Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.123 n.10 Johannesburg Oct. 2023
http://dx.doi.org/10.17159/2411-9717/2477/2023
PROFESSIONAL TECHNICAL AND SCIENTIFIC PAPERS
Electrical resistivity of heat-treated charcoal
R.D. Cromarty; S. Bharat*; D. Odendaal
Department of Materials Science and Metallurgical Engineering, University of Pretoria, Pretoria, South Africa
SYNOPSIS
The aim of this investigation was to determine the effect of high-temperature heat treatment on the electrical resistivity of charcoal. Samples of two different wood types (eucalyptus and black wattle) were pyrolised in a retort at a temperature of 700°C and the resulting charcoals heat-treated in an induction furnace at temperatures from 800°C to 1800°C and residence times from 60 to 120 minutes. After cooling, the resistivities of the samples were measured at room temperature using the four-point probe technique. It was found that as the heat treatment temperature increased the electrical resistivity of the charcoal decreased, approaching an asymptotic value at higher temperatures. Longer residence times decreased the resistivity, but this effect was not pronounced.
Keywords: charcoal, electrical resistivity, high temperatures, heat treatment.
Introduction
The ferroalloy industry is responsible for a considerable portion of the world's greenhouse gas emissions. To reduce these emissions, charcoal can be used as a replacement for coal and coke which are currently used as reductants in submerged arc furnaces. Charcoal produced from wood is considered to be a renewable source of carbon due to its short carbon cycle of 5 to 10 years (in certain climates) compared to approximately 100 million years for fossil fuels (Norgate et al., 2011).
Wood is a lignocellulosic biomass and is largely composed of lignin, cellulose, hemicellulose, and extractives (He et al., 2018; Surup, Trubetskaya, and Tangstad, 2020). The Eucalypts genus in South Africa has a growth rate of 20 to 35 m3/ha-year, which means that the carbon cycle is about ten years (Sappi, 2022). Black wattle, namely the species A. mearnsii and A. dealbata, is a fast growing invasive tree species in South Africa. It is one of the most popular species used for firewood and has a density range of 550 to 850 kg/m3. The advantage of using black wattle to produce charcoal is that it has a very low ash content (0.6%), low sulphur content (0.01%), and a low nitrogen content (0.13%) (Kosowska-Golachowska et al., 2018). The low sulphur and ash content of charcoal is beneficial to the ferroalloy industry as this reduces the amount of impurities being charged to the furnace during smelting. The sulphur content of carbon reductants in the silicon and manganese industries is required to be less than 0.6%, and the ash content less than 12% (Surup, Trubetskaya, and Tangstad, 2020). Using black wattle to produce charcoal will result in a lower slag volume and reduced sulfur levels in the product alloy.
The most common process used to produce charcoal from biomass is pyrolysis. Pyrolysis involves heating the wood to a high temperature in an inert atmosphere, which results in the wood carbonizing to form a solid charcoal product. Some of the factors that influence the pyrolysis process include residence time, maximum temperature, heating rate, and pressure (Dias Junior et al., 2020). In general, charcoal yield from pyrolysis can be optimized by using a low heating rate and long residence time (Dufourny et al., 2019).
The available literature on the electrical resistivity of charcoal is with regard to a packed bed, and not individual charcoal particles. Charcoal that has not been subject to heat treatment generally has a high volatile content and a very high electrical resistivity. Monsen et al. (2007) found that the electrical resistivity of a packed charcoal bed before heat treating was too high to measure at room temperature. The authors found that as the heat treatment temperature increased, the electrical resistivity of the bed of charcoal particles decreased.
