Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Obstetrics and Gynaecology
versión On-line ISSN 2305-8862
versión impresa ISSN 0038-2329
SAJOG vol.28 no.1 Cape Town jun. 2022
http://dx.doi.org/10.7196/SAJOG.2022.v28i1.2078
RESEARCH
A comparison of malignant histopathological diagnoses on uterine curettings and hysterectomy specimens
A IsmailI; R WadeeII
IMB BCh; Department of Anatomical Pathology, School of Pathology, Faculty of Health Sciences, University of the Witwatersrand and National Health Laboratory Service, Johannesburg, South Africa
IIMB BCh, FC Path, MMed (Anat Path), Dip RCPath, FAcadTM, MIAC, PhD; Department of Anatomical Pathology, School of Pathology, Faculty of Health Sciences, University of the Witwatersrand and National Health Laboratory Service, Johannesburg, South Africa
ABSTRACT
BACKGROUND: Endometrial carcinoma (EC) is a common gynaecological malignancy in postmenopausal females. Diagnosis is made on endometrial biopsy, where histological subtype and tumour grade are used to predict disease progression and to plan surgical management
OBJECTIVES: To determine the accuracy of preoperative biopsies compared with the final diagnosis on hysterectomy specimens
Methods. This was a retrospective, cross-sectional study in which 126 biopsies and corresponding hysterectomy specimens, collected over
a 3-year period, were reviewed. Patient demographics and histological features were recorded and statistically analysed.
RESULTS: The most prevalent tumours were endometrioid endometrial carcinoma (EEC) (48.5%), serous carcinomas (25.4%) and carcinosarcomas (16.7%). The majority (66.7%) of tumours were high-grade tumours on biopsy and hysterectomy specimens (58.7%
EECs had a poor sensitivity level (65.1%) compared with other subtypes but had a high specificity rate (90%). There was moderate agreement between biopsy and excision specimen diagnoses. High-grade tumours had a high sensitivity level (94.3%).
CONCLUSIONS: Our study showed moderate agreement between histopathological diagnoses on biopsy and excision specimens. There was
a high sensitivity level for biopsies of high-grade tumours, concordant with other studies. Accurate preoperative tumour subtyping and grading are needed to guide surgical management. It is envisaged that use of a combined histological and molecular tumour classification will better guide patient treatment and allow for reproducible results.
Endometrial carcinoma (EC) is the sixth most common gynaecological malignancy and the fourteenth leading cause of cancer-related deaths worldwide.[1] Uterine malignancies accounted for 3.8% of all malignancies in South African (SA) females in 2017.[2] EC usually presents with post-menopausal bleeding. Initial investigation includes a uterine ultrasound evaluation, with an endometrial thickness >4 mm, considered concerning for malignancy.[3] In South Africa, the Pipelle/'Z-Sample' is a cost-effective method that is performed on an outpatient basis and has a sensitivity of 84.2 - 99.0%.[4-6]
ECs have previously been grouped into Type I and Type II tumours. Endometrioid endometrial carcinoma (EEC) is a typical Type I tumour, which arises in the background of endometrial hyperplasia. Risk factors for EEC are obesity, tamoxifen usage, hormone replacement therapy and decreased fertility - all of which are associated with increased circulating oestrogen.[7,8] These tumours develop from endometrial hyperplasia without atypia, progress to atypical hyperplasia, followed by endometrial intraepithelial neoplasia (EIN) and finally to invasive carcinoma. Type II or non-endometrioid endometrial carcinoma is by definition high-grade, and is the umbrella category for serous and clear cell carcinomas. These occur in multiparous, post-menopausal women, often in the background of an atrophic endometrium. Type II tumours develop in a background of endometrial glandular dysplasia, progress to serous intraepithelial carcinoma and then become invasive serous carcinomas.[8,9] Other histopathological subtypes of ECs include carcinosarcoma, neuroendocrine carcinoma (NEC), and undifferentiated and dedifferentiated carcinomas, all of which are high grade by definition.[10]
ECs are histologically graded using the International Federation of Gynaecology and Obstetrics (FIGO) system, which aids in prognostication and guides the extent of surgical intervention.[11] Low-grade tumours are treated fairly conservatively whereas high-grade tumours require more aggressive forms of treatment, including radical lymph node dissection, chemotherapy, radiation therapy and omental biopsy.[8] Tumour staging requires assessment of para-aortic and/or pelvic lymph nodes for high-grade or locally advanced tumours.[12] Lymph node dissection may cause significant morbidity, including intraoperative complications, postoperative lymphoedema and deep vein thrombosis.[13] Apart from grade and histological subtype, which may be assigned on biopsy, most prognostic factors of EC can only be assigned following examination of the hysterectomy specimen. As such, there is a reliance on accurate grading and subtyping of endometrial biopsies to facilitate adequate planning of surgical intervention and chemoradiation.
