Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Journal of the Southern African Institute of Mining and Metallurgy
versión On-line ISSN 2411-9717
versión impresa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.123 no.12 Johannesburg dic. 2023
http://dx.doi.org/10.17159/2411-9717/3114/2023
PROFESSIONAL TECHNICAL AND SCIENTIFIC PAPERS
Selected trace element concentrations in run-of-mine coal, discard, and coal product, and environmental implications
R.M. Mashishi; O.J. Okonkwo; T. Malehase
Department of Environment, Water, Earth Sciences, Tshwane University of Technology, South Africa. ORCID: R.M Mashishi: http://orcid.org/0000-0002-5079-481X. O.J Okonkwo: http://orcid.org/0000-0001-9396-4949. T. Malehase: http://orcid.org/0000-0002-5788-3911
SYNOPSIS
The concentrations of selected trace elements (As, Cd, Hg, and Pb in run-of-mine (ROM) coal, discard, and coal product were investigated to assess the efficiency of beneficiation in reducing trace element concentrations and ascertain any implications regarding environmental compliance to regulatory frameworks. Samples were collected from a colliery in Mpumalanga Province twice a month for a period of 24 months. The samples were ashed to approximately 0.21 mm, then digested in a mixture of 70% HNO3 and 40% HF and analysed using inductively coupled plasma-mass spectrometry (ICP-MS). Except for Pb, the mean concentration of all elements decreased from the ROM stage to the coal product stage. The order in which trace elements occurred from the highest to lowest throughout the production chain was Pb>As>Hg>Cd. With the exception of Cd, the mean trace element concentrations in the discard were above the total concentration threshold (TCT) set for landfill disposal. The As, Hg, and Cd concentrations in the product were well below the thresholds for all land uses. However, Pb concentrations in the product coal were above legal limits, which is of concern with regard to environmental compliance and the performance and marketability of the product.
Keywords: trace elements, coal, ROM, discard, product, environmental compliance.
Introduction
Coal encompasses a large range of fossil fuels that were derived from the degradation of plant material (Moid, 2008). Coal is one of the worlds most inexpensive, abundant, and most accessible energy sources at present. However, its use creates extensive environmental damage (Ogbonna et al., 2012). Although nuclear and renewable energy are gradually becoming more important for power generation, the combustion of coal is still a major source of power. High-temperature coal combustion for the generation of power results in the emission of pollutants such as NOx, SOx, and particulate matter, among others. Coal also contains trace elements such as As, Hg, Se, Pb, Cr, and Cd, which are highly toxic and have drawn attention from environmental legislators.
Trace elements are nondegradable chemical elements and are emitted during the burning of fossil fuels. As, Cd, Hg, and Pb exist in trace amounts in coal (Munawer, 2018). The primary anthropogenic sources of trace elements are smelters, mines, foundries, vehicle emissions, and combustion of by-products (Moid, 2008). A study on the bioaccumulation of nutrient and trace elements in plants and soil was conducted near an abandoned coal mine in Nigeria. The study indicated that coal mining is one of the anthropogenic sources of toxic elements in the area (Ogbonna et al., 2012).
Coal mined in South Africa for the competitive international market needs to meet the numerous quality specifications of customers. This is achieved by washing coal in a dense media separation (DMS) plant. As a result, coal wastes such as discard are generated. When coal is combusted, most trace elements collect in the coal ash, which is isolated as much as possible from the environment. Despite this, trace elements are emitted to the receiving environment. These emissions are usually much less compared to the major pollutants; however, the impact of trace elements on ecosystems and human health can be quite significant (Senior et al., 2020).
South African coal is known to have a high ash or mineral content, and therefore a high trace element content. Variations in mineral content cause trace elements to vary in coal from the same seam classification (Mguni, 2015). The term 'mode of occurrence' refers to whether an element forms part of a specific mineral or is dispersed within a particular host mineral or in the coal macerals (Finkelman, 1994).
Differences in mode of occurrence are due to geological conditions during coal formation, and knowledge of the geochemistry of trace elements is important in the assessment of the environmental impact of coals (Bai, Wang, and Li, 2017). Coal discard is a major waste product from coal mining and coal washing. Coal discard consists of inorganic and organic minerals with high ash content, high pyrite content, and low heating value. Large quantities of coal discard are stockpiled, which can result in serious environmental consequences such as air pollution, and water and soil contamination. Trace elements in coal discards may be released and pose environmental problems in various degrees, depending on the type of trace element (Guo, 2017). Trace elements of concern include, but are not limited to, arsenic, cadmium, mercury, and lead.
