Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Journal of the Southern African Institute of Mining and Metallurgy
versão On-line ISSN 2411-9717
versão impressa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.123 no.10 Johannesburg Out. 2023
http://dx.doi.org/10.17159/2411-9717/2299/2023
PROFESSIONAL TECHNICAL AND SCIENTIFIC PAPERS
Contact sorption drying of chromite concentrates
C. SnymanI, *; M. le RouxI; Q.P. CampbellI; S. EngelbrechtII
INorth-West University, School of Chemical and Minerals Engineering. Potchefstroom, South Africa. ORCID: M. le Roux: http://orcid.org/0000-0002-9330-426X. ORCID: Q.P. Campbell: http://orcid.org/0000-0003-0510-6018
IIClarient Solutions Pty (Ltd), South Africa.
SYNOPSIS
Due to the ultrafine particle size required for effective processing of chromite ores, dewatering of the concentrates presents a challenge. It is not uncommon for the ore to have elevated moisture contents even after dewatering, which must be reduced to required levels of between 8% and 10% by mass for further processing. Contact sorption drying has shown promise in test work on fine coal. This method was used to study the dewatering of chromite on a laboratory scale using 3 mm spherical activated alumina ceramic beads as a sorbent. Three different sorbent-to-chromite mass ratios, namely 0.5:1, 1:1, and 2:1, were tested with different process conditions, including dewatering in a stationary and a rotatingl bed. The experimental work showed that it was possible to achieve the target moistures in less than 10 minutes, irrespective of the sorbent-to-chromite ratio used. Ratios of 1:1 or higher, however, proved to be the best. The sorbent reusability at mass ratios of 1:1 and 2:1 were therefore tested. With a 1:1 mass ratio, the sorbents could be reused for three cycles, while with 2:1 ratio, the number of cycles increased to six. The sorbent-to-chromite mass ratio used had a significant influence on the required contact time and the reusability of the sorbents.
Keywords: chromite, contact sorption drying, dewatering.
Background
South Africa is currently the global leader in chromite production, having an estimated 70% of the world's known resources, mainly located in the Bushveld Complex (Backeberg, 2021). Chromite is the only economic mineral from which chromium is obtained (King, 2016). More than 95% of global chromite consumption is for production of ferrochromium, more than 80% of which is used in the stainless steel industry (Holappa, 2013; KPMG, 2018; Murthy, Tripatjy, and Kumar, 2011). For this reason, stainless steel is the main driving factor for the demand and pricing of ferrochromium and chromite commodities (KPMG, 2018). Stainless steel prices are sensitive to changes in the costs of alloying elements since these elements are contained in large amounts (Holappa, 2013). If the costs of producing these alloying elements increase, it will inevitably lead to an increase in the production cost of stainless steel.
Chromite deposits within the Bushveld Complex are present as stratified layers which differ in purity and complexity. Where chromite is mined as the primary product, it is preferable to produce a concentrate known as lumpy, chip, or pebble ore, which varies in size between 6 mm and 150 mm (Beukes et.al, 2017). However, when it is produced as a by-product during platinum group metal (PGM) production, the complexity of the ores necessitates the reduction of chromite particle size below 1 mm to produce a concentrate known as metallurgical grade (van Staden, 2018). This is achieved using primarily spiral and cyclone technology (Murthy, Tripatjy, and Kumar, 2011). Current well-established gravity techniques are, however, inefficient and become complex when treating ultrafine particles (< 75 µm) (Murthy, Tripatjy, and Kumar, 2011). Consequently, other techniques have been investigated and utilized, such as column flotation, jigging, and wet high-intensity magnetic separation (WHIMS) (Mokoena and Nheta, 2020; Murthy, Tripatjy, and Kumar, 2011). These techniques play a major role in the recovery of chromite from tailings.
The abovementioned processes are all water-based and produce a concentrate in slurry form, which must be dewatered prior to ferrochromeium production. Several different industrial techniques are currently utilized for this purpose, namely filters (rotary drums and ceramic disc filters), different spray driers, high-temperature dryers (such as kilns), or natural drainage on stockpile heaps. This is done to reach a target moisture between 8% and 11% by mass, which is ideally suited for the downstream pelletizing process that precedes smelting (Rao, 1994; du Preez, 2018; McDougal, 2013). These processes are in general costly, environmentally unfriendly, inefficient, time-consuming, or require frequent maintenance which increases the operational costs.
