Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.123 n.8 Johannesburg Aug. 2023
http://dx.doi.org/10.17159/2411-9717/2082/2023
PROFESSIONAL TECHNICAL AND SCIENTIFIC PAPERS
Mechanical activation and physicochemical factors controlling pyrometallurgical, hydrometallurgical, and electrometallurgical processing of titanium ore: A review
H.C.S. Subasinghe; A.S. Ratnayake
Department of Applied Earth Sciences, Faculty of Applied Sciences, University, Passara Road, Badulla, Sri Lanka
SYNOPSIS
In this study, we review the role of mechanical activation in the pyrometallurgical, hydrometallurgical, and electrometallurgical processing of titanium feedstock. Mechanical activation has been shown to decrease the activation energy of chemical reactions, thus enhancing process efficiency and product quality by reducing processing time and energy consumption. Pyrometallurgical processing is energy-intensive and time-consuming. Hydrometallurgy is costly, requires high-grade feed material, and generates toxic waste. Waste generation and process complexity are the major drawbacks of electrometallurgy and solvent extraction. Bioleaching via a mechanically activated pyrometallurgical process can be identified as an alternative method, but the lengthy processing time is the major disadvantage. Mechanically activated titanium concentrate can be used in a finely tuned combined metallurgical process to overcome the challenges and drawbacks in these technologies.
Keywords: ilmenite, synthetic rutile, titanium metal, pyrometallurgy, hydrometallurgy, electrometallurgy, mechanical activation.
Introduction
Titanium is the ninth most abundant element in the Earth's crust (Das et al., 2013), and is known to be the metal of the 21st century. Titanium-rich heavy minerals such as ilmenite (40-80% TiO2), leucoxene (>65% TiO2), and rutile (approx. 95% TiO2) are the major titanium minerals used to produce refined TiO2 and titanium metal (Haverkamp, Kruger, and Rajashekar, 2016; Kothari, 1974; Shi et al., 2022; Zhu, Zhang, and Cheng, 2011; Subasinghe et al., 2022). About 95% of the annual global production of rutile (both natural and synthetic) is utilized to produce high-quality white TiO2 pigments, while the rest is mainly used in the production of titanium metal (Gázquez et al., 2014). Titanium dioxide is characterized by properties such as high transparency to visible light, iridescence, and high UV absorption. TiO2 therefore has diverse applications such as in pharmaceuticals, advanced ceramics, paints, porcelains, and rubber (Elsner, 2010; Subasinghe and Ratnayake, 2021; Subasinghe and Ratnayake, 2022). The photocatalytic activity of TiO2 has been used in advanced applications such as photovoltaic cells, gas sensors, purification filters, and electro-ceramics (Bai et al., 2014; Wang and Lin, 2010). Titanium metal finds applications in the aerospace industries, and biomedical engineering such as prosthesis (Elsner, 2010; Subasinghe and Ratnayake, 2021). Titanium minerals cannot be directly used in any of these applications. Consequently, it is essential to upgrade/process titanium ores into refined TiO2 and/or titanium metal.
Ilmenite smelting was first reported in the late 19th century in New Jersey, USA, and the production of titanium alloys was initiated in 1906 (Morley, 1981). Titanium white pigments were first produced a couple of years later, replacing the toxic Pb and Zn white paint pigments (Brooks, 2000). Since then, several routes have been developed for the conversion of low-grade titanium ores into synthetic rutile via chemical, physical, physicochemical, and thermochemical techniques (Nguyen and Lee, 2018; Zhang, Zhu, and Cheng, 2011). Processing high-grade feedstock (natural rutile) generates less waste compared to low-grade feedstock such as ilmenite and leucoxene (Subasinghe et al., 2022) and is the preferred feedstock in the titanium mineral processing industry. However, ilmenite and leucoxene became prominent feed materials to cater to the surging demand for titanium and its compounds due to the scarcity of natural rutile (Subasinghe and Ratnayake, 2021; Wang and Yuan, 2006).
Commercial production of TiO2 and titanium metal follows distinct routes (Figure 1). Nevertheless, all routes require titanium ores to be upgraded, -usually consuming large amounts of energy and utilizing concentrated acids (Gázquez et al., 2014; Takeda, Ouchi, and Okabe, 2020).
This study is intended to fill the gap in the recent literature between titanium metallurgical processes and the role of mechanical activation in each method. We outline the controlling factors in hydrometallurgical, pyrometallurgical, and electrometallurgical processes to upgrade/refine titanium ores and discuss the significance of mechanical activation for each of the processing techniques (Table I).
Role of mechanical activation
Initial mechanical activation, with or without the addition of reductants, can be advantageous for upgrading titanium ores to synthetic rutile and titanium metal. This step influences the efficiency, product quality, waste generation, and cost of subsequent unit operations. Milling increases the reaction rates by (Amade et al., 2009; Baba et al., 2013; Begin-Colin et al., 1994; Ren, Yang, and Shaw, 2000; Sasikumar et al., 2007; Subasinghe and Ratnayake, 2021; Tao et al., 2012; Tromans and Meech, 2001; Wei et al., 2009; Welham and Llewellyn, 1988):
➤ Increasing the specific surface area
➤ Breaking down crystalline structure (i.e., grain boundary disordering, polymorphic transformations, and creation of defects such as Schottky, Frenkel, or Wadsley defects along crystallographic shear planes)
➤ Promoting chemical reactions (i.e., mechanochemical reactions with order-disorder reactions and phase transformations, especially in oxides)
➤ Promoting surface amorphization.
The X-ray diffraction patterns of activated and unactivated ilmenite are similar, with no new phases forming during mechanical activation (Li et al., 2008; Li, Liang, and Wang, 2008b; Sasikumar et al., 2004, 2007; Shojaei et al., 2014; Tan, Hu, and Zhang, 2011; Wang et al., 2010; Wei et al., 2009; Wu et al., 2011a; Zhang et al., 2010). However, ilmenite milled with sulphur as a reducing agent shows weak reflections from new phases formed during attrition (Chen et al., 1996; Subasinghe and Ratnayake, 2021). Ball milling can induce alteration of the lattice structure (i.e., rearrangement of grains and increments of strain). In this case, ilmenite peaks in the diffractogram become broadened with diminished intensities (Shojaei et al., 2014).
Milling conditions
Grinding is the main method for the mechanical activation of titanium ore. Milling parameters are thus significant for effective and efficient grinding with the minimum possible energy consumption. For example, a lower ball-to-powder ratio results in less efficient grinding and longer grinding times (Begin-Colin et al., 2000). Planetary, attritor, and vibration mills are different types of ball mills based on the movement of balls and vials (Zhang, Zhu, and Wang, 2008). Impact, chipping, and abrasion are key mechanisms for the deformation of particles in these milling techniques (Kurlov and Gusev, 2007; Wu et al., 2018; Zhang, Zhu, and Wang, 2008, 2013). The median particle size (d50) and the specific surface area are generally used to assess the effectiveness of ball milling. The main milling parameters are rotation speed (r/min), size of balls, ball material (wear resistance and hardness), ball-to-powder mass ratio, milling time, medium of milling, filling ratio, milling container, additives/reducing agents, and milling atmosphere (e.g., vacuum, airtight, ambient air, or inert gas) (Table II). The selection of the milling parameters varies substantially based on feed materials (Zhang, Zhu, and Wang, 2008). However, trace contamination can occur from the balls and vials (Dworkin et al., 2018; Zhang et al., 2013).
Although the conditions listed in Table II have been used in successful bench-scale experiments, they are rarely reported to have been incorporated on an industrial scale, due to the lack of scale-up optimized process parameters for both hard-rock and placer ilmenite.
Milling time and particle size
Increased milling time decreases the particle size and increases the effective surface area. However, the rate of particle size reduction gradually decreases with time. The formation of composites can be initiated by using additives/reducing agents, during milling. Subasinghe and Ratnayake (2021) demonstrated the effect of milling time and particle size on the reduction of ilmenite. Nanoparticles were produced by ball milling a mixture of ilmenite, sulphur, and vein graphite in the weight ratio of 4:0.5:0.5 for 6 hours at room temperature.
Several studies have focused on the mechanical activation of titanium ores followed by acid leaching (Nguyen and Lee, 2018; Zhang, Zhu, and Cheng, 2011). In this regard, prolonged milling to decrease the crystallite size improved the leaching efficiency of ilmenite (Shojaei et al., 2014; Wei et al., 2009). The improvement of leaching efficiency can occur due to the hindrance of lattice structure by mechanical activation (Li et al., 2008; Li, Liang, and Wang, 2008; Zhang et al., 2010). Li, Liang, and Wang (2008) demonstrated that high-energy ball milling increases iron dissolution and the subsequent hydrolysis of titanium by HCl. However, the filterability of reactive slurries is a major drawback with fine-grained particles. Milling time can be adjusted to obtain suitable particle sizes for solid-liquid separation (Li, Liang, and Wang, 2008).
Pyrometallurgy in titanium ore processing
Solid-state reactions such as oxidation and reduction occur at elevated temperatures (Bordbar, Yousefi, and Abedini, 2017; Nguyen and Lee, 2018; Zhang, Zhu, and Cheng, 2011). However, thermal treatments do not yield only pure products (Kothari, 1974). In this case, a mixture of TiO2 and elemental iron (usually referred to as slag in titanium ore processing) is obtained. These pyrometallurgical processing routes employ combinations of thermal oxidation and reduction by roasting, leaching, and physical separation (Zhang, Zhu, and Cheng, 2011). During these processes, iron is converted to the soluble ferrous or elemental form by thermal reduction in a pre-treatment step (Nguyen and Lee, 2018; Zhang, Zhu, and Cheng, 2011), and the ore is subsequently acid-leached to obtain synthetic rutile (TiO2) (e.g., Kataoka and Yamada, 1973).
Common reducing agents
Several authors have highlighted the benefits of reducing agents during the high-energy ball milling of titanium ores (Chen et al., 1996, 2013a); Chen, Tang, and Xiao, 2015; Shahien et al., 2015; Wijewardhana, Subasinghe, and Ratnayake, 2021). For example, ilmenite undergoes sulphurization reduction (Equation [1]) with the formation of pyrite (FeS2) during prolonged milling in the presence of sulphur at room temperature (Chen et al., 1996; Subasinghe and Ratnayake, 2021). Chen et al. (1996) also claimed that milling ilmenite with sulphur for 200 hours successfully produces TiO2 at room temperature. Recently, Subasinghe and Ratnayake (2021) reduced the milling time to 6 hours by optimizing the ilmenite to sulphur ratio. Chen et al. (1996) obtained pure TiO2 powder by sulphurization reduction followed by selective leaching using HCl. Shahien et al. (2015) isolated the Fe and Ti phases as elemental Fe and TiO2 using carbothermic reduction, and leached the product to remove iron and produce pure TiO2. Wijewardhana, Subasinghe, and Ratnayake (2021) and Subasinghe and Ratnayake (2021) optimized parameters such as grinding, and carbothermic and sulphurization reduction conditions.