According to Burmistrz et al. (2018), the arsenic content in coal can vary between 0.5-80.0 mg kg-1, 0.3-16.6 mg kg-1, and 0.3-11.0 mg kg-1 as reported by Swaine 1990; Saha et al. 2016, and Dai et al. 2005 respectively. Arsenic is a known carcinogen. Arsenic occurs in three dominant forms in coal, namely pyrite, organic materials, and arsenate. Arsenic is considered an environmentally sensitive element and extensive studies have demonstrated that it is strongly associated with pyrite (Moid, 2008). Arsenic is among the top trace elements of concern due to its volatility, toxicity, and its ability to bioaccumulate in the environment. Cadmium is one of the main pollutants emitted from coal mining and coal combustion. It has been reported that even at low concentrations, Cd emitted from coal can cause reproductive system complications, cardiovascular diseases, brain malfunctions, and neurological disease. Cadmium usually occurs at trace amounts in coal, 0.1-3 mg kg-1. The element is moderately volatile during coal combustion (Cui et al., 2019).
The World Health Organization identified Hg as a chemical of concern in the highest category in 2017, because it poses a threat to human health globally. About 2000 metric tons per year according to the US EPA (https://19january2017snapshot.epa.gov/international-cooperation/mercury-emissions-global-context_.html. Approximately 2000 t of Hg is emitted to the atmosphere globally every year as a result of human activity.
According to the United National Environment Programme (UNEP), Russia, China, South Africa, the USA, Ghana, Colombia, and Indonesia contribute 56% of total anthropogenic emissions to the atmosphere (Singh, Dhyani, and Pujari, 2022). Coal washing and burning are among the major sources of anthropogenic Hg emissions (Pacyna, 1987). According to Pacyna (1987), more than 50% of Hg emissions are from coal-powered power plants. When released through emissions from power plants, Hg, like most trace elements, circulates in the atmosphere as particulates before it is deposited on vegetation, land, and water (Gade, 2015). The Minamata Convention, which was adopted in 2013, regulates the sources of Hg, trade in Hg, manufacturing methods that use Hg compounds, Hg products, artisanal and small-scale gold mining, emission into air, deposition on land and in water, storage, wastage, polluted sites. and health implications (Singh, Dhyani, and Pujari, 2022).
Lead toxicity and its effects on the human nervous system, immune system, and the environment is well documented (Wang et al., 2021). Lead is rarely found in the environment in its elemental state but occurs as Pb2+, in which form it also occurs naturally in lead minerals. Lead in the environment is very mobile and contaminates the air and water. Mining, burning of coal, and vehicular emissions are among the main sources of Pb in the environment (Munawer, 2018).
It was hypothesized that the concentrations of As, Cd, Hg, and Pb in coal products and discards may be affected by beneficiation processes. The present study was, therefore, aimed at assessing the efficacy of the beneficiation process with respect to the selected trace metals as well as evaluate the different coal fractions in terms of environmental compliance.
Geological setting
The project site was a colliery in the Thembisile Hani Local Municipality in Mpumalanga Province, South Africa. The colliery covers an area of about 4 km2 and is situated about 35 km north of Bronkhorstspruit. The site borders Tshwane Metropolitan Municipality of Gauteng Province (Figure 1). The colliery is geologically situated in the Nooitgedacht Outlier, an erosional relict of the Vryheid Formation in the Ecca Group of the Karoo Supergroup. The Karoo Supergroup hosts the largest coal deposit in South Africa, including the Witbank Coalfields (Digby Wells, 2018).
The colliery exploits two coal seams - the main seam and bottom seam. Thick layers of sandstone interbedded with clay and carbonaceous shale mostly overlie the coal seams. The main seam occurs throughout the mining area while the bottom seam is found in the southern section. The mining method employed is opencast truck-and-shovel. Overburden and coal are removed in sequential strips. Excavators and dump trucks are used to load the coal to the ROM stockpiles. The coal is crushed to 40 mm during the beneficiation process for ease of handling and compliance with marketing specifications. Product and discard are produced, with the discard being returned to the pit.
Materials and method
Reagents and materials
Trace element standard solutions of analytical grade were used. Trace metal grade HNO3 (70%) and HF (40%) were purchased from Merck and Sigma Aldrich (Germany).