According to Tripathy et al., (2019), very little has been done to study and understand the best performing dewatering circuit for ultrafine chromite concentrates. To rectify this, a study was done in India using a tailing slurry from a chromite beneficiation plant containing particles with a d80 of 102 µm and d50 of 36 µm. The study investigated the dewatering of the tailings using different combinations of unit operations generally utilized in the dewatering of tailing streams in the mineral processing industry. A conventional dewatering circuit that has proven to be effective on fine coals, which mainly consisted of thickening followed by pressure filtration, was tested, as well as other circuits where hydrocyclones were included. A final chromite concentrate moisture of 20% by mass was achieved, which is above the prescribed target moisture for pelletizing. These circuits further proved to be uneconomical, even with the introduction of hydrocyclones (Tripahy et al., 2019).
One technology that has proven to be very efficient for dewatering fine coals is contact sorption drying. Van Rensburg et al., (2020a) showed that an equivalent mass of ceramic spheres added to a rotary bed can reduce the moisture content of feed coal from around 20% by mass to inherent moisture content levels and lower within less than 10 minutes' retention time. The discharge from the bed can then be screened to separate the ceramics and coal, producing a final coal suitable for use. The study highlighted the preference for using a rotational bed to aid in particle mixing, instead of a stationary bed (although both were effective), indicative of the need for particle contact to assist in the transfer of the moisture. Le Roux et al., (2018) and le Roux, Campbell, and Hoffman (2019) showed through a series of tests that the predominant transfer mechanism is via liquid adsorption of moisture into the pores of the sorbent. Contact between the ore and the sorbent is therefore required to facilitate the transfer of moisture from the ore particle to the sorbent.
In addition to drying, it was further proven by van Rensburg et al., (2020a) that the sorption capacity of the ceramic can be regenerated in a packed bed using a high air flow at ambient conditions to drive off the moisture and ultrafine particles that diffused into the micro-pores of the spheres.
Since this method has not yet been tested on fine chromite, the aim of this investigation was to study the possibility of using contact sorption as a suitable drying method for ultrafine chromite.
Experimental
Materials
Chromite concentrate originating from the LG-6 (Lower Group 6) seam in the Rustenburg area in South Africa, was used in this study. The as-received material was air dried, split into 1 kg samples, and stored in sealed bags to be used as the feed to the rotary mixer. Prior to feeding to the mixer, water was added to the chromite to achieve an 18% moisture content. A particle size distribution (PSD) analysis of the samples was done using a Malvern Mastersizer 2000. The samples had a d50 size of 85.5 µm, and a d90 size of 192 µm.
Sorbents
Three-millimetre diameter Porocel Dryocel 848 activated alumina ceramic spheres were purchased and used as received. The spheres have a surface area of 320 m2/g, a pore volume of 0.5 cm3/g, a bulk density of 753 kg/m3, a crush strength of 20 kg, and an abrasion and attrition loss of 0.2 wt% and 0.4 wt%, respectively. The material consists of 93.5 wt% Al2O3, 0.35 wt% Na2O, and 0.15 wt% SiO2, and has a 6.00 wt% loss on ignition (1000°C).
The maximum water capacity of the sorbent was tested by drenching a sample completely in water. The moisture content was measured after 24 hours, after 48 hours, and again after 12 days' immersion. The moisture levels were measured at 29.8%, 28.7%, and 28.2%, respectively, resulting in an average maximum water capacity of 28.9% by weight.
Experimental set-up
Two set-ups were used to determine the dewatering capabilities of the ceramic beads. For the first set-up, the sorbent and chromite in mass ratios of 1:1, 0.5:1, and 2:1 were placed in four stationary vessels for a duration of two hours. The concentrate was added first, and then the sorbent, without any mixing involved. Every half hour, one vessel was emptied and sampled to determine the moisture content of the chromite. This was done to simulate the possibility of adding sorbents to a heap of chromite and leaving it to dewater by itself with minimal mixing involved.
For the second set-up, a series of plastic cylindrical vessels of length 5 cm and diameter 5.5 cm was place on rollers to act as the rotating bed. The rollers were set to rotate at three revolutions per minute to introduce a cascading motion of the bed inside the vessel. Material (again in mass ratios of 1:1, 0.5:1 and 2:1) was fed into the ten different vessels and allowed to dry before each was removed and emptied at predetermined time intervals. The content of each vessel was split into chromite and ceramic beads using a standard laboratory sieve. The chromite was then analysed to determine its moisture content. A schematic of the second set-up is shown in Figure 1.
Parameters tested
This study focused on the following:
► The effect of chromite-to-sorbent mass ratio to determine the minimum sorbent addition to the vessels that will yield a satisfactory drying performance.