Surup et al. (2020) looked at the effect of both high temperatures and particle size on the electrical resistivity of a charcoal bed. It was surmised that the high oxygen content in the charcoal coupled with the disordered carbon structures could be the reason for the high resistivity at temperatures lower than 950°C. An increase in heat treatment temperature results in thermal decomposition of organic compounds, the release of hydrogen- and oxygen-containing volatiles, and a reordering of the residual carbon. As lignin and cellulose are non-graphitizing they will form non-graphitic turbostratic carbon when carbonized at high temperature (Gimåker and Granberg, 2021). This results in a decrease in the electrical resistivity of the charcoal. Surup, Pedersen, and Tangstad (2020) also briefly considered the effect of residence time on the electrical resistivity of charcoal beds at a temperature of 1600°C. It was found that the electrical resistivity initially decreased with increasing time, but after 30 minutes the resistivity decreased at a slower rate and began to approach an asymptotic value.
Although the resistivity of particle beds, including charcoal, has been measured the material resistivity of charcoal is seldom reported. To improve understanding of how charcoal behaves in a furnace it would be useful to know how the resistivity changes with increasing temperature. Ideally, it would be best to measure the resistivity at high temperatures. However, due to the difficulties involved in high-temperature resistivity measurements, in this investigation the resistivity was measured at room temperature after heat treatment.
One of the main disadvantages of using charcoal as a reductant compared to coke is the cost. Charcoal produced in Australia, making use of Lambiotte retorts for pyrolysis, has an estimated production cost of US$386 per ton, while charcoal produced in Brazil costs US$255 per ton (Suopajärvi and Fabritius, 2013). The cost of producing coke is approximately US$ 237.50 per ton (Makgato, Falcon, and Chirwa, 2019).
Experimental plan
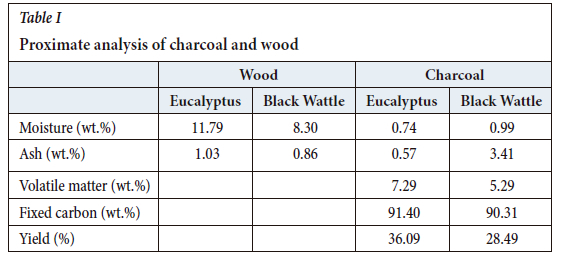
Samples of the eucalyptus and black wattle wood that were used to produce the charcoal are shown in Figure 1. The wood was cut into blocks and dried in a drying oven to remove any excess moisture present. The moisture content of the wood was determined using the ASTM D442-20 standard test method (ASTM International, 2020) and the ash content was measured following the ASTM D1102-84 standard test method (ASTM International, 2021a).
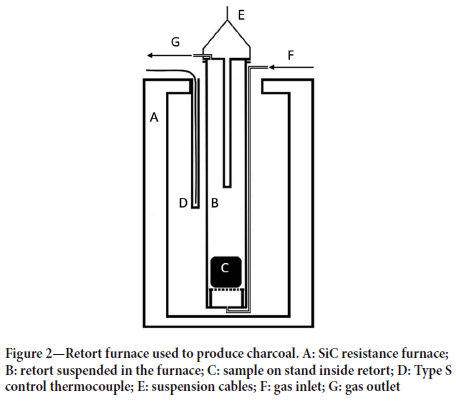
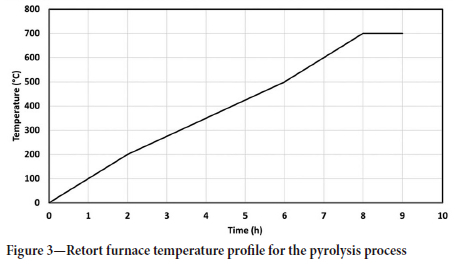
The charcoal was produced in a retort suspended in a resistance heated furnace. Figure 2 shows the general layout of the retort suspended in the furnace. Nitrogen gas was used to maintain an inert atmosphere in the retort. Furnace temperature was measured using a Type S thermocouple mounted in the furnace, outside of the retort. A programmable temperature controller was used to control the temperature of the furnace. The temperature profile of the retort used for this process is shown in Figure 3. The maximum pyrolysis temperature was 700°C. After the pyrolysis process had been completed the retort was removed from the furnace and the charcoal was allowed to cool inside the retort. Large pieces of charcoal were cut into smaller blocks for heat treatment.