Our study aimed to determine the accuracy of histopathological findings of preoperative endometrial biopsy specimens compared with pathology results of the final hysterectomy specimens. In addition, we aimed to expand on the limited SA data available on the prevalence of histological subtypes, grade and demographics in patients diagnosed with EC.
Methods
This was a retrospective, cross-sectional study. Ethical clearance was granted by the Human Research Ethics Committee (Medical) of the University of the Witwatersrand (ref. no. M180628). We reviewed 126 biopsies with corresponding hysterectomy specimens from 1 January 2015 to 31 December 2017 at the Department of Anatomical Pathology, University of the Witwatersrand/National Health Laboratory Service (NHLS) located at Charlotte Maxeke Johannesburg Academic Hospital (CMJAH). The NHLS database was searched using the Systematised Nomenclature of Medicine (SNOMED) codes for appropriate terms.
Exclusion criteria encompassed the following: cases where archived slides could not be found, incomplete or partially completed reports, and hysterectomy specimens in which the entire tumour was completely autolysed.
The slides were reviewed independently by both authors (reviewer 1 (R1) and reviewer 2 (R2)) in the absence of pathology reports. The authors compared their own results and their findings against those of the final pathology reports. Relevant, anonymised data were collected from each case.
SPPS version 23 (IBM Corp., USA) was used for statistical analysis. Categorical data were recorded as percentages and means where appropriate. In the assessment of biopsy performance statistics, all cases of atypical cells, 'not applicable' and in-situ malignancies were excluded. Biopsy sensitivity and kappa coefficient scores were used to assess agreement of grading and histological subtype between preoperative and final pathology, with 95% confidence intervals (CI). Conventional methods of interpreting kappa (k) values were used with level of agreement categories of 'poor/slight' (k = 0.0 - 0.20), 'fair' (k = 0.21 - 0.40), 'moderate' (k = 0.41 - 0.60), 'strong/substantial' (k = 0.61 - 0.80) and 'almost perfect' (k = 0.81 - 1.00).[14]
Results
Table 1 shows that most cases (96.8%) in our cohort were from post-menopausal, multiparous women between the ages of 60 and 79 years and diagnosed as 'malignant' on both biopsy (86.5%) and hysterectomy (97.6%) (p<0.001). Most biopsy specimens (86.5%) had a malignant diagnosis (Table 2). Table 3 shows that more than three-quarters of patients (79.4%) had a total abdominal hysterectomy, with a significant number (18.3%) having undergone radical hysterectomies (p<0.001). Of the 18.3% (n=23) radical hysterectomy cases, five were grade 1 EECs, two were grade 2 EECs, one was a grade 3 EEC, 10 were serous endometrial carcinomas, while the remaining five cases had insufficient tissue for a definitive tumour diagnosis or grading to be rendered. Of the three patients who had undergone subtotal hysterectomy surgery, one was a grade 1 EEC on biopsy but grade 3 EEC on the excision specimen, one showed atypical cells on biopsy but was a dedifferentiated carcinoma on the hysterectomy specimen and one was inadequate for a definitive diagnosis on biopsy but proved to be a grade 3 EEC on the hysterectomy specimen.