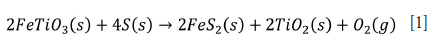
Various carbon sources (flake graphite, crystalline vein graphite, coal, anthracite, carbon black powder, activated carbon, and carbonized waste coconut shells) have been successfully used in the carbothermic reduction of ilmenite (Amer, 2002; Chen et al., 2013, 2015; Shahien et al., 2015; Subasinghe and Ratnayake, 2021; Tao et al., 2012; Tripathy, Srinivasan, and Mehrota, 2012 ; Wang et al., 2008; Wijewardhana, Subasinghe, and Ratnayake, 2021). However, the initiation of carbothermic reduction during ball milling was not observed in these investigations. Subsequent thermal treatments have produced TiCh via carbothermic reduction of ilmenite (see Equations [2]-[4]) (Chen et al, 1996, 2013a; Merk and Pickles, 1988; Run et al, 2017; Shahien et al, 2015; Thripathy et al, 2012; Wang and Yuan, 2006; Welham, 1996; Zhao, 1990).
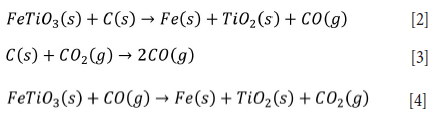
Interestingly, Subasinghe and Ratnayake (2021) obtained more promising results using commercially available sulphur and crystalline vein graphite (Figure 1).
Significance of reducing agents
The reducing agents (sulphur and carbon) improve results during pyrometallurgical processes. A sufficient mass (according to stoichiometric or weight ratios) should be added to complete the reduction (Chen et al., 1996; Shahien et al., 2015; Subasinghe and Ratnayake, 2021). Chen et al. (1996) used an ilmenite to sulphur ratio of 6:2.5 in the milling process to produce TiCh at room temperature. A mixture of graphite and titanium dioxide was used to observe polymorphic transformations and powder characteristics during high-energy ball milling (Ren, Yang, and Shaw, 2000). The authors observed the transformation of anatase to rutile and srilankite, and amorphization of TiCh.
In addition to polymorphic transformation, TiC2 and graphite particles, and crystallite refinement, agglomeration of fine particles, and mixing of TiCh and carbon on a nanometre scale were observed. Ilmenite and carbon were mixed at the stoichiometric ratio of 4:1 to provide sufficient carbon for completing ilmenite reduction (Shahien et al., 2015; Tao et al., 2012). Carbonized coconut shells mixed with ilmenite in the ratio of 1:4 have also served as a successful reducing agent (Wijewardhana, Subasinghe, and Ratnayake, 2021). Subasinghe and Ratnayake (2021) also used ilmenite with sulphur and vein graphite in three different weight ratios. A combination of ilmenite, sulphur, and vein graphite in the ratio of 4.0:0.5:0.5 produced the best results.
Oxidation and reduction during thermal treatment
In industrial practice, oxidation involves high-temperature thermal treatment in a rotary kiln in the presence of air or oxygen to convert iron in titanium ore to iron oxides (Tan, Hu, and Zhang, 2011). Oxidation enhances the reactivity of titanium minerals (Janssen and Putnis, 2011; Sarker, Rashid, and Kurny, 2006; Zhu, Zhang, and Li, 2014). The preferred oxidation products are haematite and rutile. However, the increase in crystallite size of haematite and rutile above 800°C decreases the interfacial surface area (Zhu, Zhang, and Li, 2014), which may decrease the efficiency of subsequent leaching. According to Vásquez and Molina (2012), oxidation of titanium ore at 800-1050°C increases the proportion of pseudobrookite. The oxidation rate increases gradually with temperature, and ilmenite can be completely oxidized above 800°C, as shown in Equation [5] (Zhu, Zhang, and Li, 2014).
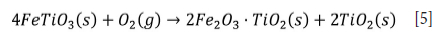
The reduction of titanium ore is relatively cheap to carry out, and is thus preferred in the industry. Titanium ore is heated in the presence of a reducing agent as described above. Numerous investigators have reported the successful reduction of titanium ore using either solid or gaseous reductants such as H2 and CC, or mixtures of both (Bordbar, Yousefi, and Abedini, 2017; Kothari, 1974; Merk and Pickles, 1988). Several techniques have been commercialized to reduce titanium ore; for instance, smelting in electric furnaces to yield titania-enriched slag and pig iron (Bordbar, Yousefi, and Abedini, 2017), removal of Fe, Mg, Si, and Mn oxides using vacuum carbothermic reduction (Run et al., 2017), and microwave heating (Wang et al., 2014). Zhao (1990) described the carbothermic reduction of titanium ores using four steps: (i) diffusion of CO into porous grains, (ii) reaction of CO with ilmenite to produce TiO2 and Fe, (iii) migration of Fe from the unreacted core towards the grain boundaries, and (iv) formation of Fe nuclei and their subsequent growth. Zhao (1990) used three stages (induction, acceleration, and deceleration) to demonstrate the mechanism of conversion of ilmenite to Fe and TiO2. The coalescence of Fe particles retards the rate of reduction (Zhao, 1990). Moreover, the annealing environment (vacuum, air, or inert gas) is also important for the reduction reactions (Merks and Pickles, 1988). However, the major disadvantage of oxidation and reduction pretreatments is the intense energy consumption (Vásquez and Molina, 2008).
Temperature and time
Temperature and time of thermal treatment (e.g., annealing, sintering, smelting, roasting) are important parameters in pyrometallurgical processes (Merks and Pickles, 1988; Run et al., 2017; Wang et al., 2014; Zhang and Ostrovski, 2001, 2002; Zhao, 1990). The temperature and time of pretreatment determine the amount and concentration of acid required and energy consumption during post-treatments such as leaching. Mechanical activation/grinding of ilmenite ore before heat treatment considerably reduces the annealing temperature and time (Subasinghe and Ratnayake, 2021). Moreover, the purity and crystallinity of the end-products depend on temperature and time of thermal treatment, whereas mechanical activation has produced better results (Subasinghe and Ratnayake, 2021). For example, high-purity TiO2 crystals can be formed by thermal treatment above 1300°C. The crystal size of rutile increases with increasing temperature (Bordbar, Yousefi, and Abedini, 2017; Subasinghe and Ratnayake, 2021; Zhang et al., 2011). Thermal treatments oxidize Ti3+ to Ti4+ with increasing temperatures from 1000°C to >1500°C (Zhang et al., 2011). It is also claimed that the grain size and volume fraction of rutile depend on melting temperature, heating time, and cooling rate. In this case, the rutile phase is precipitated rapidly during high-temperature treatment. The grain sizes are subsequently increased during the isothermal heating time and cooling stages (Zhang et al., 2011).
Possible catalysts for the carbothermic reduction of titanium ores
Carbothermic reduction of ilmenite is not preferred in the industry due to time requirements and high energy consumption. However, the addition of catalysts during mechanical activation could decrease the activation energy of reactants during heat treatment and subsequent leaching processes. Several studies have focused on enhancing the rate of solid-state reactions to reduce energy consumption and speed up the process. Alkalis, chlorides, carbonates, and oxides have been found to increase the rate of reduction of iron oxide (Barnes and Pickles, 1988; Lv et al., 2017; Singh, Kishor, and Mankhand, 2018; Taylor, 1976). Specifically, the alkali carbonates of Group 1A elements in the periodic table have been shown to increase the reaction rates of titanium ore with gases such as CO2 and CO by increasing the fluidity of the charge (Rao, Adjorlolo, and Haberman, 1982). Singh, Kishor, and Mankhand (2018) used sodium carbonate as a catalyst for the carbothermic reduction of ilmenite. Similarly, Wijewardhana, Subasinghe, and Ratnayake (2021) used powdered seashells (calcium carbonate) during milling as a possible catalyst to enhance the rate of carbothermic reduction of ilmenite. Future research should investigate better catalysts for use during mechanical activation for sulphurization and carbothermic reduction of titanium ore.
Effect of impurities and ore grade on reduction
The physical and chemical characteristics and mineralogy of titanium ores significantly affect the rate of reduction reactions (Ismail, Amarasekera, amd Kumarasinghe, 1983; Merks and Pickles, 1988, 2008; Sasikumar et al., 2004; Wang and Yuan, 2006; Welham, 1996; Welham and William, 1999; Zhang and Ostrovski, 2001). For example, the presence of manganese impurities (Mn >1.24 wt.%) in titanium ores reduces the rate of carbothermic reduction (Wang and Yuan, 2006; Wang et al., 2008). Zhang and Ostrovski (2001) investigated the effect of different Fe and TiO2 contents on the rate of reduction. The authors suggest that higher Fe contents increase the reduction rate at the induction stage. In contrast, magnesium oxide (MgO), manganese oxide (MnO), calcium oxide (CaO), and silica inhibit reduction (Merks and Pickles, 1988). The reduction rate of titanium ores is controlled by the grade and impurity level of the feedstock, and thus the purity of synthetic rutile produced using mechanical activation and pyrometallurgical routes is questionable. Mechanical activation/fine grinding followed by magnetic and gravity separation would yield a higher grade concentrate.
Hydrometallurgical processes
Extraction or leaching using solutions (liquid phases) is referred to as hydrometallurgical processing. Pyrometallurgical processes are also followed by hydrometallurgical steps to extract pure TiO2 and titanium metal. There are several hydrometallurgical processes used in the titanium industry (Figure 2).
Acid leaching
HCl and H2SO4 are the most commonly used acids for upgrading and purification of titanium ores. However, the Reachability of titanium ore using weaker acids such as oxalic acid and citric acid has also been studied (Table III). Mahmoud, Afifi, and Ibarhim (2004) classified acid leaching of titanium ores into five process routes:
(i) Smelting followed by H2SO4 or HCl leaching at elevated temperatures
(ii) Reduction of titanium ore followed by acid leaching
(iii) Reduction of iron in ilmenite to metallic iron followed by corrosion with oxygen and ammonium chloride
(iv) Oxidation of titanium ore followed by reduction and HCl leaching
(v) Roasting and magnetic separation followed by HCl leaching.
Almost all of these processes have been successful in the production of TiO2 and/or titanium metal using direct leaching techniques (Figure 3). However, there are both advantages and disadvantages to these processes (Table III), most of which can be circumvented by mechanical grinding before acid leaching.
Leaching time and temperature are significant variables affecting product quality. Therefore, future research should focus on the kinetic modelling of mechanically activated titanium ore to investigate the leaching models. If ball-milled titanium ore follows the shrinking core model during leaching, the effectiveness and process efficiency could be raised (Kuppusamy and Holuszko, 2022).
