Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.123 n.7 Johannesburg Jul. 2023
http://dx.doi.org/10.17159/2411-9717/1600/2023
PROFESSIONAL TECHNICAL AND SCIENTIFIC PAPERS
Enhancement of shear flocculation of a galena suspension by ultrasonic treatment
K. Esmeli; A. Ozkan
Department of Mining Engineering, Konya Technical University, Konya, Turkey. ORCID: K. Esmeli: http://orcid.org/0000-0001-5699-5199; A. Ozkan: http://orcid.org/0000-0002-5780-788X
SYNOPSIS
The enhancement of shear flocculation of a galena suspension by ultrasound in the presence of sodium isopropyl xanthate and potassium ethyl xanthate was investigated by studying the effects of surfactant concentration on flocculation efficiency, contact angle, and zeta potential. Strong flocculation was obtained under alkaline conditions (pH > 9) and a high ultrasound power level (150 W). Although the ultrasonic treatment increased the negativity of the zeta potential of the galena particles, this did not decrease the flocculation efficiency, thereby showing that the hydrophobic interactions between the particle were stronger than the electrical double-layer repulsion resulting from xanthate adsorption. The findings indicate that the ultrasonic treatment promoted the adsorption of surfactants onto the mineral surfaces.
Keywords: ultrasonic treatment, galena, shear flocculation, contact angle, zeta potential.
Introduction
Due to the depletion of high-grade ore reserves, finely disseminated deposite are increasingly being exploited. The fine particles formed during mineral liberation cause many problems downstream, such as during dewatering, transportation, and dust generation. Although flotation is the most common method used for the beneficiation of fine minerals, it has some disadvantages (Song and Valdivieso, 1998). Flocculation, shear flocculation, or selective flocculation techniques are used as alternatives to flotation (Warren, 1975). Flocculation is performed by creating a physical bridge between particles using high-molecular-weight organic polymers. Shear flocculation is an aggregation process that takes place under an appropriate mixing regime after fine grains are rendered hydrophobic using convenient surfactants. Shear flocculation is carried out through the application of sufficient mechanical energy (mixing of suspension) to overcome the energy barrier caused by surface charges on the particles (Warren, 1975). Surfactants known as flotation collectors are generally used to increase the hydrophobicity of particle surfaces. In shear flocculation, the hydrophobic attraction between grains and the merging of hydrocarbon chains between layers of surfactant adsorbed to the surfaces cause particles to aggregate during interparticle collisions (Warren, 1992).
Lead rarely occurs in nature in the free form, and is usually associated with other elements in minerals such as galena (PbS) and the oxides cerussite and anglesite, which are the most common lead minerals. Among these minerals, galena has the highest lead content and is the most important source of lead (Ozun and Ergen, 2019).
Ultrasound propagates in liquids as a wave consisting of successive compression and rarefaction cycles. The rarefaction cycle has negative pressure, and the following compression cycle has positive pressure. The rarefaction cycle overcomes the intermolecular forces binding the liquid, resulting in the formation of microbubbles, while the compression cycle instantaneously causes a localized burst of energy. This phenomenon, which has an important influence on any solid phase in the liquid, is known as cavitation (Onal, Ozer, and Arslan, 2003; Altun, Hwang, and Hicyilmaz, 2009).