► Stationary drying versus mixing of the chromite and sorbent to assess the effect of mixing on drying time. This would also give an indication if the addition of sorbents to a drying heap would be effective.
► The reusability and recyclability of the sorbents.
Results and discussion
Stationary tests
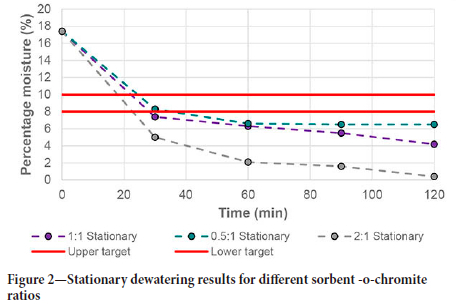
The results of the stationary bed tests are shown in Figure 2. All results reported in this paper are averages of three tests, with errors varying between 0.5 and 2.8 percentage points. The figure shows an initial rapid decrease in chromite moisture content for the first half hour of the tests, followed by a more gradual decline. The dewatering curve is similar to those published by van Rensburg et al., (2020a). The fast initial dewatering is attributed to the excess amount of moisture that is free to move between the chromite particles. When it comes in contact with the ceramic sorbent, the free moisture attaches to the surface of the sorbent and diffuses into its pores. Contact between the sorbent and chromite is important to allow for moisture transfer. This was shown to be the primary transfer mechanism between ore particles and a sorbent (van Rensburg et al., 2020b). Therefore, within a stationary set-up like the one used here, moisture has to diffuse from between the particles to come into contact with the sorbent surface. Such diffusion becomes slower as the amount of free moisture decreases. From Figure 2 it is apparent that the target moisture of 8% by mass was reached after 23 minutes for the 2:1 sorbent to chromite ratio, and approximately 30 minutes for the other ratios. Although stationary dewatering is very slow compared with rotational vessels (Figure 4), it results in a significant decrease in total dewatering time compared to heap drying, which usually takes between two and three days. The addition of sorbents to these heaps would therefore reduce the retention time of the chromite on the heaps and thus deliver a final product much quicker.
Rotating vessels
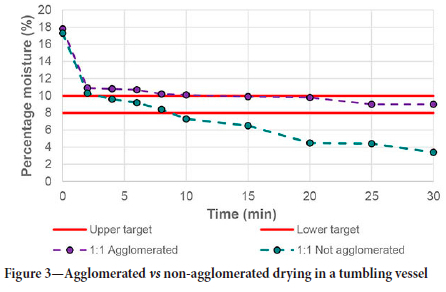
Feed mixtures with similar sorbent-to-chromite mass ratios were used to determine the drying performance of the ceramic beads when subjected to a mixing motion. The initial results of these tests are shown in Figure 3. It became evident at the start of operation that the chromite tended to agglomerate due to the ultrafine nature of the particles coupled with the moisture content. Although dewatering of the chromite was still possible and much quicker than for stationary drying, shown by the purple line in Figure 3 where the moisture target was reached in less than half the time needed for stationary drying, it was clear that thorough mixing was not achieved, and that after 30 minutes, the moisture content of the chromite was higher than for the corresponding stationary test. It was postulated that the initial quicker drying could be attributed to the ore still being free to move in the vessel prior to agglomerating to the sidewalls of the vessel. After the first two minutes of drying, most of the chromite packed against the walls of the vessel, forming a closely packed bed which contributed to stronger interparticle capillary forces and hence weak dewatering. This resulted in the final moisture content of the chromite being higher than for the stationary tests due to the increase water retention properties.
To alleviate the agglomeration of particles to the sidewalls, the vessels were each tapped against the table within the first two minutes of the test to loosen the material from the sides and ensure that better mixing would occur during operation. This would allow constant contact between the sorbent and the chromite, which promotes better dewatering. This is shown by comparison of the two data-sets in Figure 3. The non-agglomerated material reached the lower moisture target quicker than the agglomerated material took to even reach the upper moisture limit. It was able to reach the upper moisture target in just three minutes, having a final moisture content of less than 4% after 30 minutes.