The mean number of days between biopsy and hysterectomy was 86 (range 13 - 281) days. The majority of patients (87.3%) required only one biopsy, whereas 11.9% (n=16) of patients required two biopsies and one patient (0.8%), three biopsies. A mean of 2.6 (range 0 - 12) immunohistochemical stains were performed per biopsy and 1.54 (range 0 - 10) per hysterectomy.
No statistically significant differences were noted in the proportions of myometrial depth of invasion (p=0.0652). However, a statistically significant proportion of cases showed no cervical stromal involvement and no lymphovascular invasion (p<0.001) (Table 3).
Histological subtype
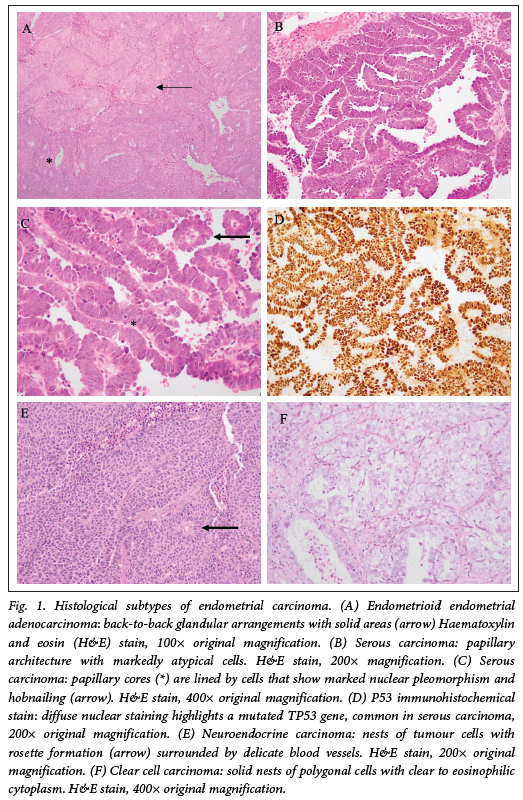
Table 2 shows that the most prevalent histological subtypes on biopsy were serous carcinoma (32.5%), followed by EEC (27%), and carcinosarcoma (9.5%). In a significant number of biopsies, no histopathological tumour subtype was specified (10.3%; p<0.001). On the excision specimens, most tumours were EEC (48.5%), 25.4% were serous carcinomas and 16.7% were carcinosarcomas (p<0.001) (Table 3; Fig. 1). Of the seven tumours diagnosed as atypical hyperplasia on biopsy, six were upgraded to a grade 1 or 2 invasive endometrioid carcinoma on the excision specimens, while one case was interpreted as atypical hyperplasia within a polyp on the hysterectomy specimen.
Histological grade
Tables 2 and 3 show that the majority of tumours were high-grade tumours on both biopsy (66.7%) and hysterectomy specimens (58.7%; p<0.001), while 7.9% of biopsies did not have a FIGO grade assigned.
Agreement
There was a k value of 0.529 when the histology subtype was compared with the final histology subtype and a value of 0.530 (moderate agreement) was obtained for comparison of FIGO grade between the biopsy and excision specimens based on the original patient pathology reports. High-grade tumours had a higher sensitivity (94.29%) and were more common than low-grade tumours. Table 4 shows the performance statistics of biopsy accuracy. An almost perfect level of agreement was found between Rl's assessment of tumour subtype on biopsy and the hysterectomy specimen (k = 0.95), whereas R2 had k scores of 0.94 and 0.89 for assessment of grade on hysterectomy specimens and on biopsies, respectively. Both Rl and R2 had substantial agreement with biopsy histological subtype (0.73 and 0.69). Substantial agreement was obtained when comparing Rl and R2's findings with the original pathology tumour subtype and grade (results not shown).