Factors such as temperature, concentration of acid, particle size, and solid to liquid ratio also affect the leaching kinetics, efficiency and quality of products (Nguyen and Lee, 2018; Zhang, Zhu, and Cheng, 2011). However, HCl and H2SO4 are consumed in dissolving impurities. Therefore, only part of the acid is used to break the strong covalent bonds of titanium minerals. This reduces the economic feasibility at an industrial scale. Simple initial leaching with dilute HNO3 can be used to dissolve the impurities, thus reducing the consumption of HCl and H2SO4 (Figure 4). A brief comparison of the advantages and disadvantages of common hydrometallurgical methods is summarized in Table IV.
H2SO4 leaching
H2SO4 leaching dissolves titanium minerals to form Ti(VI) and SO42"/ HSO4" complexes, such as TiOSO4, TiO(SO4)22-, TiO(SO4)46", and Ti(OH)3HSO4 (Nayl and Aly, 2009). Han, Rubcumintara, and Fuerstenau (1987) reported that a distinctive product layer of TiOSO4 and FeSO4 is formed at higher than 16 M H2SO4, and which is soluble in water at 98°C (see Equations [6] and [7]). Jonglertjunya and Rubcumintara (2013) identified a significant reduction of titanium dissolution in acid concentrations ranging from 12 M to 18 M at 90°C, due to the formation of titanium precipitates. Li, Liang, and Guo (2007) and Li et al. (2008) ascertianed that the leaching of titanium is very low in H2SO4 solution (concentration range from 10 to 40 wt.%) below 100°C due to the hydrolysis of dissolved titanium. Leach residues can form a compact layer on the surface of unreacted ilmenite at 150°C with sulphuric acid concentration above 40 wt.%, hindering the leaching of iron (Jia et al., 2014).
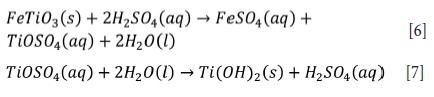
HCl leaching
Samal (2011) found that the leaching efficiency of titanium is lower than that of iron at low acid concentrartions such as 2.5 M HCl due to the precipitation of TiOCb (Equations [8] and [9]).
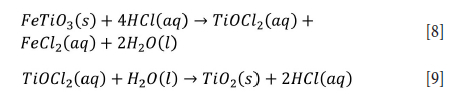
El-Hazek et al. (2007) suggested that leaching with high acid concentrations such as 12 M at 80°C is needed to suppress the hydrolysis of titanium at a high solid to liquid ratio of 1/20. The dissolution of titanium and iron increases with increasing acid concentration (Das et al., 2013; El-Hazek et al., 2007; Middlemas, Fang, and Fan, 2013; Olanipekun, 1999). The leach liquor of the pretreated titanium slag leached with 0.75-1.5 M HCl at 50°C is quite turbid and difficult to filter (Middlemas, Fang, and Fan, 2013). Clear filtrate and less finely suspended particles can be obtained at HCl concentrations above 1.5 M. However, precipitation occurs in this solution after only a few days (Middlemas, Fang, and Fan, 2013). Consequently, the hydrolysis of dissolved titanium occurs at either low acidity or low temperature (e.g., 1.5 M and 50°C).
The effect of HCl concentration on the selective leaching of iron over titanium from ilmenite has been reported in the literature (Gireesh et al., 2015; Guo et al., 2014; Lasheen, 2005; Liu et al., 2015; Mahmoud, Afifi, and Ibarhim, 2004). Iron is almost completely dissolved in 30 wt.% HCl under reducing conditions, but titanium dissolution is negligible (Mahmoud, Afifi, and Ibarhim, 2004). In addition, 20 wt.% HCl is a suitable acid concentration for the selective leaching of iron. Therefore, it enhances the TiO2 content in the residue (Guo et al., 2014). Liu et al. (2015) also indicated that the recovery of TiO2 in the residue increased with increasing acid concentration from 200 to 240 g/L. Consequently, HCl concentrations higher than 220 g/L can be used to obtain a product containing more than 92% TiO2. The leaching efficiency of iron also increased as the acid concentration increased from 2 M to 12 M, but the solubility of TiO2 seemed to be negligible in this acid concentration range (Gireesh et al., 2015; Lasheen, 2005). Furthermore, TiO2, HCl, and MgO (MgCl2 is added to increase the chloride ion concentration to enhance titanium and iron leaching) can be recovered via pyrohydrolysis/high-temperature hydrolysis. The recovered HCl is recyclable as shown in Equation [10] (Zhang, Zhu, and Cheng, 2011).
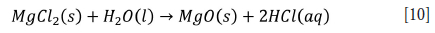
HCl is generally more efficient than H2SO4 in terms of titanium dioxide purity and recovery (Jia et al., 2014; Mehdilo and Irannajad, 2012; Razieh, 2014; Sasikumar et al., 2004). In addition, HCl can be easily recovered from waste, making it more advantageous than H2SO4 (Mehdilo and Irannajad, 2012). However, the corrosion of production equipment is less with H2SO4 in comparison to HCl (Jia et al., 2014).
Dissolution of titanium ore with weaker acids
Titanium ores can also be leached using weaker acids such as oxalic (C2H2O4) and citric (C6H8O7) acid (Jonglertjunya and Rubcumintara, 2013; Kordzadeh-Kermani et al., 2020; Nayl, Awwad, and Aly, 2009a; Nayl and Aly, 2009). The reactions are as indicated in Equations [11][13].
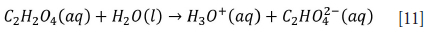
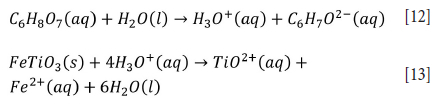
Both oxalic and citric acid are able to leach titanium, and citric acid provides effective results at low concentrations with the same physical conditions (Jonglertjunya and Rubcumintara, 2013). However, the leaching efficiencies of hydrochloric and oxalic acid are low compared to sulphuric and citric acid for lower grade ore (Jonglertjunya and Rubcumintara, 2013). Titanium leached more than iron under optimum conditions due to the precipitation of Fe(III) and Fe(II) at 90°C (Jonglertjunya and Rubcumintara, 2013). In contrast, the leaching efficiency of titanium gradually declines at temperatures above 150°C with 80 wt.% oxalic acid due to titanium hydrolysis (Nayl, Awwad, and Aly, 2009).
Although the leaching efficiency of sulphuric acid is high for low-grade ore, Jonglertjunya and Rubcumintara (2013) suggest citric acid as a better reagent due to lower acid consumption and lower leaching of iron. In addition, Nayl, Awwad, and Aly (2009) suggest oxalic acid as the most favourable reagent for the dissolution of pre-treated titanium ore due to the selectivity for titanium over iron. Oxalic acid is less corrosive, and the liquid/solid ratio is also reasonable. However, higher costs for oxalic acid and higher energy requirements compared with HCl and H2SO4 are the main disadvantages (Nayl, Awwad, and Aly, 2009).
Alkaline/caustic leaching
Alkaline/metal hydroxide/caustic leaching is also used to process titanium ores. Ilmenite is decomposed in an alkaline medium such as concentrated KOH or NaOH under atmospheric pressure to obtain an intermediate product with lower iron and higher titanium concentrations as shown in Equations [14] and [15], (Han et al., 2021; Nguyen and Lee, 2018; Zhang, Zhu, and Cheng, 2011).
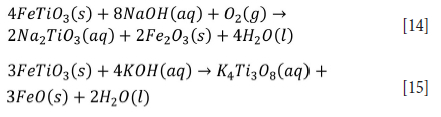
Amer (2002) developed a method to dissolve titanium from mechanically activated Rosetta ilmenite (a prominent deposit located in Egypt) using sodium hydroxide. About 90% of the titanium could be leached under optimum conditions of 0.3 M NaOH, 200°C, 90 minutes, and an oxygen partial pressure of 10 bar (Amer, 2002). Subsequently, several researchers have prepared TiO2 from titanium slag using alkaline leaching and obtained intermediates (e.g., Na2TiO3 and IQTi3O8) with high titanium and low iron contents (Han et al., 2021; Liu et al., 2006; Qi et al., 2005; Xue et al., 2009). For example, the intermediate product (K4Ti3O8) can be hydrolysed in acid (HCl and H2SO4) of pH 2.0 at 25°C for 1 hour. The resulting hydrous TiO2 is calcined at 400°C to obtain well-crystallized anatase (TKO2) with a purity of 99.3% (Zhang, Zhu, and Cheng, 2011). Alkaline leaching is usually used for the dissolution of titanium from raw ilmenite or hydrolysed titania after the removal of iron (Wu et al., 2011a, 2011b). Finely powdered titanium slag can be completely dissolved with 10 M NaOH at a NaOH to TiO2 ratio of 4:1 at a temperature of 220°C to obtain Na4Ti3O8. This intermediate has been acidified and subjected to cation exchange in the pH range <1.20 at 100°C to form TKO2 (Zhang et al., 2009).
NH3.H2O has also been employed along with H2O2 as an oxidizing agent to provide O22- ions (O22- reacts with titanium to form peroxide (TiO32-) and pertitanate (TiO62-) groups) for dissolving titanium from hydrolysed titania (Wu et al., 2011a, 2011b). However, the final TiO2 obtained was less pure due to the presence of SKO2 (Wu et al., 2011a). In this case, Wang et al. (2010, 2013) proposed two-step alkaline leaching (re-leaching the first filtrate to dissolve SiO2) and leaching with a mixture of NaOH and H2O2, respectively to obtain titanium precursors with less SiO2.
Interestingly, the alkaline leaching processes require less energy than conventional methods of titanium ore processing (Zhang, Zhu, and Cheng, 2011). These processes can be carried out at comparatively low temperatures and pressures.
Thermochemical reduction and dissolution
Chlorination at elevated temperatures (900-950°C) is the major commercialized thermochemical process for producing pure titanium tetrachloride (TiCk) (Equations [16] and [17]). This method is also employed to produce high-purity TiO2 and titanium metal from titanium slag or synthetic rutile (Zhang, Zhu, and Cheng, 2011). Thermochemical processing was initiated immediately after the beginning of ilmenite smelting at end of the 19th century. For example, the Hunter process (reduction of TiCl4 in an inert atmosphere with sodium at 1000°C and re-leaching the salt with dilute HCl) was developed in 1887 to produce titanium metal via reduction of TiCk in a molten bath of sodium (Zhang, Zhu, and Cheng, 2011).
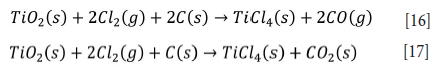
The Kroll process, in which TiCl4 obtained by carbochlorination is purified and reduced with magnesium, followed by electrolysis of MgCh to recycle Mg, is now industrially mature (Fatollahi-Fard and Pistorius, 2017). This method was introduced in 1940 to replace Hunter process, and is relatively cheap compared with the Hunter process. The Kroll process reduces TiCl4 at 900°C in molten magnesium (Nagesh et al., 2004). However, production of the TiCU feed material is capital intensive due to the use of petroleum coke and chlorine gas, and quite environmentally unfriendly due to the release of dioxins, fiirans, and bulky organic pollutants (Jackson and Dring, 2006). Several studies have been carried out incorporating modifications to overcome these drawbacks using thermochemical reduction and dissolution (Zhang, Zhu, and Cheng, 2011). However, cost reduction of these thermochemical processes (the, Hunter and Kroll processes) remains a challenge.