Proximate analysis was conducted to determine the differences in the charcoal produced from the two wood types. The proximate analysis determined the moisture, ash, fixed carbon, and volatile matter contents. The proximate analysis was conducted according to the ASTM standard test method for the chemical analysis of wood charcoal (ASTM D1762-84) (ASTM International, 2021b).
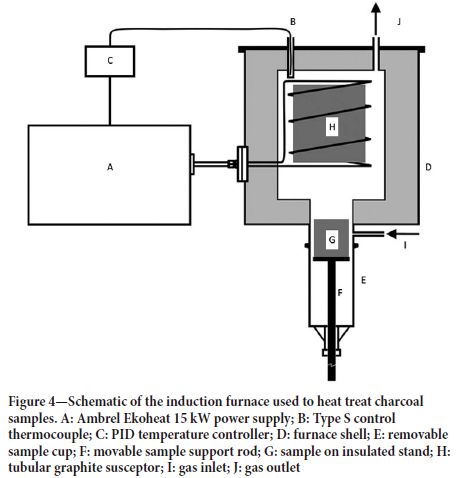
The charcoal samples were subjected to heat treatment cycles in an induction furnace. A schematic of the furnace is shown in Figure 4. The samples were placed in a graphite crucible which was then raised into a preheated graphite susceptor. An inert atmosphere was maintained inside the furnace using argon gas. Heat treatment temperature and residence time were varied while all other factors were fixed. After heat treatment the sample was lowered into the sample cup and allowed to cool in an argon atmosphere.
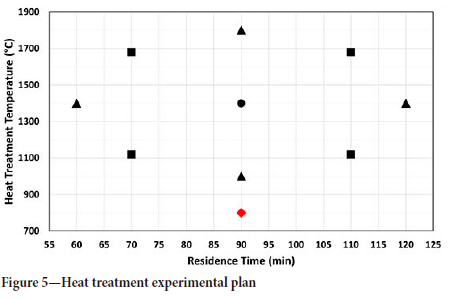
A central composite experimental design was used to allow for a wide range of heat treatment temperatures and times to be investigated while minimizing the number of experiments. The heat treatment plan is shown in Figure 5. An additional heat treatment at 800°C for 90 minutes, indicated in red in Figure 5, does not form part of the central composite experimental design and was simply a further heat treatment that was conducted to obtain more data at lower temperatures. The centre point was repeated three times while all other tests were done once.
The electrical resistivity of the charcoal samples was measured by the four-probe measurement technique. Resistivity was measured in two directions: with current flowing parallel to the wood grain and perpendicular to the grain.
The diagram in Figure 6 shows the set-up that was used for measuring the electrical resistivities. A Velleman DC Lab power
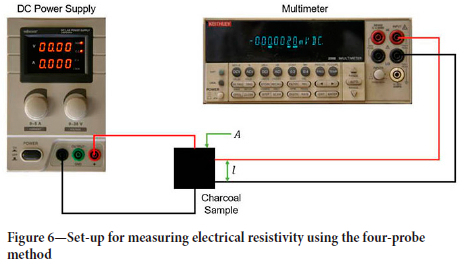
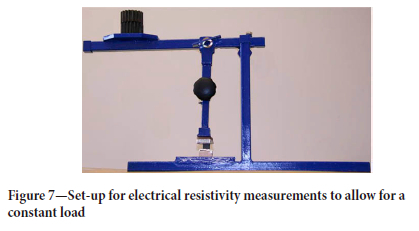
The measurement process was as follows. The probes were first connected to the charcoal sample as shown in Figure 6. The sample was placed between two pieces of aluminium foil backed by polyethylene foam. A constant load was applied to the charcoal sample using the set-up shown in Figure 7. This arrangement ensured good electrical contact across the whole area of the sample and thus a uniform current distribution, as well as reducing any variance between the measurements. The voltage drop across the sample was measured using a set of pins that were a fixed distance apart. The current was first increased from 0.5 A to 4.5 A in 1 A increments, and the corresponding voltage drop recorded. The current was then decreased from 5 A to 1 A in 1 A increments, with the corresponding voltage drop being recorded. These measurements were used to calculate the resistance using Ohm's Law. Making use of several current and voltage readings for each measurement ensured accurate estimates of the sample resistance. The electrical resistivity was then calculated by using the resistance and the sample dimensions as shown in Equation [1].