Discussion
Our study showed moderate overall agreement between diagnosis on endometrial curettings and the final excision specimen with regards to biopsy grade and histological subtype, respectively, which is concordant with or better than other studies.[13] We showed a high level of sensitivity when comparing all biopsies that had highgrade tumours (94.29%). However, the sensitivity of grade 1 and 2 EEC (42 - 46%) was lower than that reported in international studies, likely due to the comparatively low prevalence of low-grade tumours in our population.[16,13,15] Helpman et al.[11] showed similar levels of agreement with our study and concluded that preoperative endometrial sampling was a modest predictor of final surgical pathology findings.
Visser et al.[15] showed a higher degree of overall agreement, with the lowest agreement rate for EECs, which is similar to our findings. This study showed that 25% of tumours were histologically downgraded to grade 1 or 2 tumours and 21% were upgraded to high-grade tumours. In our study, of the six tumours that were histologically upgraded, three were upgraded from EEC grade 1 to EEC grade 3. Four tumours were downgraded significantly, with three of these having been downgraded from high-grade serous carcinoma to EEC grade 1. One tumour was downgraded from EEC grade 3 to EEC grade 1. For both histological upgrades and downgrades, this was due to better tissue preservation and visual representation of the tumour on the hysterectomy specimens. In addition, the larger tumour volume on the excision specimens allowed for improved assessment of cytomorphological features and solid or papillary growth patterns, which are integral to assigning a tumour FIGO grade.
Despite EEC having had a relatively poor sensitivity (65.12%) compared with other subtypes, EEC had a high specificity rate (90%) and was the most prevalent tumour subtype, whereas NEC and dedifferentiated carcinoma had sensitivity and specificity levels of 100% but were the most uncommon malignancies. Of the most prevalent subtypes, serous carcinoma had the highest sensitivity (77.68%) compared with EEC and carcinosarcoma. EEC had a comparatively poor biopsy sensitivity, but had a high specificity compared with serous carcinoma. This suggests that interpretation of the level of agreement is most meaningful for highgrade tumours and highly prevalent tumour subtypes.
Although EECs were the most common subtype on excision specimens, serous carcinomas were the most commonly diagnosed subtype on biopsy (32.5%). Thus, EECs and carcinosarcomas are under-diagnosed while serous carcinomas are over-diagnosed on biopsy specimens at our institution. The most likely reason for this is that the solid areas seen in EECs are better assessed on hysterectomy specimens and may not be adequately sampled and represented on biopsy specimens. Highgrade EEC may also show papillary-like growth patterns and nuclear 'hobnailing', which may account for the tumour being diagnosed as a serous carcinoma on biopsy, despite the final diagnosis being that of highgrade EEC. In the case of carcinosarcoma, sarcomatous areas and heterologous elements are more likely to be sampled and better represented on the excision specimen. In addition, there may be a difference in diagnostic thresholds for patterns of growth, nuclear grade or immunohistochemical staining between pathologists. Gilks et al.[16] showed a considerable level of disagreement (up to 35.8%) when comparing histological diagnoses by various pathologists. The most common disagreements were between serous and high-grade EEC.[16]
Only one other SA study to date has assessed concordance rates between endometrial biopsies and excision specimens. Mhlongo et al.[4] showed high sensitivities (93.8%) for low-grade endometrioid tumours and 99.2% for high-grade endometrioid tumours. Our study, however, showed higher sensitivities (65.65%) for serous carcinoma compared with Mhlongo et al.[4] (42.9%). This may be due to the larger sample size in our present study. Our cohort showed that EECs form a significantly lower proportion (48%) of total cases, with an increased prevalence of serous carcinoma in our population, consistent with findings by Mhlongo et al.[4] This evidence contrasts with other studies, which state that EEC accounts for 80 - 90% of cases,[4,8] but is consistent with the fact that serous carcinomas are more common in multiparous females and in African populations. While risk factors for the development of serous carcinoma are not well established, TP53 mutations are the underlying molecular abnormality. Risk factors such as previous pelvic radiation and germline mutations - for example, mutations in the breast cancer gene (BRCA) - warrant further investigation in our population.[10]