Samples and sample preparation
The ROM coal was the initial step of sample collection. ROM coal is in its natural state before any processing takes place. Therefore, the results of the ROM samples serve as a benchmark to indicate whether trace element concentrations increase or decrease during beneficiation. Two samples at each processing stage ROM, product, and discard were collected per month over a 24-month period.
ROM samples were collected from the ROM conveyor belt using an auto-mechanical sampler and placed in 50 kg containers. An auto-mechanical sampler was also used to collect product samples from the final product conveyor. The discard sample was collected mechanically at the feed end of the conveyor belts. The maximum sample size was 50 kg. All sample details were initially logged into the system and each sample was allocated a unique sample number for internal tracking.
For digestion of the crushed/solid samples, HNO3 (70%) and HCl (40%) were used. A standard operating procedure (SOP) for general microwave digestion of soil and coal samples was employed. About 10 g of solid sample was initially crushed into a fine powder with a mortar and pestle and passed through a mesh sieve. The solid samples were crushed to 25 mm, later reduced to 6 mm and finally crushed to approximately 0.21 mm. The ashed coal samples were placed in a clean vessel of 7 mL capacity.
The sample was then digested in a mixture of 5 mL 70% HNO3 and 2 mL 40% HF. Ten millilitres of 70% concentrated HNO3 was then added into a 120 mL volumetric container to maintain pressure in the vessel. The loosely closed 7 mL vessel was then placed into the large 120 mL vessel. The vessels were, thereafter, placed into the microwave oven and heated for 1 h at a temperature of 250°C. After the digestion, the samples were transferred into 25 mL calibrated flasks and diluted with HNO3 to give 2-3% solutions. The yellowish solutions were filtered to remove the remaining solids. After dissolution of the samples, the solutions were submitted for analysis using ICP-MS.
Instrumental analysis
A Perkin Elmer Elan 6100 DRC ICP-MS was used for analysis. The percentage recovery of each element was calculated and compared with the values in the certified reference material (CRM). The experimental value is the value calculated from instrumental measurement and the final dilution factor of the extraction. The calibration values gave correlation coefficients that ranged from 0.95 to 0.99.
Quality assurance and quality control (QA/AC)
All glassware was thoroughly washed and rinsed with deionized water and dried between samples. All prepared standard solutions were kept at 4°C in a refrigerator before analysis. Each sample was digested and analysed three times. Blank reagents and reference materials were analysed three times. Blank reagents and reference materials for coal were used in each sample batch to verify the accuracy digestion and analysis.Blank samples and a digested reference material, South African Reference Material (SARM 19) were included to check the accuracy of the digestion and analytical methods.
The recovery rates for the selected trace elements in the reference material (SARM 19) were within the certified ranges of 96%, 97%, 99%, and 99% for As, Cd, Hg, and Pb respectively. A multi-element standard solution including the elements of interest was used to prepare calibration solutions for trace element concentrations. The calibration curves for the selected trace elements were linear and within the expected ranges.
Statistical analysis
The two sets of data (from the first and second samples taken each month) were combined to produce a monthly average. MS Excel and Pearson's correlation coefficient were used to test for relationship and significance.
Results and discussion
The results of the study are presented in Table I. Except for Pb, the mean concentration of all elements was lower in the product than in the ROM coal. As, Cd, Hg, and Pb mean concentrations and standard deviation (SD) in the ROM were 8.71 ± 0.479, 0.13 ± 0.017, 0.38 ± 0.098, and 28.35 ± 2.74 mg kg-1, respectively, whereas in discard, As, Cd, Hg, and Pb concentrations were 31.55 ± 3.935, 0.15 ± 0.018, 1.49 ± 0.106, and 31.15 ± 1.597 mg kg-1 respectively. The mean concentrations of As, Cd, Hg, and Pb in the coal product were 2.93 ± 0.464, 0.12 ± 0.011, 0.20 ± 0.058, and 29.42 ± 2.191 mg kg-1, respectively.
ROM samples
The arsenic concentration in ROM coal (8.7 mg kg-1) was found to be lower than the 13.14 mg kg-1 reported by Hlatswayo and Wagner (2005). However, it is higher than the values of 4.96 mg kg-1, 4.6 mg kg-1,4.7 mg kg-1, and 5 mg kg-1 reported by Mohammed (2010), Cairncross, Hart, and Willis (1990), Bergh (2009), and Zhang et al. (2004) respectively.