Recently, the use of ultrasound waves in mineral processing has increased, and studies have been conducted on its effect on grinding, leaching, and especially flotation (Onal, Ozer, and Arslan, 2003; Altun, Hwang, and Hicyilmaz, 2009; Lemanowicz, Kus and Gierczycki, 2010; Ozkan and Gungören, 2012). In flotation, ultrasound treatment enhances the performance of the reagents used by distributing them more uniformly throughout the suspension (Altun, Hwang, and Hicyilmaz, 2009; Celik, 1989). In addition, distortions at solid-liquid interfaces alter the surface properties of the mineral particles, which affects the adsorption of collectors and thus the flotation performance (Slaczka, 1987). Although there are few studies of the influence of ultrasonic processes on the sedimentation of minerals, the beneficial effect of ultrasound has-also been noted in this regard (Onal, Ozer, and Arslan 2003; Demir et al., 2021). Onal, Ozer, and Arslan (2003) reported that flocculant consumption and settling time decreased as a result of the use of ultrasound in clay flocculation. Burat, Sirkeci, and Onal (2014) and Yang et al. (2022) found similar results in their studies focusing on the effect of ultrasonic treatment on the dewatering of fine coal, and stated that ultrasonic treatment improved flocculation and filtration performance. Few studies, however, have been conducted to investigate the effect of ultrasonic treatment on the shear flocculation of minerals with surfactants. The research reported here is aimed at elucidating the effect of ultrasonic treatment on the shear flocculation of galena using sodium isopropyl xanthate and potassium ethyl xanthate as surfactants, by means of measurements of zeta potential and contact angle.
Materials and methods
Materials
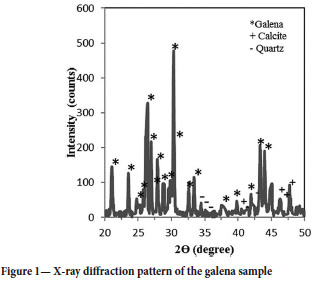
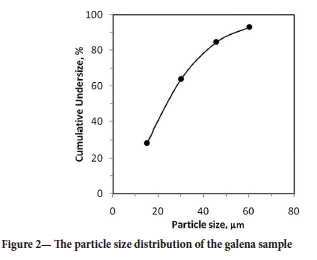
The sample of galena concentrate used in the experiments was obtained from the Koyulhisar sulphide ore concentrator in Sivas, Turkey. The XRD spectrum of the sample is shown in Figure 1. The analysis revealed that calcite, quartz, and clay were present in low amounts. The sample was dry-ground for 3 hours using a ceramic ball mill. Particle size of the ground sample was determined using a laser diffractometer (Malvern Mastersizer 2000, UK), and the particle size distribution is shown in Figure 2. The prepared sample was 80% passing 40 |im. Sodium isopropyl xanthate (C4H7NaOS2) and potassium ethyl xanthate (CH3CH2OCS2K) were used as anionic surfactants for galena. The reagents were obtained from ECS Chemistry (Istanbul, Turkey) and had a purity of 99.5%. Hydrochloric acid (HCL, Merck) and sodium hydroxide (NaOH, Merck) solutions were used as pH adjusters. The ultrasonic homogenizer (Bandelin HD 3200, Germany) used in the experimental studies is shown in Figure 3. The constant frequency of the device is 20 kHz, and its maximum output power value is 200 W.

Shear flocculation experiments and ultrasonic treatment
In the flocculation experiments, 1 g ground galena cocentrate and 300 cm3 distilled water were added to a glass beaker fitted with fourbaffles and mixed with a magnetic stirrer at a rotational speed of 500 r/min for 2 minutes. Surfactant was then added and the suspension was conditioned by the ultrasound device for 5 minutes. Following this, the suspension was again stirred with a magnetic stirrer for 2 minutes at the same rotational speed. Then, the suspension was allowed to settle for 1 minute. Finally, a suspension sample of 20 cm3 was taken at a constant depth of 5 cm below the air-liquid interface using a special system for turbidity measurements. The average of three measurements made on this sample was recorded as the turbidity value, and the measurement error was found to be approximately 5%. The degree of flocculation was determined using Equation [1]:
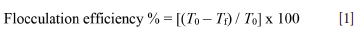
where To is the turbidity of the original suspension and Tf is the turbidity of the sample after sedimentation assisted by a surfactant.
The flocculation tests were conducted at a pH of 8.4 (natural pH). The ultrasonic treatment was carried out in batch mode (successive pulsations on for 5 seconds and off for 10 seconds), with the probe centrally located at an immersion depth of 2 cm. The shear flocculation values were determined to be within an experimental error of ± 3%.
Measurements of turbidity, zeta potential, contact angle, and SEM
A digital turbidimeter (Velp Scientifica, Usmate Velate, Italy) was used to measure the turbidity, and the turbidity values were reported as nephelometric turbidity units (NTU).