The Cancer Genome Atlas (TCGA) has now classified ECs into four groups according to their molecular makeup, which has been prognostically reproducible and allows for better prediction of patient outcomes than the traditional dualistic classification.[17] The TCGA groups include: POLE mutant (POLEmut) that is associated with a good prognosis; mismatch repair deficient (MMR-d); low-copy-number alterations (both of which are associated with an intermediate prognosis); and high-copy number variant and p53 mutant (p53mut), which are associated with poor prognosis.[17-19] Unfortunately, a full molecular workup and classification in a resource-limited setting such as in state hospitals is not always feasible. However, partial classification is possible with the use of immunohistochemical (IHC) stains such as p53 and MMR proteins, which are available in some SA anatomical pathology laboratories. These may be used to classify ECs as serous carcinomas or MMR-deficient carcinomas and thus aid in prognostication. More immunohistochemical stains were used for biopsies compared with hysterectomy specimens in our study, as these stains are required for initial diagnosis and are usually not needed if the findings on hysterectomy are morphologically consistent with those of the biopsy. Of the seven cases that had significant changes in tumour grades, only one case had p53 and MMR immunohistochemistry. Studies have shown that the use of IHC significantly improves agreement between biopsy and hysterectomy.l18] P53 is an immunohistochemical stain that serves as a surrogate marker for underlying TP53 mutation and is currently the most frequently used stain to identify TP53 mutant neoplasms such as serous carcinomas. Polymerase E (Pol E) assessment is not performed in most state hospitals. As stated previously, Pol E ECs have the best prognosis, while p53 mutant ECs the worst, whereas MMRd and copy-number low tumours have an intermediate prognosis.l19] Using p53 and MMRd IHC stains routinely may help improve the accuracy of diagnosis, especially in the case of EECs. An integrated clinical, morphological and molecular classification, where available, may identify previously uncategorised high-risk patients who require individualised treatment such as fertility-sparing surgery and an integrated risk stratification that guides use of brachytherapy, external beam radiation and chemotherapy for high-risk tumours.[20]
Our study showed that 73.7% of tumours were high-grade ECs on biopsy but only 19% of patients underwent radical surgical intervention. The low rate of radical surgery for high-grade tumours in our present study suggests that the assigned stage using the American Joint Committee on Cancer (AJCC) and the Union for International Cancer Control's Tumour-Nodes-Metastasis (TNM) Classification of Malignant Tumours may not be an accurate reflection of the actual tumour stage in our population as the lymph nodes had not been surgically excised and were therefore not microscopically examined. Although our study was performed at a quaternary hospital complex, our department provides a pathology service to many hospitals and clinics in the southern Gauteng region and not all such healthcare facilities have gynaecological oncology services or personnel with experience to perform radical surgeries, which may account for the low number of lymph node dissections in patients with highgrade ECs. Other factors which may explain the low number of radical hysterectomies include the age of patients, as elderly patients with comorbidities may not tolerate the risk of radical surgery. These are all factors that may be discussed at multidisciplinary team meetings to establish the most beneficial, definitive management plans for patients.
Our study serves as a form of quality assurance as it highlights delays in surgery, suboptimal biopsies, and unspecified grades (7.9%). It underscores the need for ancillary tests such as IHC to ensure more accurate, reproducible results, especially if grade or subtype are different on hysterectomy specimens. Only 16% of cases required more than one biopsy before a diagnosis was made, with only one patient needing three biopsies, inferring that a diagnosis should be assigned by at least two biopsy specimens. The mean period between biopsy and hysterectomy was 86 (13 - 281) days. Delays in turnaround time of reports, the need for repeat biopsies and lack of surgical resources may have contributed to the relatively lengthy interval of biopsy to surgery. This may contribute to increasing the morbidity and mortality of patients. Other causes of delayed turnaround time include trainee/registrar training factors (due to the necessity of teaching by pathologists and co-existing responsibilities of registrars), tissue processing factors (delays in IHC stains) and incomplete clinical information supplied.[21] These may be addressed by improved sampling methods and training to attain adequate biopsies and better communication between pathologists and gynaecologists by raising issues of concern at multidisciplinary team meetings. Complete clinical details and contact numbers should be filled out on the patient requisition forms that accompany tissue specimens and a request to process biopsy specimens as urgent specimens, as opposed to routine cases, may also improve workflow and result in faster laboratory processing and reporting.