There are three commercially established technologies to produce TiCU, namely the fluidized bed process (most prominent), shaft furnace process, and chlorination in a molten salt bath (used in Japan and the former USSR). These thermochemical processes require high-grade feed material (over 90% TiO2) from natural/ synthetic rutile or high-grade titanium slag (Zhang, Zhu, and Cheng, 2011). However, the wastes generated in these processes are more disposable than those generated by the sulphate processes.
Solvothermal/hydrothermal conversion
Solvothermal and hydrothermal techniques are closely associated. They only differ in their precursor solutions (Zhang, Zhu, and Cheng, 2011). Several authors have used hydrothermal conversion to decompose titanium minerals into iron and titanium using acidic or alkaline solutions (Li, Wang, and Li, 2016; Manhique, Focke, and Madivate, 2011; Nayl et al., 2009a, 2009b; Nayl and Aly, 2009; Xiong et al., 2013). In particular, the minerals are decomposed to obtain solid or liquid phase TiO2 using sulphuric acid solution (Li, Wang, and Li, 2016; Sui and Zhai, 2014; Xiong et al., 2013). Xiong et al. (2013) decomposed ilmenite with 80-85 wt.% H2SO4 at 150°C, followed by leaching with water to obtain titanium sulphate. The major drawbacks of this method can be summarized as the requirement for highly concentrated 80-85 wt.% H2SO4 (Xiong et al., 2013), energy for heating (Sui and Zhai, 2014), and generation of toxic gases such as H2S, SO2, and SO3 (Baba et al., 2013). However, titanium minerals are successfully decomposed at optimum conditions of 13.5 M H2SO4, at 160°C for 2 hours to yield titanium with minimal generation of unfriendly by-products (Li, Wang, and Li, 2016).
Titanium dissolution has been observed in concentrated KOH under atmospheric pressure. In this method, potassium titanate and iron oxide are obtained (Liu et al., 2006; Nayl and Aly, 2009). In addition, Baba et al. (2013) reported that NaOH is a better roasting agent than Na2CO3 in terms of the capacity for Na2O generation for reacting with titanium minerals to form NaFeTiO4, Na2TiO3, NaFeO2, and NaFeTi3O8. Moreover, this method enhances the leaching efficiency of titanium using sulphuric acid, enabling complete dissolution to be achieved (Baba et al., 2013).
Solvothermal techniques can be employed to synthesize titanium-based nanomaterials (Xie and Shang, 2007). These methods have some merits over other synthetic processes, such as the use of mild chemical conditions at relatively low temperatures and the formation of non-agglomerated nanomaterials (Zhan, Zhu, and Cheng, 2011).
Biohydrometallurgical process
In this method, titanium minerals are leached using microorganisms such as bacteria and fungi. Jonglertjunya and Rubcumintara (2013) compared acid leaching and bioleaching in terms of titanium and iron extraction. The experimental results indicated very low iron and titanium dissolutions even after 35 days of leaching in both pure (A. niger, P. citrinum, and B. megaterium) and mixed (A. niger and P. citrinum) culture media (Jonglertjunya and Rubcumintara, 2013). Besides, Acidithiobacillus ferrooxidans (A. ferrooxidans), an iron oxidizing bacterium and Pseudomonas mendocina (P. mendocina), an iron scavenging bacterium have been used to extract titanium from ilmenite (Navarrete et al., 2013). Bioleaching is thus less effective due to very low iron and titanium dissolutions even after extensive leaching times. However, the combination of pyrometallurgy and biohydrometallurgy would be the best solution to enhance productivity while being environmentally acceptable. In this regard, bioleaching should effectively incorporate mechanical activation of raw ore for better results. Metal scavenging bacteria would attack smaller particles more rapidly than coarser particles. This will also increase efficiency and decrease leaching times. Therefore, practicing this on a large scale is likely to produce better results than traditional bioleaching techniques. Moreover, the titanium mineral processing industry can test such alternative methods for their suitability in terms of reaching the United Nations Sustainable Development Goals.
Factors controlling hydrometallurgical process Acid concentration
The literature suggests that leaching recovery gradually increase with increasing acid concentration (Jonglertjunya and Rubcumintara, 2013; Nayl, Awwad, and Aly, 2009a; Nayl and Aly, 2009; Sasikumar et al., 2007; Zhang and Nicol, 2010). For example, dissolved titanium is hydrolysed in the presence of 10-40% H2SO4, which has an adverse effect on the leaching process (Li, Liang, and Guo, 2007, Li et al., 2008). In addition, the leaching of iron and titanium in pre-treated titanium ore increases considerably with HCl concentrations up to 9 M (Nayl and Aly, 2009).
Temperature
The leaching rate of ilmenite using sulphuric acid is extremely sensitive to reaction temperature. Titanium and iron are dissolved simultaneously by concentrated sulphuric acid, and the leaching recoveries of these metals increase with temperature (Nayl et al., 2009; Sasikumar et al., 2007; Zhang and Nicol, 2010). Titanium and iron can be separated in the leaching process (using dilute H2SO4) by controlling reaction temperature (Jia et al., 2014; Li, Liang, and Guo, 2007). Leaching of iron increased with temperature using 20% H2SO4, while that of titanium decreased due to the instability of titanium in the solution at high temperatures (Li, Liang, and Guo, 2007). Jia et al. (2014) also reported that most of the iron from Panzhihua (the main deposit located in Sichuan, southwest China) ilmenite was selectively leached using 20% H2SO4 at 150°C, while leaching of Ti was less than 1%. Hydrolysis of the dissolved titanium ion occurs simultaneously during leaching at 125-200°C, resulting in a decrease in the leaching efficiency of titanium (Jia et al., 2014).
The leaching of titanium and iron from ilmenite using HCl increases significantly as the temperature increases from 25 to 80°C (Das et al., 2013; El-Hazek et al., 2007; Lasheen, 2005). High leaching efficiency of ilmenite was obtained at higher temperatures (>80°C). However, several drawbacks can be identified, such as increased loss of HCl vapour and hydrolysis of dissolved titanium (Das et al., 2013; Tao et al., 2012). El-Hazek et al. (2007) and Lasheen (2005) reported low leaching efficiencies of iron and titanium at room temperature due to the low reactivity of ilmenite. Nevertheless, the leaching recovery of iron increased more rapidly than that of titanium as the temperature increased from 20 to 50°C, owing to the partial hydrolysis of titanium. The mobility of ions increases with temperature, enhancing the interaction between reactants in solids and liquids (Gireesh et al., 2015). However, reaction temperatures above 100°C adversely affect the leaching of titanium due to high polymerization and hydrolysis of titanium without affecting iron (El-Hazek et al., 2007). Leaching temperature is quite important for the production of high-purity TiO2 using moderate (pH 3-5) HCl concentrations (Guo et al., 2014; Lasheen, 2005; Liu et al., 2015; Mahmoud, Afifi, and Ibarhim, 2004; Razieh, 2014; Tao et al., 2012). The leaching of iron rapidly increased with enhanced temperature up to 90°C using 4 M HCl, leaving most TiO2 in the residue at any temperature (Lasheen, 2005). The hydrolysis reaction of TiOCl2 is known to be greatly enhanced at higher temperatures. Consequently, the recovery of TiO2 from the ilmenite residue using a moderate HCl concentration increases with temperature (Guo et al., 2014; Liu et al., 2015; Mahmoud, Afifi, and Ibarhim, 2004; Razieh, 2014). Middlemas, Fang, and Fan (2013) reported that the dissolved titanium can react with water to form insoluble hydrates such as orthotitanic acid, Ti(OH)4 or TiO2.2H2O at low temperatures (25-80°C), and metatitanic acid, Ti(OH)2 or BO2.H2O at higher temperatures (80-110°C).
Ultrasound (>16 kHz) can also be used to reduce leaching time and temperature. Specifically, the reaction rate is enhanced due to high frequency, wave intensity, duration of ultrasound irradiation, and the physical characteristics of the lixiviant and nature of titanium ore (Narayana et al., 1997; Swamy et al., 1995).
The reduction of chlorine content during the leaching of titanium-bearing minerals is another advantage of using ultrasound (Narayana et al., 1997).
Contact time
Long contact times between the solution/acid and the feed material increase the dissolution of iron (El-Hazek et al., 2007; Gireesh et al., 2015; Jia et al., 2014; Li, Liang, and Guo, 2007, Li et al., 2008; Li, Liang, and Wang, 2008; Nayl and Aly, 2009; Samal, 2011).
Nevertheless, hydrolysis of dissolved titanium delays the leaching process (Li, Liang, and Guo, 2007; Zhang, Zhu, and Cheng, 2011). Moreover, the production of H+ during the hydrolysis of titanium ions enhances the ionic strength and the reactivity of the acid. In this regard, the dissolution of iron becomes rapid (Zhang et al., 2010). The literature reveals that the percentage dissolution of iron and the enhancement of TiO2 in the residue increases with time (Li, Liang, and Wang, 2008b; Mahmoud, Afifi, and Ibarhim, 2004; Razieh, 2014). However, minimizing the contact time between acid (both HCl and H2SO4) and feed material by adjusting the other parameters would add more economic benefits (Zhang, Zhu, and Cheng, 2011).
Effect of particle size
Several researchers describe how the ilmenite particles break down during reduction/oxidation as a result of the separation of the iron from the TiO2. A decrease in particle size increases the effectiveness of leaching. The leaching of titanium increases at particle sizes below 150 |im and mostly decreases above >200 |im (El-Hazek et al., 2007; Mehdilo and Irannajad, 2012; Samal, 2011). The reduction of particle size increases the effective surface area and enhances the leaching activity (El-Hazek et al., 2007; Samal, 2011). The dissolution of iron and titanium occurs generally for any particle size in the presence of HCl (van Dyk, Vegter, and Pistorius, 2002). Fine particles show faster reaction rates (El-Hazek et al., 2007; Nurul, 2016). However, reduction of particle size to less than 105 |im weakens iron dissolution (Nayl, Awwad, and Aly, 2009; Nayl, Ismail, and Aly, 2009; Nayl and Aly, 2009).
Additives and catalysts
Reducing agents have been found to improve leaching efficiency (Nguyen and Lee, 2018). The effect of additives and catalysts can be summarized as follows.