A ZetaPlus apparatus (Brookhaven, USA) was used for the zeta potential measurements. The device measures the zeta potential of the sample using the electrophoresis method (Hunter, 1981). Zeta potential measurements were conducted under the same experimental conditions as the flocculation tests. A sample of supernatant was taken and placed in a cell made of plastic. A total of 12 runs for each sample were used for the zeta potential measurements, and the average value recorded.
The contact angle values were measured using a goniometer device (KSV CAM 101, KSV Instruments Finland). The flocs formed during flocculation tests were removed by filtration and dried. Then, cylindrical pellets with a height of 2-3 mm and a diameter of 1.2 cm were prepared from a 0.5 g dried sample by using a hydraulic press. The pellets were prepared using a constant pressure of 25 MPa. A drop of liquid was placed on the pellet surface with a syringe and the device recorded this image. Two angle values (0leftand 0right) were determined for each sample, and the contact angle was taken as the average of these two values.
A Zeiss EVO LS10 scanning electron microscope was used to examine the surface morphologies of the ultrasound-treated and non-treated galena particles.
Results and discussion
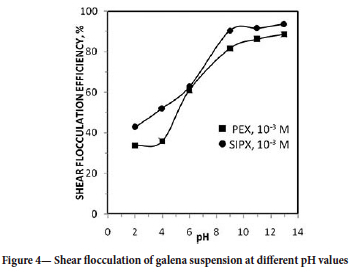
Figure 4 illustrates the effect of pH on shear flocculation efficiency of the galena suspension at an ultrasound power of 150 W. Flocculation with both sodium isopropyl xanthate (SIPX) and potassium ethyl xanthate (PEX) increased as pH increased, with atrong flocculation obtaines in the pH range of 9-13. The flotation of sulphide minerals with xanthates depends strongly on the pH of the suspension, and because the anionic monomers of xanthates (X-) are more dominant at neutral-alkaline pH levels, high flotation recoveries of galena are usually obtained at moderately alkaline pH values (Ozun and Ergen, 2019). A similar result was evident in the current study, even at high pH. SIPX, the longer-chain surfactant, was more effective than PEX. Long-chain surfactants are known to be more effective in flocculation because the hydrocarbon chain association depends on the carbon number of the adsorbed surfactant chain (Zollars and Ali, 1986; Ozkan, Ucbeyiay, and Aydogan, 2006).
The effects of SIPX and PEX concentration on the flocculation of galena suspension at 40 and 150 W ultrasound power are shown in Figure 5. Flocculation was enhancemed at the higher power setting of 150 W, but at 40 W the flocculation efficiencies were lower than those obtained without ultrasonic treatment. The physical and chemical effects of ultrasonication are known to be complex.
Therefore, different ultrasound intensities and exposure times cause different results (Chen et al., 2020). Flocculation of galena initially increased depending on the surfactant concentrations, and then plateaus were obtained for all three conditions.
Figure 6 presents the effects of ultrasound on the contact angle and zeta potential of galena at various SIPX and PEX concentrations. The contact angle increased with increases in both surfactant concentrations. Song et al. (2000) noted that in the hydrophobic flocculation of galena, the hydrophobicity of the particle surfaces increased as the adsorption of the surfactant increased, which leads to an increase in the contact angle. In our study, the ultrasonic treatment further increased the contact angle values, resulting in more successful flocculation (see Figure 7). Similar results have been observed in some flotation studies. Gungoren et al. (2019) examined the effect of ultrasound on quartz flotation and found that ultrasonic treatment increased the contact angle of quartz with amine. Gungoren et al. (2020) also noted that higher contact angle values were obtained for galena with PEX under optimal ultrasound conditions, but increased ultrasound power and long treatment times adversely affected both contact angle and flotation performance. Xu et al. (2017) stated that the contact angle values of coal increased with the application of a certain ultrasound treatment time, but that ultrasonic treatment in the presence of SIPX and PEX increased the magnitude of the negative zeta potential of galena. Ozkan (2012) also noted that ultrasonic treatment increased the negativity of the zeta potential of hard coal slime samples.