where: ρ: Electrical resistivity (Ω-m)
A: Cross-sectional area of the sample (m2)
I: Current flowing through the sample (A)
L: Length of the sample (m)
V: Voltage drop across the sample [V]
Results
Wood and charcoal proximate analysis
One of the problems encountered was that it was difficult to produce charcoal at the set temperature, 700°C, without cracks forming. The charcoal produced from black wattle is shown in Figure 8. It can be seen that most of the cracks formed in the direction parallel to the wood grain, along the ray cells. Due to these cracks, the charcoal had to be cut into smaller cubes before heat treating, as shown in Figure 9.
The proximate analyses of the two different types of charcoal, as well as the moisture and ash contents of the two wood feedstock materials, are shown in Table I.
Electrical resistivity
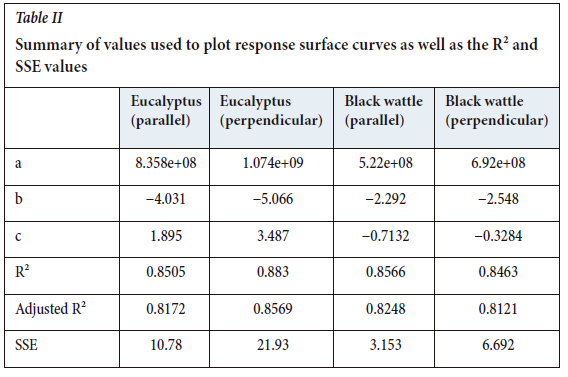
The resistivity measurements are summarized in four plots based on the wood type and the direction of measurement. The empirical equation which was used to describe the response surface plots is shown in Equation [2], and the values which were substituted in for each plot are summarized in Table II. One of the main causes of variance in the measured resistivities was the cracks present in the samples.

where: X1 Temperature (°C)
X2:Time (min)
a, b, c: Constants
Figures 10 and 11 shows the response surface plots for the electrical resistivity of eucalyptus charcoal in the direction parallel to the wood grain and perpendicular to the wood grain respectively. The adjusted R2 values were above 0.8 for the two response surfaces, which indicates that the fit of the models accurately represents the measured values.
Figures 12 and 13 show the response surface plots for the electrical resistivity of black wattle charcoal in the directions parallel to and perpendicular to the wood grain respectively. The adjusted R2 values were above 0.8 for the two response surfaces, which indicates that the fit of the models accurately represents the measured values.
An attempt was made to measure the resistivity of the charcoal produced at 700°C prior to any heat treatment. Using a two-point technique, the resistance exceeded 200 MΩ, the maximum range of the instrument used. This indicates a resistivity of at least 2 MΩ-m. A two-point measurement method was used in this case as the current that could be passed through the sample was too low to accurately measure using the available instrumentation.
SEM analysis
A JEOL JSM-IT300 scanning electron microscope was used in secondary electron mode to view the charcoal samples. SEM images were taken of both eucalyptus and black wattle charcoal to determine if there was any change in the microstructure due to the heat treatments. Figure 14 shows the structures of eucalyptus and black wattle charcoal parallel to the wood grain, and Figure 15 shows the structures perpendicular to the wood grain.
Discussion
The results show that in general, the electrical resistivity of the charcoal samples decreased with an increase in heat treatment temperature and residence time, although this decrease became less pronounced as the temperature increased. The greatest decrease in resistivity took place between 700°C, the temperature at which the charcoal was produced, and 800°C.