(i) The leaching of titanium increases, and that of iron decreases, with increasing FeSO4 (addition of Fe2+ ions) concentration during H2SO4 leaching (Jia et al., 2014)
(ii) The presence of Ti(III) ions and SO2 gas in sulphuric acid reduces iron (III) in titanium minerals via a redox reaction and aids dissolution of titanium (Zhang and Nicol, 2010)
(iii) Addition of metallic iron powder increases titanium leaching efficiency via reduction of iron(III) during HCl leaching
(iv) The HCl leaching efficiency of titanium ores depends on the chloride ion concentration, and the effect has been found to be in the order CaCl2 > MgCl2 > NaCl (Das et al., 2013)
(v) The presence of sulphate ions in HCl solution also increases the efficiency of iron dissolution (Gireesh et al., 2015)
(vi) Leaching efficiency increases in the presence of hydrogen peroxide (H2O2) and ultraviolet (UV) light (Jayaweera et al., 2011).
However, the increment of iron powder also increases acid consumption (El-Hazek et al., 2007). The presence of 6% iron powder enhances iron dissolution in HCl solution, and increases TiO2 content (Lasheen, 2005). Furthermore, the presence of sulphates/sulphate ions such as CaSO4, MgSO4, Na2SO4, and K2SO4 during HCl leaching enhances the purity of titanium. Specifically, monovalent metal sulphates (Na2SO4 and K2SO4) are less effective than divalent metal sulphates (CaSO4 and MgSO4). Moreover, an excess of reducing agents might reduce Ti(IV) to Ti(III) and decrease the tendency of Fe(II) ions to be oxidized to Fe(III) (Mahmoud, Afifi, and Ibarhim, 2004).
Oxidation and reduction of titanium ore during hydrometallurgical processing
Oxidation induces micro-cracks, and micro-pores. Therefore, it enhances the rate of leaching at 1000°C (Grey et al., 2007; Sarker, Rashid, and Kurny, 2006; Zhu, Zhang, and Li, 2014). However, oxidation at 900-1000°C significantly decreases the leaching efficiency due to the formation of pseudobrookite (Zhu, Zhang, and Li, 2014). Oxidation below 800°C has little effect on iron dissolution, as the solubility of ferric iron is low during acid leaching (Janssen and Putnis, 2011).
Titanium ore upgrading processes such as the Becher process employ iron oxidation followed by reduction (Farrow, Ritchie, and Mangono, 1987). Methods such as the Murso process oxidize titanium ore in fluidized beds at temperatures ranging from 900 to 950°C (Sinha, 1973), and the ferric iron formed is reduced using a reducing agent such as H2 gas. Similarly, in the Laporte process titanium ore is pre-oxidized in a fluidized bed at 950°C and reduced in a rotary kiln using coal at 900°C (Robinson et al., 1977). Iron is oxidized according to Equation [5] followed by reduction (see Equation [18]) in a rotary kiln with a mixture of pseudobrookite (Fe2O3-TiO2), coal, and sulphur at >1200°C to convert iron oxide to metallic iron (Zhang, Zhu, and Cheng, 2011).
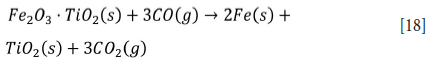
The produced metallic iron is re-oxidized (see Equation [19]) and precipitated as a slime in large vessels using 1% ammonium chloride solution at 80°C during the aeration 'rusting' step (Zhang, Zhu, and Cheng, 2011).
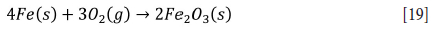
Electrometallurgical/electrochemical processes
Electrometallurgical/electrochemical processing (i.e., use of electrical energy to extract metals by electrolysis) of titanium minerals became more common in the 21st century due to the invention of electro-deoxidization in molten salt in 2000 (Liu et al., 2012). Several studies have focused on producing titanium metal and alloys via electrometallurgical routes (Table V). Disadvantages such as low productivity and lengthy times required for impurity removal could be circumvented by mechanically grinding the titanium ore. This would simultaneously increase the recovery of TiO2 in products.
The use of electrical energy in conjunction with chemicals has also focused on reducing the production cost of TiO2 and titanium metal. Most of the electrochemical methods are automated, and thus utilize continuous production lines (Fatollahi-Fard and Pistorius, 2017; Zhang, Zhu, and Cheng, 2011). However, reactive products and associated problems in redox recycling are the main limitations of this method (Chen, Fray, and Farthing, 2000). Consequently, several methods have been introduced for direct reduction, such as Electro-slag electrolysis (ESE) and the Fray-Farthing-Chen (FFC) process. The FFC process, with TiO2 as feed material, is the more efficient. These methods enable the production of titanium metal in one step (Suzuki, Teranuma, and Ono, 2003; Takenaka et al., 1999). The cost of production can be significantly controlled by using well-ground TiO2 as the feed material in these processes.
Conclusions
Pyrometallurgy, hydrometallurgy, and electrometallurgy are prominent methods to cater to the escalating global demand for TiO2 and titanium metal. Mechanical activation (ball milling with or without reductants) of titanium ore is an important method to reduce the activation energy required for interacting with the strong covalent bonding in titanium ores. This increases the efficiency of subsequent metallurgical processes. Pyrometallurgical treatment produces low-purity synthetic rutile (TiO2) and slag. In this case, an additional leaching step is required to separate synthetic rutile. Two major hydrometallurgical routes, namely the chloride and sulphate processes, have been commercialized.
Low-grade feed material such as ilmenite or leucoxene can be used in sulphate route leaching. However, a greater amount of waste is generated at the expense of high energy requirements. In contrast, the chloride process yields highly pure products with less waste generation. This process requires high-grade feed material such as natural or synthetic rutile or titanium slag. Thermochemical processes such as the Kroll and Hunter processes require high-grade feed material such as TiCl4. Consequently, these processes have proven to be less efficient, even with the existing technology. Electrochemical methods are comparatively feasible. However, the generation of highly concentrated solutions, redox recycling, feeding, and controlling heat balance are the main drawbacks. Direct leaching technologies have proven to be more effective than thermochemical and electrochemical techniques.
The use of combined metallurgical techniques such as pyro-and hydrometallurgy to increase process efficiency and purity of products, and reduce energy consumption and waste generation, should be a future focus of the titanium ore processing industry. Pyrometallurgical processing of mechanically activated titanium ore followed by microbial leaching/biohydrometallurgy can constitute an unconventional method to enhance production while being environmentally friendly.
Acknowledgements
We gratefully acknowledge the financial support of an Accelerating Higher Education Expansion and Development (AHEAD) Operation of the Ministry of Higher Education, funded by the World Bank.
Conflict of interest statement
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
Amade, R., Heitjans, P., Indris, S., Finger, M., Haeger, A., and Hesse, D. 2009. Defect formation during high-energy ball milling in TiO2 and its relation to the photocatalytic activity. Journal of Photochemistry and Photobiology A: Chemistry, vol. 207. pp. 231-235. https://doi.org/10.1016/j.jphotochem.2009.07.015 [ Links ]
Amer, A.M. 2002. Alkaline pressure leaching of mechanically activated Rosetta ilmenite concentrate. Hydrometallurgy, vol. 67. pp. 125-133. https://doi.org/10.1016/S0304-386X(02)00164-0 [ Links ]
Baba, A.A., Swaroopa, S., Ghosh, M.K., and Adekola, EA. 2013. Mineralogical characterization and leaching behaviour of Nigerian ilmenite ore. Transactions of Nonferrous Metals Society of China, vol. 23. pp. 2743-2750. [ Links ]
Bai, Y., Mora-Sero, I., De Angelis, F., Bisquert, J., and Wang, P. 2014. Titanium dioxide nanomaterials for photovoltaic applications. Chemical Reviews, vol. 114, no. 19. pp. 10095-10130. https://doi.org/10.1021/cr400606n [ Links ]
Barnes, C. and Pickles, C.A. 1988. A thermogravimetric study of the catalytic effect of alkali carbonates on the reduction of ilmenite. High Temperature Technology, vol. 6.pp. 195-201. https://doi.org/10.1080/02619180.1988.11753400 [ Links ]
Begin-Colin, S., Girot, T., le Caër, G., and Mocellin, A. 2000. Kinetics and mechanisms of phase transformations induced by ball-milling in anatase TiO2. Journal of Solid State Chemistry, vol. 149., pp. 41-48. https://doi.org/10.1006/jssc.1999.8491 [ Links ]
Begin-Colin, S., Le Caër., g., Mocellin, A., and Zandona, M. 1994. Polymorphic transformations of titania induced by ball milling. Philosophical Magazine Letters, vol. 69, no. 1, pp. 1-7. http://dx.doi.org/10.1080/09500839408242430 [ Links ]
Bordbar, H., Yousefi, A.A., and Abedini, H. 2017. Production of titanium tetrachloride (TiCl4) from titanium ores: A review. Polyolefins Journal, vol. 4. no. 2. pp. 149-173. https://dx.doi.org/10.22063/poj.2017.1453 [ Links ]
Brooks, D.R. 2000. Reclamation of land disturbed by mining of heavy minerals. Reclamation of Drastically Disturbed Lands, vol. 41. pp. 725-754. https://doi.org/10.2134/agronmonogr41.c29 [ Links ]