The electrical double layer describes the electrical potential change near a surface, which gives very important information about the behaviour of colloidal particles in contact with a solution. Therefore, the electrical double layer interactions between mineral fines are important in the aggregation process (Verwey and Overbeek, 1948). It can be expected that the increase in the zeta potential will strengthen the energy barrier that prevents particle aggregation. However, the increase in the surface load by xanthate adsorption on the galena surfaces with and without ultrasonic treatment did not suppress the flocculation under the experimental conditions studied. Similar results have also been recorded for various minerals in some studies where ultrasonic treatment was not used (Song et al., 2000, 2001; Duzyol and Ozkan, 2010). As a result, it can be said that xanthate adsorption onto the galena surfaces strengthens the hydrophobic interactions more than the electrical double-layer repulsions. Also, the negative zeta potential and contact angle of galena were further increased as the concentration of SIPX and PEX increased, due to the adsorption of xanthates on the galena surfaces and the increased adsorption density, depending on the xanthate concentration. It is clear that the flocculation efficiency of galena with or without ultrasonic treatment responded to collector concentration in the same way as the contact angle, which suggests that there is a significant correlation between flocculation and surface hydrophobicity.
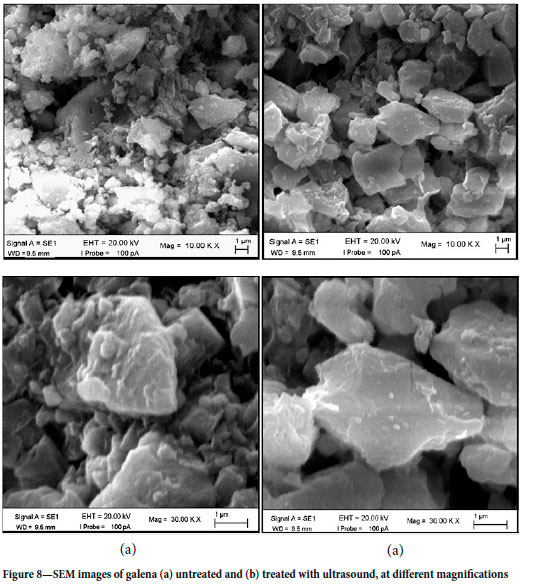
The scanning electron micrographs of galena particles treated with and without ultrasound (150 W power, 300 secndso batch treatment, and 10-3 M SIPX concentration) are shown in Figure 8. The images show that the galena surfaces became smoother following ultrasonic treatment. The relationship between the hydrophobicity of various minerals and the surface roughness was studied by Ulusoy and Yekeler (2005). They found that as the surface smoothness increased, the hydrophobicity of the minerals improved. Xu et al. (2017) also reported that the surface of oxidized coal became smoother with increased ultrasound exposure time, which enhanced flotation by removing the oxidized layer. However, with prolonged ultrasonic treatment the surface roughness increased again. Gurpinar and Sonmez (2013) stated that ultrasonic treatment improved collector adsorption by exposing clean surfaces.
The effects of the ultrasonic treatment on the flocculation efficiency, contact angle, and zeta potential of galena at xanthate concentration of 10-3 M are summarized in Figure 9. As seen in the figure, ultrasound enhanced the shear flocculation efficiency of galena with SIPX and PEX. In addition, higher contact angle values were reached using the ultrasonic treatment in the presence of these surfactants, and this led to more successful flocculation. On the other hand, in the presence of SIPX and PEX, the ultrasonic treatment increased the negativity of the zeta potential of the galena, bu this did not cause a decrease in the flocculation of the suspension.