Study limitations, strengths and recommendations
Our study was conducted at a quaternary academic institution within the public sector and, as such, may not be extrapolated to the entire SA population as data from the private sector were not included. Other study limitations include the fact that clinical information was sourced only from the histopathology reports, which is a reflection of the details provided by the requesting clinician. As such, the type of biopsy equipment used, the type of surgical procedure or other clinical details and patient risk factors were often not routinely included. Our study assessed agreement of overall tumour grade and not only grades assigned for EEC as other studies have done. This study contributes to the current sparse data from the African continent and ours is the largest cohort to date which has compared biopsy and hysterectomy specimen findings. While advances are made in the molecular classification of tumours, the agreement of biopsy with hysterectomy findings will continue to be important in resource-limited settings.
Conclusion
Our study showed moderate agreement between histopathological findings on biopsy (including subtype and grade) and the final hysterectomy specimen results. Gynaecological surgeons rely on the preoperative biopsy findings for patient management and therefore are dependent on accurate tumour subtyping and grading. It is envisaged that a combined histological and molecular tumour classification should be used, which may better guide overall patient management by producing more accurate and reproducible findings.
Declaration. This study was done in partial fulfilment of the degree requirements of an MMed (Anat Path) for AI.
Acknowledgements. We would like to thank Dr Gill Hendry for her assistance with the statistical analysis, and Ms Fadila Ebrahim and Mr Abel Ndlovu for their assistance with retrieval of slides for review.
Author contributions. AI performed the data collection and wrote the manuscript. AI and RW reviewed the slides. RW conceived the study, assisted with the write-up, and critically reviewed the manuscript. Both the authors approved the final version of the manuscript for publication.
Funding. None.
Conflicts of interest. None.
References
1. Colombo N, Creutzberg C, Amant F, et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, treatment and follow-up. Ann Oncol 2016;27(1):16-41. https://doi.org/10.1093/annonc/mdv484 [ Links ]
2. National Cancer Registry. Cancer in South Africa 2017 Full Report. Pretoria: NICS, 2020. https://www.nicd.ac.za/wp-content/uploads/2020/12/NCR_2017_Final_02dec2020.pdf (accessed 7 March 2021) [ Links ]
3. Clark TJ, Voit D, Gupta JK, Hyde C, Song F, Khan KS. Accuracy of hysteroscopy in the diagnosis of endometrial cancer and hyperplasia: A systematic quantitative review. JAMA 2002;288(13):1610-1621. https://doi.org/10.1001/jama.288.13.1610 [ Links ]
4. Mhlongo SE, Naidoo TD, Makhathini BS. Discrepancy between preoperative endometrial sampling and hysterectomy diagnosis in endometrial cancer. South Afr J Gynaecol Oncol 2020;12(1):13-16. https://doi.org/10.1080/20742835.2020.1754659 [ Links ]
5. Machado F, Moreno J, Carazo M, León J, Fiol G, Serna R Accuracy of endometrial biopsy with the Cornier pipelle for diagnosis of endometrial cancer and atypical hyperplasia. Eur J Gynaecol Oncol 2003;24(3-4):279-281. [ Links ]