Chen, Y. 1997. Low-temperature oxidation of ilmenite (FeTiO3) induced by high energy ball milling at room temperature. Journal of Alloys and Compounds, vol. 257, no. 1-2. pp. 156-160. https://doi.org/10.1016/S0925-8388(97)00012-1 [ Links ]
Chen, G.Z., Fray, D.J., and Farthing, T.W. 2000. Direct electrochemical reduction of titanium dioxide to titanium in molten calcium chloride. Nature, vol. 407. pp. 361-363. https://doi.org/10.1038/35030069 [ Links ]
Chen, Y., Marsh, M., Williams, J.S., and Ninham, B. 1996. Production of rutile from ilmenite by room temperature ball-milling-induced sulphurisation reaction. Journal of Alloys and Compounds, vol. 245. pp. 54-58. https://doi.org/10.1016/S0925-8388(96)02483-8 [ Links ]
Chen, g., Song, Z., Chen, J., Peng, J., and Srinivasakannan, C. 2013. Evaluation of the reducing product of carbonthermal reduction of ilmenite ores. Journal of Alloys and Compounds, vol. 577. pp. 610-614. https://doi.org/10.1016/j.jallcom.2013.06.038 [ Links ]
Chen, M., Tang, A., and Xiao, X. 2015. Effect of carbothermic reduction of ilmenite. Transactions of the Nonferrous Metals Society of China, vol. 25. p p. 4201-4206. https://doi.org/10.1016/S1003-6326(15)64070-5 [ Links ]
Chen, L., Wen, S., Xu, G., and Xie, H. 2013. A novel process for titanium sand by magnetic separation and gravity concentration. Mineral Processing and Extractive Metallurgy Review, vol. 34. pp. 139-150. https://doi.org/10.1080/08827508.2011.623749 [ Links ]
Das, G.K., Pranolo, Y., Zhu, Z., and Cheng, C.Y. 2013. Leaching of ilmenite ores by acidic chloride solutions. Hydrometallurgy, vol. 133. pp. 94-99. https://doi.org/10.1016/j.hydromet.2012.12.006 [ Links ]
Dworkin, J.P., Adelman, L.A., Ajluni, T., Andronikov, A.V., Aponte, J.C., Bartels, A.E., and Burton, A.S. 2018. OSIRIS-REx contamination control strategy and implementation. Space Science Reviews, vol. 214, no. 1. pp. 19. https://doi.org/10.1007/s11214017-0439-4 [ Links ]
El-Hazek, N., Lasheen, T.A., El-Sheikh, R., and Zaki, S.A. 2007. Hydrometallurgical criteria for TiO2 leaching from Rosetta ilmenite by hydrochloric acid. Hydrometallurgy, vol. 87. pp. 45-50. https://doi.org/10.1016/j.hydromet.2007.01.003 [ Links ]
Elsner, H. 2010. Heavy Minerals of Economic Importance. Bundesanstalt für Geowissenschaften und Rohstoffe (BGR). Federal Institute for Geosciences and Natural Resources, Hanover, Germany. [ Links ]
Farrow, J.B., Ritchie, LM., and Mangono, P. 1987. The reaction between reduced ilmenite and oxygen in ammonium chloride solution. Hydrometallurgy, vol. 18. pp. 21-38. https://doi.org/10.1016/0304-386X(87)90014-4 [ Links ]
Fatollahi-Fard, F. and Pistorius, P.C. 2017. Comparison of methods for electrochemical iron removal from titanium ores. Journal of Sustainable Metallurgy, vol. 3, no. 4. pp. 711-719. https://doi.org/10.1007/s40831-017-0132-6 [ Links ]
Gázquez, M.J., Bolívar, J.P., Garcia-Tenorio, R., and Vaca, F. 2014. A review of the production cycle of titanium dioxide pigment. Materials Sciences and Applications, vol. 5. pp. 441-458. https://doi.org/10.4236/msa.2014.57048 [ Links ]
Gireesh, V.S., Vinod, V.P., Krishnan Nair, S., and Ninan, G. 2015. Catalytic leaching of ilmenite using hydrochloric acid: A kinetic approach. International Journal of Mineral Processing, vol. 134., pp. 36-40. https://doi.org/10.1016/j.minpro.2014.11.004 [ Links ]
Grey, I., McDonald, K., Fisher-White, M., and de Vries, M. 2007. Hydrogen reduction of preoxidised ilmenite in fluidised bed and packed bed reactors. Mineral Processing and Extractive Metallurgy, vol. 116. pp. 209-216. https://doi.org/10.1179/174328507X198717 [ Links ]
Guo, Y., Liu, S., Jiang, T., Qiu, G., and Chen, F. 2014. A process for producing synthetic rutile from Panzhihua titanium slag. Hydrometallurgy, vol. 147-148. pp. 134-141. https://doi.org/10.1016/j.hydromet.2014.05.009 [ Links ]
Han, K.N., Rubcumintara, T., and Fuerstenau, M.C. 1987. Leaching behaviour of ilmenite with sulphuric acid. Metallurgical Transactions B, vol. 18. pp. 325-330. [ Links ]
Han, J., Zhang, J., Zhang, J., Chen, X., Zhang, L., and Tu, G. 2021. Extraction of vanadium and enrichment of titanium from modified Ti-bearing blast furnace slag. Hydrometallurgy, vol. 201. pp. 105577. https://doi.org/10.1016/j.hydromet.2021.105577. https://doi.org/10.1007/BF02656150 [ Links ]
Hao, X., Lü, L., Liang, B., Li, C., Wu, P., and Wang, J. 2012. Solvent extraction of titanium from the simulated ilmenite sulphuric acid leachate by trialkylphosphine oxide. Hydrometallurgy, vol. 113-114. pp. 185-191. https://doi.org/10.1016/j.hydromet.2011.12.023 [ Links ]
Haverkamp, R.G., Kruger, D., and Raiashekar, R. 2016. The digestion of New Zealand ilmenite by hydrochloric acid. Hydrometallurgy, vol. 163. pp. 198-203. https://doi.org/10.1016/j.hydromet.2016.04.015 [ Links ]
Ismail, M.G.M.U., Amarasekera, J., and Kumarasinghe, J.S.N. 1983. The upgrading of ilmenite from Sri Lanka by the oxidation-reduction-leach process. International Journal of Mineral Processing, vol. 10, no. 2, pp. 161-164. https://doi.org/10.1016/03017516(83)90040-6 [ Links ]
Jackson, M. and Dring, K. 2006. A review of advances in processing and metallurgy of titanium alloys. Material Science and Technology, vol. 22, no. 8, pp. 881-887. https://doi.org/10.1179/174328406X111147 [ Links ]
Janssen, A. and Putnis, A. 2011. Processes of oxidation and HCl leaching of Tellnes ilmenite. Hydrometallurgy, vol. 109. pp. 194-201. https://doi.org/10.1016/j.hydromet.2011.07.004 [ Links ]
Jayaweera, P.M., Jayaweera, P.V.V, Jayasundara, U.L., Jayaweera, C.D., Peiris, G.S., and Premalal, E.V.A.P. 2011. Photo induced reductive leaching of iron from ilmenite in hydrochloric acid solutions. Mineral Processing and Extractive Metallurgy, vol. 120. pp. 191-196. https://doi.org/10.1179/1743285511Y.0000000018 [ Links ]
Jia, L., Liang, B., Lü, L., Yuan, S., Zheng, L., Wang, X., and Li, C. 2014. Beneficiation of titania by sulphuric acid pressure leaching of Panzhihua ilmenite. Hydrometallurgy, vol. 150., pp. 92-98. https://doi.org/10.1016/j.hydromet.2014.09.016 [ Links ]
Jonglertiunya, W and Rubcumintara, T. 2013. Titanium and iron dissolutions from ilmenite by acid leaching and microbiological oxidation techniques. Asia-Pacific Journal of Chemical Engineering, vol. 8, no. 3. pp. 323-330. https://doi.org/10.1002/apj.1663 [ Links ]
Kataoka, S. and Yamada, S. 1973. Acid leaching upgrades ilmenite to synthetic rutile. Chemical Engineering, vol. 80, no. 7. pp. 92-93. [ Links ]
Kordzadeh-Kermani, V., Schaffie, M., Rafsaniani, H.H., and Ranibar, M. 2020. A modified process for leaching of ilmenite and production of TiO2 nanoparticles. Hydrometallurgy, vol. 198. pp. 105507. https://doi.org/10.1016/j.hydromet.2020.105507 [ Links ]
Kothari, N.C. 1974. Recent developments in processing ilmenite for titanium. International Journal of Mineral Processing, vol. 1. pp. 287-305. https://doi.org/10.1016/0301-7516(74)90001-5 [ Links ]
Kuppusamy, V.K. and Holuszko, M. 2022. Sulfuric acid baking and water leaching of rare earth elements from coal tailings. Fuel, vol. 319. 123738. https://doi.org/10.1016/j.fuel.2022.123738 [ Links ]
Kurlov, A.S. and Gusev, A.I. 2007. Effect of ball milling parameters on the particle size in nanocrystalline powders. Technical Physics Letters, vol. 33, no. 10. pp. 828-832. https://doi.org/10.1134/S1063785007100070 [ Links ]
Lasheen, T. A.I. 2005. Chemical benefication of Rosetta ilmenite by direct reduction leaching. Hydrometallurgy, vol. 76. pp. 123-129. https://doi.org/10.1016/j.hydromet.2004.10.002 [ Links ]
Li, C., Liang, B., and Guo, L.H. 2007. Dissolution of mechanically activated Panzhihua ilmenites in dilute solutions of sulphuric acid. Hydrometallurgy, vol. 89. pp. 1-10. https://doi.org/10.1016/j.hydromet.2007.04.002 [ Links ]
Li, C., Liang, B., Song, H., Xu, J.Q., and Wang, X.Q. 2008. Preparation of porous rutile titania from ilmenite by mechanical activation and subsequent sulphuric acid leaching. Microporous and Mesoporous Materials, vol. 115. pp. 293-300. https://doi.org/10.1016/j.micromeso.2008.01.045 [ Links ]
Li, C., Liang, B., and Wang, H.Y. 2008. Preparation of synthetic rutile by hydrochloric acid leaching of mechanically activated Panzhihua ilmenite. Hydrometallurgy, vol. 91. pp. 121-129. https://doi.org/10.1016/j.hydromet.2007.11.013 [ Links ]
Li, Z., Wang, Z., and Li, G. 2016. Preparation of nano-titanium dioxide from ilmenite using sulphuric acid-decomposition by liquid phase method. Powder Technology, vol. 287. pp. 256-263. https://doi.org/10.1016/j.powtec.2015.09.008 [ Links ]
Liu, X., Hu, M., Bai, C., and Lv, X. 2012. The electro-deoxidation process for ilmenite concentrate in molten salt. Proceedings of the T.T. Chen Honorary Symposium on Hydrometallurgy, Electrometallurgy and Materials Characterization. Wang, S., Dutrizac, J.E., Free, M.L., Hwang, J.Y., and Kim, D. (eds). Wiley, Hoboken, NJ. pp. 613-620. [ Links ]
Liu, Y., Qi, T., Chu, J., Tong, Q., and Zhang, Y. 2006. Decomposition of ilmenite by concentrated KOH solution under atmospheric pressure. International Journal Mineral Processing, vol. 81. pp. 79-84. https://doi.org/10.1016/j.minpro.2006.07.003 [ Links ]
Liu, S., Zhu, K., Xiang, J., and Huang, P. 2015. Upgrading ilmenite by an oxidation-magnetic separation-pressure leaching process. Bulg Chemical Communicable, vol. 47. pp. 1118-1123. [ Links ]