Conclusions
Galena flocculation was successfully enhanced using ultrasonic treatment at a high power level in conjunction with sodium isopropyl xanthate and potassium ethyl xanthate. The flocculation results were in good agreement with the improvement in the particle surface hydrophobicity, as measured by the contact angle values. The longer-chain surfactant (sodium isopropyl xanthate) was more effective in the shear flocculation, imparting higher contact angles. Although the ultrasonic treatment increased the surface charge of galena particles with these surfactants, this did not cause a decrease in the flocculation efficiency. These results show that the hydrophobic interactions between the mineral fines that resulted from the adsorption of the xanthate ions were stronger than the electrical double-layer repulsion. In other words, this process is closely related to the hydrophobicity of particle surfaces, as in the flotation process. The beneficial effects of ultrasound energy in the flotation process are also observed in the flocculation of galena with these surfactants.
Acknowledgment
The present study was financially supported by Konya Technical University OYP Fund for project no. 2015-OYP-015.
References
Altun, N.E., Hwang, J.Y., and Hicyilmaz C. 2009. Enhancement of flotation performance of oil shale cleaning by ultrasonic treatment. International Journal of Mineral Processing, vol. 91. pp.1-13. https://doi.org/10.1016/j.minpro.2008.10.003 [ Links ]
Burat, F., Sirkeci, A.A., and Onal, G. 2014. Improved fine coal dewatering by ultrasonic pretreatment and dewatering aids. Mineral Processing and Extractive Metallurgy Review, vol. 36, no. 2. pp. 129-135. [ Links ]
Celik, M.S. 1989. Effect of ultrasonic treatment on the floatability of coal and galena. Separation Science and Technology, vol. 24. pp.1159-1166. [ Links ]
Chen,Y., Truong, V.N.T., Bu, X., and Xie, G. 2020. A review of effects and applications of ultrasound in mineral flotation. Ultrasonics Sonochemistry, vol. 60. pp. 104739. https://doi.org/10.1016/j.ultsonch.2019.104739 [ Links ]
Demir, I., Gungoren, C., Baktarhan, Y.,Yücel, M., Ünver, I.K., Çinku, K., and Ozkan, S.G. 2021. Ultrasound supported flocculation of borate tailings with differently charged flocculants. Boron, vol. 6, no. 3. pp. 348-58. https://doi.org/10.30728/boron.971892 [ Links ]
Duzyol, S. and Ozkan, A. 2010. Role of hydrophobicity and surface tension on shear flocculation and oil agglomeration of magnesite. Separation and Purification Technology, vol. 72. pp. 7-12. https://doi.org/10.1016/j.seppur.2009.12.011 [ Links ]
Gungoren, C., Baktarhan, Y., Demir, I., and Ozkan, S.G. 2020. Enhancement of galena-potassium ethyl xanthate flotation system by low power ultrasound. Transactions of Nonferrous Metals Society of China, vol. 30. pp.1102-1110. [ Links ]
Gungoren, C., Ozdemir, 0., Wang, X., Ozkan, S., and Miller, J. 2019. Effect of ultrasound on bubble-particle interaction in quartz-amine flotation system. Ultrasonics Sonochemistry, vol. 52. pp. 446-454. https://doi.org/10.1016/j.ultsonch.2018.12.023 [ Links ]
Gurpinar, G., Sonmez, E., and Bozkurt, V 2013. Effect of ultrasonic treatment on flotation of calcite, barite and quartz. Mineral Processing and Extractive Metallurgy, vol. 113. pp. 91-95. https://doi.org/10.1179/037195504225005796 [ Links ]
Hunter, R.J. 1981. Introduction to Modern Colloid Science, Oxford Science. [ Links ]
Lemanowicz, M., Kus, A., and Gierczycki, A.T. 2010. Influence of ultrasonic conditioning of flocculant on the aggregation process in a tank with turbine mixer. Chemical Engineering and Procesing, vol. 49. pp. 205-211. https://doi.org/10.1016/j.cep.2009.12.007 [ Links ]