Cadmium exhibited a mean concentration of 0.13 mg kg-1 in the ROM, which is marginally higher than the 0.10 mg kg-1 reported by Mohammed (2010) for South African coals. Cadmium concentration in the ROM was, however, much lower than that in Witbank coals (0.3 mg kg-1, Bergh, 2009), Highveld Coalfield no. 4 Seam (0.44 mg kg-1, Hlatshwayo and Wagner, 2005), and global coals (0.6 mg kg-1, Zhang et al., 004).
The mean concentration of Hg in the ROM was 0.38 mg kg-1, a value either below or equal to the South African and global mean values. On the other hand, the Pb concentration was 28.35 mg kg-1, the highest of all the trace elements in the ROM.
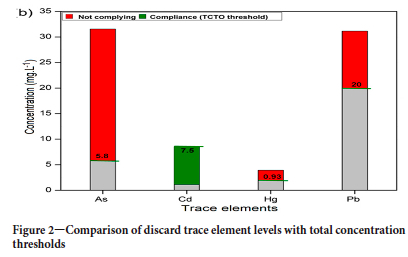
The order of concentrations of the trace elements in the ROM was Pb>As>Hg>Cd.
Discard samples
Arsenic concentration in the discard was 31.55 mg kg-1, which is substantially higher than in the ROM (7.8 mg kg-1). A typical South African washing plant makes use of dense medium separation (DMS) to separate good quality coal from waste material, including ash-forming and sulphur-bearing minerals. As a result, the wastes have a higher ash content and thus a higher elemental concentration than the ROM coal. Moyo (2018) stated that the concentrations of trace elements in coal processing wastes depend on their concentrations and modes of occurrence in the ROM and the nature of the processing operations.
A Department of Minerals and Energy report (DMRE, 2001) indicated that the discards from the DMS plant, which contains most unwanted impurities in the coal, contain more ash-forming minerals and higher sulphur concentrations than the ROM coal (Moyo, 2018). This may explain the elevated trace elements in the discard.
The arsenic concentration in the discard (31. 55 mg kg-1) was higher than the 6.94 mg kg-1 reported for Witbank coal discard. However, the mean Cd concentration, 0.15 mg kg-1, was lower than the 0.30 mg kg-1 reported in Witbank coal. Lead mean concentration in the Witbank discard (33.24 mg kg-1) was slightly higher than that in the present study discard (31.15 mg kg-1).
Coal product samples
Trace element concentrations in the coal product were compared with those in soils around coal-fired power stations. Although coal and soil are two different materials, the combustion emissions ultimately settle in the soil near a coal-fired power station. The As concentration, 2.93 mg kg-1, was either slightly above or in line with the limits for South African soils, but much lower than arsenic concentration (17.5 mg kg-1) in Bangladesh soils (Sahoo, Equeenuddin, and Powell, 2016). The cadmium concentration, 0.12 mg kg-1, was lower than that the 1.22 mg kg-1 in soils near a coal-fired station in Nigeria (Sahoo, Equeenuddin, and Powell, 2016). However, the Pb concentration, 29.42 mg kg-1, was higher than in South African and Nigerian soils (Sahoo et al., 2016), but significantly lower than Pb concentrations in Bangladesh soils (Sahoo, Equeenuddin, and Powell, 2016).
The varying concentrations of trace elements in the final coal product and soils near the coal-fired powered power plants are attributed to geological differences in the coalfields.
Therefore, it is suggested that Pb in the present study ROM may be in the organic fraction, and hence not easily removed, while in the comparative study by Dalton and Feig (2018) Pb may have existed in the inorganic fraction, hence the low Pb concentrations found in the soil.
Coal product
The National Environmental Management Waste Act (2008) and National Environmental Management Act (2008) require land to be investigated if hazardous substances are likely to have been used in or on the land. The use of the soil screening values as an indication of a 'safe' or 'clean' site is valid in the absence of a specific risk exposure scenario. The soil screening values are used to compare trace element concentrations observed in the present study. As, Hg, and Cd concentrations in the product were well below all the screening values, as indicated in Figure 3.