Lv, W, Lv, X., Xiang, J., Zhang, Y., Li, S., Bai, C., Song, B., and Han, K. 2017. A novel process to prepare high-titanium slug by carbothermic reduction of pre-oxidized ilmenite concentrate with the addition of Na2SO4. International Journal of Mineral Processing, vol. 167. pp. 68-78. https://doi.org/10.1016/j.minpro.2017.08.004 [ Links ]
Mahmoud, M.H.H., Afifi, A.A., and Ibarhim, LA. 2004. Reductive leaching of ilmenite ore in hydrochloric acid for preparation of synthetic rutile. Hydrometallurgy, vol. 73. pp. 99-109. https://doi.org/10.1016/j.hydromet.2003.08.001 [ Links ]
Manhique, A.J., Focke, W.W., and Madivate, C. 2011. Titania recovery from low-grade titanoferrous minerals. Hydrometallurgy, vol. 109. pp. 230-236. https://doi.org/10.1016/j.hydromet.2011.07.008 [ Links ]
Mehdilo, A. and Irannaiad, M. 2012. Iron removing from titanium slag for synthetic rutile production. Physicochemical Problems in Mineral Processing, vol. 48. pp. 425-439. [ Links ]
Merk, R. and Pickles, CA. 1988. Reduction of ilmenite by carbon monoxide. Canadian Metallurgical Quarterly, vol. 27, no. 3. pp. 179-185. https://doi.org/10.1179/cmq.1988.27.3.179 [ Links ]
Middlemas, S., Fang, Z.Z., and Fan, P. 2013. A new method for production of titanium dioxide pigment. Hydrometallurgy, vol. 131-132. pp. 107-113. https://doi.org/10.1016/j.hydromet.2012.11.002 [ Links ]
Middlemas, S., Fang, Z.Z., and Fan, P. 2015. Life cycle assessment comparison of emerging and traditional titanium dioxide manufacturing processes. Journal of Cleaner Production, vol. 89. pp. 137-147. https://doi.org/10.1016/j.jclepro.2014.11.019 [ Links ]
Morley, I.W. 1981. Black Sands: A History of the Mineral Sand Mining Industry in Eastern Australia. University of Queensland Press, St. Lucia, Qld [ Links ]
Nagesh, C.R.V.S., Rao, C.S., Ballal, N.B., and Rao, P.K. 2004. Mechanism of titanium sponge formation in the Kroll reduction reactor. Metallurgical and Materials Transactions B, vol. 35. pp. 65-74. https://doi.org/10.1007/s11663-004-0097-2 [ Links ]
Narayana, K.L., Swamy, K.M., Sarveswara, Rao, K., and Murty, J.S. 1997. Leaching of metals from ores with ultrasound. Mineral Processing and Extractive Metallurgy Reviews, vol. 16. pp. 239-259. https://doi.org/10.1080/08827509708914137 [ Links ]
Navarrete, JU., Cappelle, I.J., Schnittker, K., and Borrok, D.M. 2013. Bioleaching of ilmenite and basalt in the presence of iron-oxidizing and iron-scavenging bacteria. International Journal of Astrobiology, vol. 12, no. 2. pp. 123-134. https://doi.org/10.1017/S1473550412000493 [ Links ]
Nayl, A.A. and Aly, H.F. 2009. Acid leaching of ilmenite decomposed by KOH. Hydrometallurgy, vol. 97. pp. 86-93. https://doi.org/10.1016/j.hydromet.2009.0L0n [ Links ]
Nayl, A.A., Awwad, N.S., and Aly, H.F. 2009. Kinetics of acid leaching of ilmenite decomposed by KOH. Part 2. Leaching by H2SO4 and C2H2O4. Journal of Hazardous Materials, vol. 168. pp. 793-799. https://doi.org/10.1016/j.jhazmat.2009.02.076 [ Links ]
Nayl, A.A., Ismail, LM., and Aly, H.F. 2009. Ammonium hydroxide decomposition of ilmenite slag. Hydrometallurgy, vol. 98. pp. 196-200. http://dx.doi.org/10.1016%2Fj.hydromet.2009.04.011 [ Links ]
Nguyen, T.H. and Lee, M.S. 2018. A review on the recovery of titanium dioxide from ilmenite ores by direct leaching technologies. Mineral Processing and Extractive Metallurgy Review, vol. 40, no. 4. pp. 231-247. https://doi.org/10.1080/08827508.2018.1502668 [ Links ]
Nurul, A. 2016. Chemical and electrochemical leaching studies of synthetic and natural ilmenite in hydrochloric acid solution. PhD thesis, Murdoch University, Perth, Western Australia. [ Links ]
Olanipekun, E. 1999. A kinetic study of the leaching of a Nigerian ilmenite ore by hydrochloric acid. Hydrometallurgy, vol. 53. pp. 1-10. https://doi.org/10.1016/S0304-386X(99)00028-6 [ Links ]
Qi, T., Liu, Y.M., Chu, J.L., Li, H.J. and Li, Z.H. 2005. Preparation of potassium titanate using sub-molten salt method. CN patent 200510059715.5. [ Links ]
Rao, Y.K., Adjorlolo, A., and Haberman, J.H. 1982. On the mechanism of catalysis of the Boudouard reaction by alkali-metal compounds. Carbon, vol. 20. pp. 207-212. https://doi.org/10.1016/0008-6223(82)90022-7 [ Links ]
Razieh, R. 2014. Production of nanosized synthetic rutile from ilmenite concentrate by sonochemical HCl and H2SO4 leaching. Iran Journal Chemical Engineering, vol. 33. pp. 29-36. https://dx.doi.org/10.30492/ijcce.2014.10749 [ Links ]
Ren, R., Yang, Z., and Shaw, L.L. 2000. Polymorphic transformation and powder characteristics of TiO2 during high energy milling. Journal of Materials Science, vol. 35. pp. 6015-6026. https://doi.org/10.1023/A:1026751017284 [ Links ]
Robinson, M., Clamp, F., Mobbs, D.B., and Pearse, R.V. 1977. The Laporte high-efficiency ilmenite beneficiation process. Advances in Extractive Metallurgy. Jones, M.J. (ed.). Institution of Mining and Metallurgy, London. pp. 89-96. [ Links ]
Roche, E.G., Stuart, A.D., and Grazier, P.E. 2004. Production of titania. WO patent 2004035841-A1. [ Links ]
Run, H., Pengsheng, L., Yuehui, Y., and Jinzhu, Z. 2017. Vacuum carbothermic reduction of Panzhihua ilmenite concentrate: A thermodynamic study. Mineral Processing and Extractive Metallurgy Reviews, vol. 38. pp. 193-198. https://doi.org/10.1080/08827508.2017.1281129 [ Links ]
Samal, S. 2011. The dissolution of iron in the hydrochloric acid leach of titania slag obtained from plasma melt separation of metalized ilmenite. Chemical Engineering Researcher Design, vol. 89. pp. 2190-2193. https://doi.org/10.1016/j.cherd.2011.01.026 [ Links ]
Sarker, M.K., Rashid, A.K.M.B., and Kurny, A.S.W. 2006. Kinetics of leaching of oxidized and reduced ilmenite in dilute hydrochloric acid solutions. International Journal of Mineral Processing, vol. 80. pp. 223-228. https://doi.org/10.1016/j.minpro.2006.04.005 [ Links ]
Sasikumar, C., Rao, D.S., Srikanth, S., Mukhopadhyay, N.K., and Mehrotra, S.P. 2007. Dissolution studies of mechanically activated Manavalakurichi ilmenite with HCl and H2SO4. Hydrometallurgy, vol. 88. pp. 154-169. https://doi.org/10.1016/j.hydromet.2007.03.013 [ Links ]
Sasikumar, C., Rao, D.S., Srikanth, S., Ravikumar, B., Mukhopadhyay, N.K., and Mehrotra, S.P. 2004. Effect of mechanical activation on the kinetics of sulphuric acid leaching of beach sand ilmenite from Orissa, India. Hydrometallurgy, vol. 75. pp. 189-204. https://doi.org/10.1016/j.hydromet.2004.08.001 [ Links ]
Shahien, M.G., Khedr, M.M.H., Maurice, A.E., Farghali, A.A., and Ali, R.A.M. 2015. Synthesis of high purity rutile nanoparticles from medium-grade Egyptian natural ilmenite. Journal of Basic and Applied Sciences, vol. 4. pp. 207-213. http://dx.doi.org/10.1016/j.bjbas.2015.05.013 [ Links ]
Shi, J., Qiu, Y., Yu, B., Xie, X., Dong, J., Hou, C., Li, J., and Liu, C. 2022. Titanium extraction from titania-bearing blast furnace slag: A review. Journal of the Minerals, Metals & Materials Society, vol. 74. pp. 654-667. https://doi.org/10.1007/s11837-021-05040-y [ Links ]
Shojaei, v., Schaffie, M., Mohebbi, A., and Ranjbar, M. 2014. Upgrading of ilmenite using KOH sub-molten salt process assisted by mechanical activation. Materials and Manufacturing Processes, vol. 29. pp. 1284-1288. https://doi.org/10.1080/10426914.2014.941485 [ Links ]
Singh, K.K., Kishor, B., and Mankhand, T.R. 2018. Reduction of Manavalakurichi ilmenite by activated charcoal in presence of catalyst. Transactions of Indian Institute of Metals, vol. 71, no. 12. pp. 2993-3001. https://doi.org/10.1007/s12666-018-1400-2 [ Links ]
Sinha, H.N. 1973. MURSO process for producing rutile substitute. Titanium Science and Technology. Springer, Boston, MA. pp. 233-245. [ Links ]
Subasinghe, H.C.S. and Ratnayake, A.S. 2021. Processing of ilmenite into synthetic rutile using ball milling induced sulphurisation and carbothermic reduction. Minerals Engineering, vol. 173. pp. 107197. https://doi.org/10.1016/j.mineng.2021.107197 [ Links ]
Subasinghe, H.C.S. and Ratnayake, A.S. 2022. General review of titanium ores in exploitation: present status and forecast. Comunicações Geológicas, vol. 109. pp. 21-31. https://doi.org/10.34637/aab4-mk81 [ Links ]
Subasinghe, C.S., Ratnayake, A.S., Roser, B., Sudesh, M., Wijewardhana, D.U., Attanayake, N., and Pitawala, J. 2022. Global distribution, genesis, exploitation, applications, production, and demand of industrial heavy minerals. Arabian Journal of Geosciences, vol. 15. pp. 1-28. https://doi.org/10.1007/s12517-022-10874-0 [ Links ]
Sui, L.L. and Zhai, Y.C. 2014. Reaction kinetics of roasting high-titanium slag with concentrated sulphuric acid. Transactions of the Nonferrous Metals Society of China, vol. 24. pp. 848-853. https://doi.org/10.1016/S1003-6326(14)63134-4 [ Links ]
Suzuki, R.O., Teranuma, K., and Ono, K. 2003. Calciothermic reduction of titanium oxide and in-situ electrolysis in molten CaCh. Metallurgical and Materials Transactions B, vol. 3. pp. 287-295. https://doi.org/10.1007/s11663-003-0074-1 [ Links ]
Swamy, K.M., Sarveswara, Rao, K., Narayana, K.L., Murty, J.S., and Ray, H.S. 1995. Application of ultrasound in leaching. Mineral Processing and Extractive Metallurgy Reviews, vol. 14. pp. 179-192. https://doi.org/10.1080/08827509508914124 [ Links ]