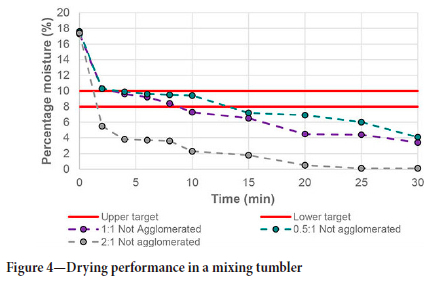
Figure 4 shows the effect of mass feed ratio of the sorbent and chromite. An increase in the ratio of sorbent to chromite will increase the sorbent area available for moisture transfer, which in theory will increase the initial rate of moisture diffusion from the chromite to the sorbent, as well as the final moisture content. Both these assumptions hold true when the grey curve in Figure 4 is compared to the other two curves. The increase in available sorbent surface area, either with proper mixing and/or an increase in sorbent amounts, provides more spaces for moisture to adsorb onto the surface of the sorbent. When the sorbent moisture content was measured at the end of the 30-minute cycle, the 0.5:1 ratio tests yielded sorbents with a moisture content just below 20% by mass, while the 1:1 and 2:1 ratios gave 12% and 7% respectively. This implies that the sorbents used in the 0.5:1 ratio need to be regenerated they can be recycled and reused, since the uptake of moisture closely relates to the ceramic's maximum moisture-holding capacity of 28.9% by mass.
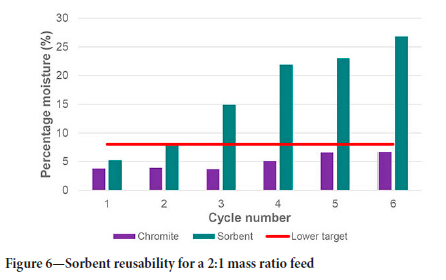
The same does not apply to the 1:1 and 2:1 mass ratio tests. The increase in sorbent area ensures that the amount of moisture adsorbed is not near to the maximum capacity, which allows for the recycling of the sorbents even without regeneration. This concept was tested by feeding the tumbler with either 1:1 or 2:1 sorbent-to-chromite mass ratio material and allowing dewatering to take place for 15 minutes' retention time. After the time elapsed, the sorbents were separated from the chromite and added in the same ratio with fresh moist chromite for a second dewatering test. This was repeated until the sorbent reached its maximum moisture-holding capacity or when the final moisture content of the chromite was above the upper target of 10%. For the 1:1 mass ratio feed, this was recorded to be three cycles, while the 2:1 mass ratio feed reached this point after six cycles, as shown in Figure 5 and Figure 6 respectively.
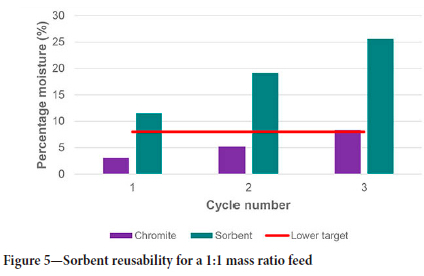
For the 1:1 mass ratio feed, the total chromite moisture was reduced by 83%, from 18.5% to 3.1%, in the first cycle. In the second and third cycles, the total chromite moisture was reduced by 72% and 55%, respectively. After three cycles, the sorbents reached a moisture content of 25.6%, which is approximately 88% of their total moisture-holding capacity. During the last cycle, some chromite started to adhere to the sorbent surface due to the moisture content approaching the maximum capacity. More moisture was present on the sorbent surface, which caused some chromite to agglomerate on the sorbent surface. It was, however, mostly removed by thoroughly shaking the sorbent on the laboratory sieve during the separation step. The capillary forces of the sorbent pores were reaching equilibrium due to saturation, which caused the diffusion rate of the moisture from the sorbent surface into the pores to slow down. Reusing the sorbents for more than three cycles will result in a higher chromite moisture content than desired, and some of the chromite could be lost due to it adhering to the sorbents. It is, however, evident that the sorbent's drying efficiency decreased with each cycle it was reused. This is due to the lower concentration gradient between the sorbent and chromite as a result of the higher saturation of the sorbent after each cycle.
The same effect was seen for the 2:1 mass ratio feed, although it took six cycles to reach this point, as evident from Figure 6.
Conclusions
This study demonstrated the efficacy of using contact sorption drying for dewatering ultrafine chromite. Commercially available ceramic spheres were used as sorbent and showed the potential to dewater the chromite from 20% to a target moisture content between 10% and 8% by mass within a residence time of less than 10 minutes. Since the transport mechanism is dominated by surface contact, the addition of ceramic spheres to the chromite directly influences the dewatering rate and total moisture adsorption. For sorbent-to-chromite ratios of 1:1 or greater, it was possible to reuse the ceramic spheres without regeneration for three to six cycles. This research has demonstrated a promising method to help reduce the turnaround time for dewatering ultrafine chromite. It is, however, imperative to test the proposed method on a larger scale and conduct a financial viability study before a final verdict is possible.