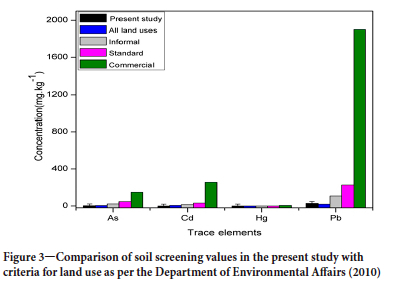
Therefore, if the coal in the current study were to be combusted, emissions would not result in non-compliance or adversely impact water resource and surrounding soils. The lead concentration in the coal product is higher than the limit set for all land uses (Figure 3) but below all other soil screening values. Emissions from combustion would result in negative impacts from Pb on the environment.
Conclusion
Trace element concentrations in the coal product were lower than in the ROM coal, with the exception of Pb. Therefore, the efficiency of coal beneficiation in reducing impurities, including trace elements, was demonstrated. In terms of legislative guidelines, discard mean concentrations of As, Hg, and Pb were above the TCTs set for landfill disposal; whereas the Cd concentration was below the TCT. The concentrations of As, Cd, and Hg in the coal product were all compliant, but Pb was higher than set thresholds for all land uses. The failure of the beneficiation process to reduce to Pb concentrations to below the legal limits is of concern with regard to the quality and marketability of the coal.
Acknowledgements
The authors gratefully acknowledge the financial and technical support from HCI Coal which made this work possible.
References
Bai, X., Wang, Y., and Li, W. 2017. Distribution and occurrence of trace elements in the No.14 coal from the Huolinhe mine. International Journal of Coal Science & Technology, vol. 4. pp. 199-213. https://doi.org/10.1007/s40789-017-0174-1 [ Links ]
Bergh, J.P. 2009. The partitioning of trace elements in the No.4 seam of the Witbank coalfield. MSc thesis, University of the Witwatersrand, Johannesburg. [ Links ]
Burmistrz, P., Wierońska, F., Marczak, M., and Makowska, D. The possibilities for reducing mercury, arsenic and thallium emission from coal conversion processes. Earth and Environmental Science, vol. 174. pp.1-9. https://doi:10.1088/1755-1315/174/1/012003 [ Links ]
Cairncross, B., Hart, R.J., and Willis, J.P. 1990. Geochemistry and sedimentology of coal seams from the Permian Witbank Coalfield, South Africa, a means of identification. International Journal of Coal Geology, vol. 16, pp. 309-325. https://doi.org/10.1016/0166-5162(90)90056-5 [ Links ]
Cui, W., Meng, Feng, Zhou, I., Cui, Y., and Li, W. 2019. Occurrence and release of cadmium, chromium, and lead from stone coal combustion. International Journal of Coal Science & Technology, vol. 6. pp. 586-595. https://doi.org/10.1007/s40789-019-00281-4 [ Links ]
Dai, S., Ren, D., Tang, Y., Yue, M., and Hao, I. 2005. Concentration and distribution of elements in Late Permian coals from western Guizhou Province, China. International Journal of Coal Geology, vol. 61 pp. 119-137. https://doi.org/10.1016/j.coal.2005.09.001 [ Links ]
Dalton, A., Feig, T.G., and Barber, K. 2018. Trace metal enrichment observed in soils around a coal fired powerplant in South Africa. Clean Air Journal, vol. 28, no. 2. pp. 1-9. https://cleanairjournal.org.za/article/view/6945 [ Links ]
Department of Minerals and Energy. 2001. National Inventory discard and duff coal 2001 Summary report. Pretoria, South Africa. [ Links ]
Deurbrouck, A.W. and Cavalaro, J.A. 1978. A washability and analytical evaluation of potential pollution from trace elements in coal. https://www.osti.gov/servlets/purl/6811424 [accessed 17 March 2023]. [ Links ]
Digby Wells. 2018. Palesa waste characterization geochemistry report. Johannesburg. [ Links ]
Finkelman, R.B. 1994. Modes of occurrence of potentially hazardous elements in coal: Levels of confidence. Fuel Processing Technology, vol. 39. pp. 21-34. https://doi.org/10.1016/0378-3820(94)90169-4 [ Links ]
Gade, D. 2015. Mercury emissions from power plants. Environmental Management and Risk Assessment (PH 560). Paper 4. Western Kentucky University. https://digitalcommons.wku.edu/pubh_560/4/ [ Links ]