The trend in electrical resistivity in the charcoal produced from eucalyptus varied slightly between the grain-parallel and grain-perpendicular directions. The resistivities measured in the perpendicular direction (Figure 11) were slightly higher than those measured in the parallel direction (Figure 10). The resistivity measured in the perpendicular direction decreased from 15.3 mΩ-m at 800°C to 0.4 mΩ-m at 1800°C, and in the parallel direction from 9.6 mΩ-m at 800°C to 0.3 mΩ-m at 1800°C. The difference between the two directions, however, was not as significant as was originally expected. It was expected that there would be a change in resistivity in the two directions due to the difference in structure. However, the SEM analysis showed that the eucalyptus charcoal has a similar structure and porosity in both directions. This can be seen when comparing Figure 14 (a) and (c), the structure parallel to the wood grain, to Figure 15 (a) and (c), the structure in the perpendicular direction.
The trend in resistivity in the charcoal produced from black wattle showed a similar response. The resistivities measured in the perpendicular direction (Figure 13) were once again found to be slightly higher than those measured in the parallel direction (Figure 12). The electrical resistivity measured in the grain-perpendicular direction decreased from 7.1 mΩ-m at 800°C to 0.3 mΩ-m at 1800 °C, and in the grain-parallel direction from 4.7 mΩ-m at 800°C to 0.2 mΩ-m at 1800°C. Comparison of the charcoal structures in the two different directions showed that black wattle exhibits a more distinct structural difference than eucalyptus. The charcoal was more porous in the parallel direction, as seen in Figure 14 (b) and (d), compared to the perpendicular direction (Figure 15 b and d).
The resistivity model presented in Equation [2] is a purely empirical model. An attempt was made to fit models based on the rate of recrystallization of the carbon, But none of the models provided an acceptable fit to the measured data. The data was fitted to an Arrhenius plot without taking the effect of time into account. Using the average resistivity for each temperature it was found that the activation energy for resistivity change was 59.3 kJ/mol and 51.6 kJ/mol for the eucalyptus and black wattle charcoals respectively.
The microstructures of the two types of charcoal did not change significantly with temperature, as seen in Figures 14 and 15. Changes may have occurred to the structure of the charcoal at an atomic level, but these changes would not be visible in the SEM images.
Conclusions
The effect of heat treatment temperature and residence time on the electrical resistivity of charcoal made from two different types of wood was investigated. The following conclusions were drawn from the results.
► The electrical resistivity of both eucalyptus and black wattle charcoal decreased with an increase in heat treatment temperature and residence time.
► In both cases the resistivities measured in the grain-perpendicular direction were higher than those in the grain parallel direction.
► The cracks present in the charcoal blocks would have had a significant effect on the resistivity, thus affecting the results obtained.
► SEM analysis showed that there was no significant change in the microstructure of the charcoal with increasing temperature. There could possibly be changes at an atomic level that would not be visible in the images.
Recommendations
In future projects related to this topic, it is recommended that lower temperatures be used for the pyrolysis process to reduce the amount of cracking that occurs. Additional heat treatments could be conducted in the temperature range of 700°C to 1000°C to gain a better understanding of the trend in resistivity. It is also recommended that the ash of the charcoal produced should be analysed to get a better understanding of the composition of the material. This could be done using either ICP-OES or XRF analysis.