References
Backeberg, N. 2021. Chromium: Ferrochrome prices back up, but for how long? https://roskill.com/news/chromium-ferrochrome-prices-back-up-but-for-how-long/ [accessed 13 August 2021]. [ Links ]
Beukes, J.P., du Preez, S.P., van Zyl, P.G., Paktunc, D., Fabritius, T., Päätalo, M., and Cramer, M. 2017. Review of Cr (VI) environmental practices in the chromite mining and smelting industry - relevance to development of the Ring of Fire, Canada. Journal of Cleaner Production, vol. 165. pp. 874-889. [ Links ]
Du Preez, S.P. 2018. Ferrochrome waste management - addressing current gaps. PhD thesis, North-West University, Potchefstroom. [ Links ]
Holappa, L. 2013. Basics of ferroalloys. Handbook of Ferroalloys: Theory and Technology. Gasik, M. (ed). Elsivier, UK. pp. 9-28. https://doi.org/10.1016/B978-0-08-097753-9.00002-2 [accessed 20 July 2021]. [ Links ]
King, H.M. 2016. Chromite. https://geology.com/minerals/chromite.shtml [accessed 20 July 2021]. [ Links ]
KPMG. 2018. KPMG Commodity Insights Bulletin - Chromite. https://assets.kpmg/content/dam/kpmg/xx/pdf/2018/11/kpmg-commodity-insights-bulletin-chromite.pdf [accessed 13 August 2021]. [ Links ]
Le Roux, M., Campbell, Q.P., van Rensburg, M.J., and Peters, E.S. 2018. Moisture transport during contact sorption of coal fines. Proceedings of the Seventeenth Australian Coal Preparation Conference, Brisbane, Australia. Australian Coal Preparation Society. [ Links ]
Le Roux, M., Campbell, Q.P., and Hoffman, J. 2019 Mechanism of drying coal fines by means of contact sorption. Proceedings of the XIX International Coal Preparation Congress, New Delhi, India. Woodhead, New Delhi. [ Links ]
McDougall, I. 2013. Ferroalloys processing equipment. Handbook of Ferroalloys: Theory and Technology. Gasik, M. (ed.). Elsivier, UK. pp. 83-138. [ Links ]
Murthy, Y.R., Tripathy, S.K., and Kumar, C.R. 2011. Chrome ore beneficiation challenges & opportunities - A review. Minerals Engineering, vol. 24, no. 5. pp. 375-380. [ Links ]
Mokoena, T. and Nheta, W 2020. Beneficiation of South African chromite tailings using magnetic separation. Proceedings of the 6th World Congress on Mechanical, Chemical, and Material Engineering (MCM20). https://avestia.com/MCM2020_Proceedings/files/paper/MMME/MMME_138.pdf [ Links ]
Rao, P.V.T. 1994. Agglomeration and prereduction of ores. 4th Refresher Course on Ferro Alloys, Jamedepur, India. http://eprints.nmlindia.org/5783/1/3.01-3.15.PDF [accessed 22 October. 2021]. [ Links ]
Tripathy, S.K., Murthy, Y.R., Farrokhpay, S., and Filippov, L.O. 2019. Design and analysis of dewatering circuits for a chromite processing plant tailing slurry. Mineral Processing and Extractive Metallurgy Review, vol. 42, no. 2. pp. 102-114. [ Links ]
Van Rensburg, M.J., le Roux, M., Campbell, Q.P., and Peters, E.S. 2020a. Contact sorption: A method to reduce the moisture content of coal fines. International Journal of Coal Preparation and Utilization, vol. 40, no. 4. pp. 266-280. [ Links ]
Van Rensburg, M.J., le Roux, M., Campbell, Q.P., and Peters, E.S. 2020b. Moisture transport during contact sorption drying of coal fines. International Journal of Coal Preparation and Utilization, vol. 40, no. 4-5. pp. 281-296 [ Links ]
Van Staden, Y. 2018. The impact of raw material selection on damring formation and pre-reduction during ferrochrome production. PhD thesis, North-West University, Potchefstroom. [ Links ]
 Correspondence:
Correspondence:
M. le Roux
Email: marco.leroux@nwu.ac.za
Received: 31 Aug. 2022
Revised: 10 Feb. 2023
Accepted: 10 Jun. 2023
Published: October 2023
* Paper written on project work carried out in partial fulfilment of BEng (Chemical Engineering) degree.