6. Huang GS, Gebb JS, Einstein MH, Shahabi S, Novetsky AP, Goldberg GL. Accuracy of preoperative endometrial sampling for the detection of high-grade endometrial tumors. Am J Obstet Gynecol 2007;196(3):243.e1-e5. https://doi.org/10.1016/j.ajog.2006.09.035 [ Links ]
7. Ghanbari Andarieh M, Agajani Delavar M, Moslemi D, Esmaeilzadeh S. Risk factors for endometrial cancer: Results from a hospital-based case-control study. Asian Pac J Cancer Prev APJCP 2016;17(10):4791-4796. https://doi.org/10.22034/apjcp.2016.17.10.4791 [ Links ]
8. Goldblum JR, Lamps LW, McKenney JK, Myers JL, Ackerman LV, Rosai J, editors. Rosai and Ackerman's surgical pathology. Philadelphia: Elsevier; 2018. [ Links ]
9. Felix AS, Weissfeld JL, Stone RA, et al. Factors associated with Type I and Type II endometrial cancer. Cancer Causes Control 2010;21(11):1851-1856. https://doi.org/10.1007/s10552-010-9612-8 [ Links ]
10. Kurman RJ, Carcangiu ML, Herrington CS, Young RH. WHO classification of tumours of female reproductive organs France: International Agency for Research on Cancer, 2020. [ Links ]
11. Helpman L, Kupets R, Covens A, et al. Assessment of endometrial sampling as a predictor of final surgical pathology in endometrial cancer. Br J Cancer 2014;110(3):609-615. https://doi.org/10.1038/bjc.2013.766 [ Links ]
12. Soslow RA, Tornos C, Park KJ, et al. Endometrial carcinoma diagnosis: Use of FIGO grading and genomic subcategories in clinical practice: Recommendations of the International Society of Gynecological Pathologists. Int J Gynecol Pathol Off J Int Soc Gynecol Pathol 2019;38(1):S64-S74. https://doi.org/10.1097/pgp.0000000000000518 [ Links ]
13. A Yilmaz CH. Concordance of preoperative and postoperative histological grades in endometrioid type endometrial cancer. Eur J Gynaecol Oncol 2020;41(2):208. [ Links ]
14. Fleiss JL. Measuring nominal scale agreement among many raters. Psychol Bull 1971;76(5):378-382. [ Links ]
15. Visser NCM, Reijnen C, Massuger LFAG, Nagtegaal ID, Bulten J, Pijnenborg JMA. Accuracy of endometrial sampling in endometrial carcinoma: A systematic review and meta-analysis. Obstet Gynaecol 2017;130(4):803-813. https://doi.org/10.1097/aog.0000000000002261 [ Links ]
16. Gilks CB, Oliva E, Soslow RA. Poor interobserver reproducibility in the diagnosis of highgrade endometrial carcinoma. Am J Surg Pathol 2013;37(6):874-881. https://doi.org/10.1097/pas.0b013e31827f576a [ Links ]
17. Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynaecol Oncol 1983;15(1):10-17. https://doi.org/10.1016/0090-8258(83)90111-7 [ Links ]
18. Hoang LN, McConechy MK, Kóbel M, et al. Histotype-genotype correlation in 36 high-grade endometrial carcinomas. Am J Surg Pathol 2013;37(9):1421-1432. https://doi.org/10.1097/pas.0b013e31828c63ed [ Links ]
19. Carlson J, McCluggage WG. Reclassifying endometrial carcinomas with a combined morphological and molecular approach: Curr Opin Oncol 2019;31(5):411-419. https://doi.org/10.1097/cco.0000000000000560 [ Links ]
20. Marnitz S, Walter T, Schómig-Markiefka B, Engler T, Kommoss S, Brucker SY. A modern approach to endometrial carcinoma: Will molecular classification improve precision medicine in the future? Cancers 2020;12(9):2577. https://doi.org/10.3390%2Fcancers12092577 [ Links ]
21. Atanda AT, Yusuf I, Haruna MS. Perceived and real histopathology turnaround time: A teaching hospital experience. Niger J Surg Off Publ Niger Surg Res Soc 2017;23(2):98-101. https://doi.org/10.4103/njs.njs_4_17 [ Links ]
 Correspondence:
Correspondence:
A Ismail
docismail88@gmail.com
R Wadee
reubinawadee@gmail.com
Accepted 22 March 2022