Gluskoter, H.J. 1975. Mineral matter and trace elements in coal. Trace Elements in Fuel, vol. 141. pp. 1-22. https://doi10.1021/ba-1975-0141.ch001 [ Links ]
Guo. S. 2017. Trace elements in coal gangue: A review. Contributions to Mineralization. InTech. https://www.intechopen.com/chapters/57250 [ Links ]
Hlatshwayo, B. and Wagner, J. 2005. The occurrence of potentially hazardous trace elements in five Highveld coals, South Africa. International Journal of Coal Geology, vol. 63, no. 3-4. pp. 228-246. https://doi.org/10.1016/j.coal.2005.02.014 [ Links ]
Mguni, N.G. 2015. Determination of hazardous trace elements in select Hwange, Zimbabwe coal samples with a comparison to select South African coal samples. MSc thesis, University of the Witwatersrand, Johannesburg. [ Links ]
Mohammed, R. 2010. The path of trace elements in a combustion process: from feed coal to ash products. MSc thesis, University of the Witwatersrand, Johannesburg. [ Links ]
Moid, M.C. 2008. The determination of heavy metals in coal ash. BSc (Hons) thesis, University of Malaysia Sarawalk, Malaysia. [ Links ]
Moyo, A. 2018. Characterising the environmental risks of coal preparation wastes: A study of coal slurry waste and discards from South African collieries. MSc thesis. University of Cape Town. [ Links ]
Munawer, M.E. 2018. Human health and environmental impacts of coal combustion and post-combustion wastes. Journal of Sustainable Mining, vol. 17. pp. 87-96. https://doi.org/10.1016/j.jsm.2017.12.007 [ Links ]
Ogbonna, P.C., Anigor, T.O., Jamie, A., and Da Silva, T. 2012. Bioaccumulation of nutrients and heavy metals in plants at a coal mine. Terrestrial and Aquatic Environmental Toxicology, vol. 6. pp.127-131. [ Links ]
Pacyna, J.M. 1987. Lead, mercury, cadmium and arsenic in the environment. Journal of Applied Toxicology, vol. 8. pp. 69-87. https://doi.org/10.1007/978-94-010-0403-9_4 [ Links ]
Saha, D., Chakravarty, S., Shome, D., Basariya, MR., Kumari, A., and Kundu, AK. 2016. Distribution and affinity of trace elements in Samaleswari coal, Eastern India. Fuel, vol. 181 pp. 376-88. https://doi.org/10.1016/j.fuel.2016.04.134 [ Links ]
Sahoo, P.K., Equeenuddin, M.D., and Powell, M.A. 2016. Trace elements in soils around coal mines: Current scenario, impact and available techniques for management. Current Pollution Reports, vol. 2. pp.1-14. https://doi.org/10.1007/s40726-016-0025-5 [ Links ]
Senior, C., Granite, E., Linak., W., and Seames, W. 2020. Chemistry of trace inorganic elements in coal combustion systems: A century of discovery. Energy Fuels. pp. 15141-15168. https://doi.org/10.1021/acs.energyfuels.0c02375 [ Links ]
Singh, S., Dhyani, S., and Putari, P.R. 2022. Coal-fired thermal power plants and mercury risks: status and impacts to realize Minamata Convention promises. Anthropocene Science, vol. 1. pp. 419-427. https://doi.org/10.1007/s44177-023-00042-8 [ Links ]
South Africa. 2008. National Environmental Management: Waste Act: National Norms and Standards for Disposal of Waste to Landfill, Act No 59 of 2008. Government Printer, Pretoria. [ Links ]
Swaine DJ. 1990. Trace elements in coal. London: Butterworths, pp. 292. https://doi.org/10.1016/0378-3820(94)90176-7 [ Links ]
Wang, Y., Hu, Wang, X, Liu, H., Dong, I., Luo, G., Zhao, Y., and Yao, H. 2021. A critical review on lead migration, transformation and emission control in Chinese coal-fired power plants. Journal of Environmental Sciences, vol. 124. pp. 397-413. https://doi.org/10.1016/j.jes.2021.09.039 [ Links ]
Zhang, J., Ren, D., Zhu, Y., Chou, C-I., Zeng, R., and Zheng, B. 2004. Mineral matter and potentially hazardous trace elements in coals from Qianxi Fault Depression Area in south-western Guizhou, China. International Journal of Coal Geology, vol. 57, no. 1. pp. 49-61. https://doi.org/10.1016/j.coal.2003.07.001 [ Links ]
 Correspondence:
Correspondence:
O.J Okonkwo
Email: OkonkwoOJ@tut.ac.za
Received: 27 Aug. 2023
Accepted: 5 Oct. 2023
Published: December 2023