Takeda, O., Ouchi, T., and Okabe, T.H. 2020. Recent progress in titanium extraction and recycling. Metallurgical and Materials Transactions B, vol. 51, no. 4. pp. 1315-1328. https://doi.org/10.1007/s11663-020-01898-6 [ Links ]
Takenaka, T., Suzuki, T., Ishikawa, M., Fukasawa, E., and Kawakami, M. 1999. The new concept for electrowinning process of liquid titanium metal in molten salt. Electrochemistry, vol. 67, no. 6. pp. 661-668. https://doi.org/10.5796/electrochemistry.67.661 [ Links ]
Tan, P., Hu, H.P., and Zhang, L. 2011. Effects of mechanical activation and oxidation-reduction on hydrochloric acid leaching of Panxi ilmenite concentration. Transactions of the Nonferrous Metals Society of China, vol. 21. pp. 1414-1421. https://doi.org/10.1016/S1003-6326(11)60875-3 [ Links ]
Tao, T., Chen, Q.Y., Hu, H.P., Yin, Z.L., and Chen, Y. 2012. TiO2 nanoparticles prepared by hydrochloric acid leaching of mechanically activated and carbothermic reduced ilmenite. Transactions of the Nonferrous Metals Society of China, vol. 22. pp. 1232-1238. https://doi.org/10.1016/S1003-6326(11)613101 [ Links ]
Taylor, 1976. Process for ilmenite ore reduction. US patent 3,966,455. [ Links ]
Tripathy, M., Srinivasan, T., and Mehrota, S.P. 2012. Investigations on reduction of ilmenite ore with different sources of carbon. IMM Transactions Section C: Mineral Processing and Extractive Metallurgy, vol. 121, no. 3. pp. 147-155. doi: 10.1179/1743285512Y.0000000007 [ Links ]
Tromans, D. and Meech, J.A. 2001. Enhanced dissolution of minerals: stored energy, amorphism and mechanical activation. Minerals Engineering, vol. 14. pp. 1359-1377. https://doi.org/10.1016/S0892-6875(01)00151-0 [ Links ]
Van Dyk, J.P., Vegter, N.M., and Pistorius, P.C. 2002. Kinetics of ilmenite dissolution in hydrochloric acid. Hydrometallurgy, vol. 65. pp. 31-36. https://doi.org/10.1016/S0304-386X(02)00063-4 [ Links ]
Vásquez, R. and Molina, A. 2008. Leaching of ilmenite and pre-oxidized ilmenite in hydrochloric acid to obtain high grade titanium dioxide. International Journal of Mineral Processing, vol. 68. pp. 13-21. [ Links ]
VÁSQüEZ, R. and Molina, A. 2012. Effects of thermal preoxidation on reductive leaching of ilmenite. Minerals Engineering, vol. 39. pp. 99-105. https://doi.org/10.1016/j.mineng.2012.05.003 [ Links ]
Verhulst, D., Sabachy, H., Spitler, T., and Prochazka, J. 2003. New developments in the Altair hydrochloride TiO2 pigment process. Hydrometallurgy 2003 Proceedings of the 5th International Conference in Honour of Professor Ian M. Ritchie, Vancouver, BC. Vol. 1. TMS, Warrendale, PA. pp. 565-575. [ Links ]
Wang, J. and Lin, Z. 2010. Dye-sensitized TiO2 nanotube solar cells with markedly enhanced performance via rational surface engineering. Chemistry of Materials, vol. 22, no. 2. pp. 579-584. https://doi.org/10.1021/cm903164k [ Links ]
Wang, X., Li, W., Yang, B., Guo, S., Zhang, L., Chen, G., Peng, J., and Luo, H. 2014. Microwave-absorbing of carbothermic reduced products of ilmenite and oxidized ilmenite. Journal of Microwave Power and Electromagnetic Energy, vol. 48. pp. 192-202. https://doi.org/10.1080/08327823.2014.11689883 [ Links ]
Wang, X., Li, X., Wang, Z., Wu, L., Yue, P., Guo, H., Wu, F., and Ma, T. 2010. Preparation and characterization of Li4Ti5d2 from ilmenite. Powder Technology, vol. 204. pp. 198-202. https://doi.org/10.1016/j.powtec.2010.07.030 [ Links ]
Wang, Y. and Yuan, Z. 2006. Reductive kinetics of the reaction between a natural ilmenite and carbon. International Journal of Mineral Processing, vol. 81, no. 3. pp. 133-140. https://doi.org/10.1016/j.minpro.2006.07.010 [ Links ]
Wang, Y.M., Yuan, Z.F., Guo, Z.C., Tan, Q.Q., Li, Z.Y., and Jiang, W.Z. 2008. Reduction mechanism of natural ilmenite with graphite. Transactions of the Nonferrous Metals Society of China, vol. 18. pp. 962-968. https://doi.org/10.1016/S1003-6326(08)60166-1 [ Links ]
Wei, L., Hu, H., Chen, Q., and Tan, J. 2009. Effects of mechanical activation on the HCl leaching behaviour of plagioclase, ilmenite and their mixtures. Hydrometallurgy, vol. 99. pp. 39-44. https://doi.org/10.1016/j.hydromet.2009.06.003 [ Links ]
Welham, N.J. 1996. A parametric study of the mechanically activated carbothermic reduction of ilmenite. Minerals Engineering, vol. 9, no. 12. pp. 1189-1200. https://doi.org/10.1016/S0892-6875(96)00115-X. [ Links ]
Welham, N.J. and Llewellyn, D.J. 1988. Mechanical enhancement of the dissolution of ilmenite. Minerals Engineering, vol. 11, no. 9. pp. 827-841. https://doi.org/10.1016/S0892-6875(98)00070-3 [ Links ]
Welham, N.J. and Williams, J.S. 1999. Carbothermic reduction of ilmenite (FeTiO3) and rutile (TiO2). Metallurgical and Materials Transactions B, vol. 30, no. 6. pp. 1075-1081. https://doi.org/10.1007/s11663-999-0113-7 [ Links ]
Wijewardhana, T.D.U., Subasinghe, H.C.S., and Ratnayake, A.S. 2021. Value addition to ilmenite using carbonized waste coconut shells: A mechanochemical approach aided with powdered seashells as a rate raiser. Mining, Metallurgy & Exploration, vol. 38, no. 3. pp. 1573-1587. https://doi.org/10.1007/s42461-021-00420-z [ Links ]
Wu, F., Li, X., Wang, Z., Wu, L., Guo, H., Xiong, X., Zhang, X., and Wang, X. 2011a. Hydrogen peroxide leaching of hydrolysed titania residue prepared from mechanically activated Panzhihua ilmenite leached by hydrochloric acid. International Journal of Mineral Processing, vol. 98. pp. 106-112. https://doi.org/10.1016/j.minpro.2010.10.013 [ Links ]
Wu, F., Li, X., Wang, Z., Guo, H., Wu, L., Xiong, X., and Wang, X. 2011b. Preparation of TiO2 nanosheets and LiTi5O12 anode material from natural ilmenite. Powder Technology, vol. 213. pp. 192-198. https://doi.org/10.1016/j.powtec.2011.07.034 [ Links ]
Wu, Z., Liang, Y., Fu, E., Du, J., Wang, P., Fan, Y., and Zhao, Y. 2018. Effect of ball milling parameters on the refinement of tungsten powder. Metals, vol. 8, no. 4. pp. 281. https://doi.org/10.3390/met8040281 [ Links ]
Xie, R. and Shang, J.K. 2007. Morphological control in solvothermal synthesis of titanium oxide. Journal of Materials Science, vol. 42. pp. 6583-6589. https://doi.org/10.1007/s10853-007-1506-0 [ Links ]
Xiong, X., Wang, Z., Wu, F., Li, X., and Guo, H. 2013. Preparation of TiO2 from ilmenite using sulphuric acid decomposition of the titania residue combined with separation of Fe3+ with EDTA during hydrolysis. Advanced Powder Technology, vol. 24. pp. 60-67. https://doi.org/10.1016Zj.apt.2012.02.002 [ Links ]
Xue, T., Wang, L., Qi, T., Chu, J., Qu, J., and Liu, C. 2009. Decomposition kinetics of titanium slag in sodium hydroxide system. Hydrometallurgy, vol. 95. pp. 22-27. https://doi.org/10.1016/j.hydromet.2008.04.004 [ Links ]
Zhang, L., Hu, H., Wei, L., Chen, Q., and Tan, J. 2010. Hydrochloric acid leaching behaviour of mechanically activated Panxi ilmenite (FeTiO3). Separation and Purification Technology, vol. 73. pp. 173-178. https://doi.org/10.1016/j.seppur.2010.03.022 [ Links ]
Zhang, P., Jia, D., Yang, Z., Duan, X., and Zhou, Y. 2013. Influence of ball milling parameters on the structure of the mechanically alloyed SiBCN powder. Ceramics International, vol. 39, no. 2. pp. 1963-1969. https://doi.org/10.1016/j.ceramint.2012.08.047 [ Links ]
Zhang, G. and Ostrovski, O. 2001. Reduction of ilmenite concentrates by methane containing gas: Part I. Effects of ilmenite composition, temperature and gas composition. Canadian Metallurgical Quarterly, vol. 40, no. 3. pp. 317-326. https://doi.org/10.1179/cmq.2001.40.3.317 [ Links ]
Zhang, G. and Ostrovski, O. 2002. Effect of preoxidation and sintering on properties of ilmenite concentrates. International Journal of Mineral Processing, vol. 64, no. 4. pp. 201-218. https://doi.org/10.1016/S0301-7516(01)00055-2 [ Links ]
Zhang, S. and Nicol, M.J. 2010. Kinetics of the dissolution of ilmenite in sulphuric acid solutions under reducing conditions. Hydrometallurgy, vol. 103. pp. 196-204. https://doi.org/10.1016/j.hydromet.2010.03.019 [ Links ]
Zhang, W., Zhu, Z., and Cheng, C.Y. 2011. A literature review of titanium metallurgical processes. Hydrometallurgy, vol. 108. pp. 177-188. https://doi.org/10.1016/j.hydromet.2011.04.005 [ Links ]
Zhang, F.L., Zhu, M., and Wang, C.Z. 2008. Parameters optimization in the planetary ball milling of nanostructured tungsten carbide/cobalt powder. International Journal of Refractory Metals and Hard Materials, vol. 26, no. 4. pp. 329-333. https://doi.org/10.1016/j.ijrmhm.2007.08.005 [ Links ]
Zhao, Y. and Shadman, F. 1990. Kinetics and mechanism of ilmenite reduction with carbon monoxide. AIChE Journal, vol. 36, no. 9. pp. 1433-1438. https://doi.org/10.1002/aic.690360917 [ Links ]
Zhu, Q., Zhang, J., and Li, H. 2014. Influence of phase and microstructure on the rate of hydrochloric acid leaching in pretreated Panzhihua ilmenite. Particuology, vol. 14. pp. 83-90. https://doi.org/10.1016/j.partic.2013.08.002 [ Links ]
Zhu, Z., Zhang, W., and Cheng, C.Y. 2011. A literature review of titanium solvent extraction in chloride media. Hydrometallurgy, vol. 105. pp. 304-313. https://doi.org/10.1016/j.hydromet.2010.11.006 [ Links ]
 Correspondence:
Correspondence:
A.S. Ratnayake
Email: as_ratnayake@uwu.ac.lk
Received: 16 Apr. 2021
Revised: 17 Oct. 2022
Accepted: 24 Nov. 2022
Published: August 2023
ORCID:
A.S. Ratnayake http://orcid.org/0000-0001-7871-2401














