babies are evaluated for CS and are followed up. At the same time, the National Department of Health must meet with funders of medical aids and representatives of private health professional organisations to ensure that national guidelines for testing of syphilis, like HIV, are fully funded. The lack of systematic follow-up testing for the exposed neonates suggests an underestimated rate of CS.
Declaration. None.
Acknowledgements. None.
Author contributions. Equal contributions. All authors approved the final version of the manuscript for publication.
Funding. None.
Conflicts of interest. None.
References
1. Watson-Jones D, Oliff M, Terris-Prestholt F, et al. Antenatal syphilis screening in sub-Saharan Africa: Lessons learned from Tanzania. Trop Med Int Health 2005;10(9):934-943. https://doi.org/10.1111/j.1365-3156.2005.01473.x [ Links ]
2. Kuznik A, Lamorde M, Nyabigambo A, Manabe YC. Antenatal syphilis screening using point-of-care testing in sub-Saharan African countries: A cost-effectiveness analysis. PLoS Med 2013;10(11):e1001545. https://doi.org/10.1371%2Fjournal.pmed.1001545 [ Links ]
3. Gomez GB, Kamb ML, Newman LM, Mark J, Broutet N, Hawkes SJ. Untreated maternal syphilis and adverse outcomes of pregnancy: A systematic review and meta-analysis. Bull World Health Organ 2013;91:217-226. https://doi.org/10.2471%2FBLT.12.107623 [ Links ]
4. World Health Organization. The global elimination of congenital syphilis: Rationale and strategy for action. Geneva: WHO, 2007. [ Links ]
5. Taylor YJ. Social determinants of prenatal care use in sub-Saharan Africa: Exploring equity in content and outcomes of care: The University of North Carolina at Charlotte; 2013. [ Links ]
6. Serafim AS, Moretti GP, Serafim GS, et al. Incidence of congenital syphilis in the south region of Brazil. Rev Soc Bras Med Trop 2014;47(2):170-178. https://doi.org/10.1590/0037-8682-0045-2014 [ Links ]
7. Smock L, Caten E, Hsu K, DeMaria Jr A. Economic disparities and syphilis incidence in Massachusetts, 2001 - 2013. Public Health Rep 2017;132(3):309-315. https://doi.org/10.1177/0033354916688269 [ Links ]
8. Braveman PA, Cubbin C, Egerter S, Williams DR, Pamuk E. Socioeconomic disparities in health in the United States: What the patterns tell us. Am J Public Health 2010;100(S1):S186-S196. https://doi.org/10.2105/ajph.2009.166082 [ Links ]
9. Pampel FC, Krueger PM, Denney JT. Socioeconomic disparities in health behaviors. Annu Rev Soc 2010;36:349-370. https://doi.org/10.1146%2Fannurev.soc.012809.102529 [ Links ]
10. Kim MK, Lee SM, Bae S-H, et al. Socioeconomic status can affect pregnancy outcomes and complications, even with a universal healthcare system. Int J Equity Health 2018;17(1):2. https://doi.org/10.1186/s12939-017-0715-7 [ Links ]
11. Hughes G, Field N. The epidemiology of sexually transmitted infections in the UK: Impact of behavior, services and interventions. Future Microbiol 2015;10(1):35-51. https://doi.org/10.2217/fmb.14.110 [ Links ]
12. Zimbalist Z. Analysing post-apartheid poverty trends by geo-type, 1997 - 2012: The understated role of urbanisation and social grants. Development Southern Africa. 2017;34(2):151-167. https://doi.org/10.1080/0376835X.2016.1259989 [ Links ]
13. Ataguba JE, Akazili J, McIntyre D. Socioeconomic-related health inequality in South Africa: Evidence from general household surveys. Int J Equity Health 2011;10(1):48. https://doi.org/10.1186/1475-9276-10-48 [ Links ]
14. Turok I, Visagie J. Inclusive urban development in South Africa: What does it mean and how can it be measured? IDS Working Paper 512. Brighton: IDS, 2018. [ Links ]
15. Akhatar F, Rehman S. Prevention of congenital syphilis through antenatal screenings in Lusaka, Zambia: A systematic review. Cureus 2018;10(1).e2078 https://doi.org/10.7759%2Fcureus.2078 [ Links ]
16. Gratrix J, Plitt S, Lee BE, et al. Impact of reverse sequence syphilis screening on new diagnoses of late latent syphilis in Edmonton, Canada. Sex Transm Dis 2012;39(7):528-530. https://doi.org/10.1097/olq.0b013e31824e53f7 [ Links ]
17. Matsitse TB, Helberg E, Meyer JC, Godman B, Massele A, Schellack N. Compliance with the primary healthcare treatment guidelines and the essential medicines list in the management of sexually transmitted infections in correctional centres in South Africa: Findings and implications. Expert Rev Anti-infect Ther 2017;15(10):963-972. https://doi.org/10.1080/14787210.2017.1382354 [ Links ]
18. Department of Health. National antenatal sentinel HIV and syphilis prevalence survey in South Africa, 2009. Pretoria: NDoH, 2010. [ Links ]
19. Naidoo S, Wand H, Abbai NS, Ramjee G. High prevalence and incidence of sexually transmitted infections among women living in KwaZulu-Natal, South Africa. AIDS Res Ther 2014;11(1):31. https://doi.org/10.1186/1742-6405-11-31 [ Links ]
20. Patra S. Socio-cultural correlates and risky sexual behaviour influencing prevalence of HIV/AIDS and STIs in Uganda: A gender perspective. Cogent Soc Sci 2016;2(1):1166472. https://doi.org/10.1080/23311886.2016.1166472 [ Links ]
21. Lin C-Y, Broström A, Nilsen P, Pakpour AH. Using extended theory of planned behavior to understand aspirin adherence in pregnant women. Pregnancy Hypertens 2018;12:84-89. https://doi.org/10.1016/j.preghy.2018.04.001 [ Links ]
22. Trivedi S, Williams C, Torrone E, Kidd S. National trends and reported risk factors among pregnant women with syphilis in the United States, 2012 - 2016. Obstet Gynaecol 2019;133(1):27. https://doi.org/10.1097/aog.0000000000003000 [ Links ]
23. Slutsker JS, Hennessy RR, Schillinger JA. Factors contributing to congenital syphilis cases - New York City, 2010 - 2016. Morb Mort Weekly Rep 2018;67(39):1088. https://doi.org/10.15585/mmwr.mm6739a3 [ Links ]
24. Schneider H, Blaauw D, Dartnall E, Coetzee D, Ballard R. STD care in the South African private health sector. S Afr Med J 2001;91(2):151-156. [ Links ]
25. Morshed MG, Singh AE. Recent trends in the serologic diagnosis of syphilis. Clin Vaccine Immunol 2015;22(2):137-147. https://doi.org/10.1128/cvi.00681-14 [ Links ]
26. Nah E-H, Cho S, Kim S, Cho H-I, Chai J-Y. Comparison of traditional and reverse syphilis screening algorithms in medical health checkups. Ann Lab Med 2017;37(6):511-515. https://doi.org/10.3343%2Falm.2017.37.6.511 [ Links ]
27. World Health Organization. WHO guideline on syphilis screening and treatment for pregnant women. Geneva: WHO, 2017. [ Links ]
 Correspondence:
Correspondence:
O A Onyangunga
onyangunga51@gmail.com
Accepted 17 May 2022