References
ASTM International. 2020. ASTM D4442-20. Standard test methods for direct moisture content measurement of wood and wood-based materials. https://doi.org/10.1520/D4442-20 [ Links ]
ASTM International. 2021a. ASTM D1102-84. Standard test method for ash in wood. https://doi.org/10.1520/D1102-84R21 [ Links ]
ASTM International. 2021b. ASTM D1762-84. Standard test method for chemical analysis of wood charcoal. https://www.astm.org/d1762-84r21.html [ Links ]
Dias Junior, A.F., Esteves, R.P., da Silva, Á.M., Sousa Júnior, A.D., Oliveira, M.P., Brito, J.O., Napoli, A., and Braga, B.M. 2020. Investigating the pyrolysis temperature to define the use of charcoal. European Journal of Wood and Wood Products, vol. 78, no. 1. pp. 193-204. https://doi.org/10.1007/s00107-019-01489-6 [ Links ]
Dufourny, A., van de Steene, L., Humbert, G., Guibal, D., Martin, L., and Blin, J. 2019. Influence of pyrolysis conditions and the nature of the wood on the quality of charcoal as a reducing agent. Journal of Analytical and Applied Pyrolysis, vol 137. pp. 1-13. https://doi.org/10.1016/j.jaap.2018.10.013 [ Links ]
Gimáker, M. and Granberg, H. 2021. Graphite materials - Production from biomass? https://urn.kb.se/resolve?urn=urn:nbn:se:ri:diva-58964 [ Links ]
He, C., Tang, C., Li, C., Yuan, J., Tran, K.Q., Bach, Q.V, Qiu, R., and Yang, Y. 2018. Wet torrefaction of biomass for high quality solid fuel production: A review. Renewable and Sustainable Energy Reviews, vol. 91. pp. 259-271. https://doi.org/10.1016/j.rser.2018.03.097 [ Links ]
Keithley Instruments. 2000. Model 2000 Multimeter User's Manual. https://download.tek.com/manual/2000-900_J-Aug2010_User.pdf [ Links ]
Kosowska-Golachowska, M., Magdziarz, A., Wolski, K., and Luckos, A. 2018. A study into the combustion process of Acacia mearnsii (black wattle) in a circulating fluidized bed. Proceedings of the 23rd International Conference on Fluidized Bed Conversion, Seoul, South Korea. https://www.researchgate.net/publication/325273201 [ Links ]
Makgato, S., Falcon, R.M.S., and Chirwa, E.M.N. 2019. Reduction in coal fines and extended coke production through the addition of carbonisation tar: Environmentally clean process technology. https://repository.up.ac.za/bitstream/handle/2263/74833/Makgato_Reduction_2019.pdf?sequence=1&isAllowed=y [ Links ]
Monsen, B., Tangstad, M., Solheim, I., Syvertsen, M., Ishak, R., and Midtgaard, H. 2007. Charcoal for manganese alloy production. Proceedings of INFACON XI, New Delhi, India, 18-21 February 2007. pp. 297-310. https://www.pyrometallurgy.co.za/InfaconXI/297-Monsen.pdf [ Links ]
Norgate, T., Haque, N., Somerville, M., and Jahanshahi, S. 2011. The greenhouse gas footprint of charcoal production and of some applications in steelmaking. Proceedings of the 7th Australian Conference on Life Cycle Assessment. Australian Life Cycle Assessment Society. https://publications.csiro.au/rpr/download?pid=csiro:EP104971&dsid=DS3 [ Links ]
Sappi. 2022. Frequently asked questions about eucalypts. https://cdn-s3.sappi.com/sfs-public/Sappi-FAQs-Eucalypts-6.pdf [ Links ]
Suopajärvi, H. and Fabritius, T. 2013. Towards more sustainable ironmaking - An analysis of energy wood availability in Finland and the economics of charcoal production. Sustainability (Switzerland), vol. 5, no. 3. pp. 1188-1207. https://doi.org/10.3390/su5031188 [ Links ]
Surup, G.R., Pedersen, T.A., Chaldien, A., Beukes, J.P., and Tangstad, M. 2020. Electrical resistivity of carbonaceous bed material at high temperature. Processes, vol. 8, no. 8. https://doi.org/10.3390/PR8080933 [ Links ]
Surup, G.R., Trubetskaya, A., and Tangstad, M. 2020. Charcoal as an alternative reductant in ferroalloy production: A review. Processes,vol. 8, no. 11. pp. 1-41. https://doi.org/10.3390/pr8111432 [ Links ]
Velleman Group. 2022. Velleman LABPS3005N User Manual. Gavere, Belgium. [ Links ]
 Correspondence:
Correspondence:
R.D. Cromarty
Email: robert.cromarty@up.ac.za
Received: 29 Nov. 2022
Revised: 31 Aug. 2023
Accepted: 5 Oct. 2023
Published: October 2023
* Paper written on project work carried out in partial fulfilment of BEng (Metallurgical Engineering) degree