Onal, G., Ozer, M., and Arslan, F. 2003. Sedimentation of clay in ultrasonic medium. Minerals Engineering, vol. 16. pp.129-134. https://doi.org/10.1016/S0892-6875(02)00309-6 [ Links ]
Ozkan, A., Ucbeyiay, H., and Aydogan, S. 2006. Shear flocculation of celestite with anionic surfactants and effects of some inorganic dispersants. Colloids and Surfaces A: Physicochemical and Engineering Aspects, vol. 281. pp.92-98. https://doi.org/10.1016/j.colsurfa.2006.02.020 [ Links ]
Ozkan, S.G. 2012. Effects of simultaneous ultrasonic treatment on flotation of hard coal slimes. Fuel, vol. 93. pp.576-580. https://doi.org/10.1016/j.fuel.2011.10.032 [ Links ]
Ozkan, S.G. and Gungoren, C. 2012. Enhancement of colemanite flotation by ultrasonic pre-treatment. Physicochemical Problems In Mineral Processing, vol. 48. pp. 455-462. [ Links ]
Ozun, S. and Ergen, G. 2019. Determination of optimum parameters for flotation of galena: Effect of chain length and chain structure of xanthates on flotation recovery. ACS Omega, vol. 4. pp. 1516-1524. https://doi.org/10.1021/acsomega.8b02841 [ Links ]
Slaczka, A. 1987. Effect of an ultrasonic field on the flotation selectivity of barite from a barite-fluorite-quartz ore. International Journal of Mineral Processing, vol. 20. pp.193-210. https://doi.org/10.1016/0301-7516(87)90066-4. [ Links ]
Song, S. and Lopez-Valdivieso, A. 1998. Hydrophobic flocculation flotation for beneficiating fine coal and minerals. Separation Science and Technology, vol. 33. pp.1195-1212. [ Links ]
Song, S., Lopez-Valdivieso, A., Reyes-Bahena, J.L., and Bermejo-Perez, H.I. 2001. Hydrophobic flocculation of sphalerite fines in aqueous suspensions induced by ethyl and amyl xanthates. Colloids and Surfaces A: Physicochemical and Engineering Aspects, vol. 181. pp.159-169. https://doi.org/10.1016/S0927-7757(00)00789-5 [ Links ]
Song, S., Lopez-Valdivieso, A., Reyes-Bahena, J.L., Bermejo-Perez, H.I., and Trass, 0. 2000. Hydrophobic flocculation of galena fines in aqueous suspensions. Journal of Colloid and Interface Science, vol. 227. pp.272-281. https://doi.org/10.1006/jcis.2000.6857 [ Links ]
Ulusoy, U. and Yekeler, M. 2005. Correlation of the surface roughness of some industrial minerals with their wettability parameters. Chemical Engineering And Processing, vol. 44. pp. 557-565. https://doi.org/10.1016/j.cep.2004.08.001 [ Links ]
Verwey, E.J.W and Overbeek, J.Th.G. 1948. Theory of the Stability of Lyophobic Colloids. Elsevier, Amsterdam. [ Links ]
Warren, L.J. 1975. Shear flocculation of ultrafine scheelite in sodium oleate solutions. Journal of Colloid and Interface Science, vol. 50. pp. 307-318. https://doi.org/10.1016/0021-9797(75)90234-9 [ Links ]
Warren, L.J. 1992. Shear flocculation. Colloid Chemistry in Mineral Processing. Laskowski, J.S. and Ralston, J. (eds). Elsevier, New York. pp. 309-329. [ Links ]
Xu, M., Xing, Y., Gui, X., Cao, Y., Wang, D., and Wang, L. 2017. Effect of ultrasonic pretreatment on oxidized coal flotation. Energy Fuels, vol. 31, no. 12. pp. 1436714373. https://doi.org/10.1021/acs.energyfuels.7b02115 [ Links ]
Yang, A., Liao, Y., An, M., Cao, Y., Yang, Z., Ren, H., Su, H., Zou, Q., and Chen, L. 2022. Effect of ultrasonic pretreatment on flocculation filtration of low-rank coal slurry. Molecules, vol. 27. 6460 p. [ Links ]
Zollars, R.I. and Ali, S.I. 1986. Shear coagulation in the presence of repulsive interparticle forces. Journal of Colloid and Interface Science, vol. 114. pp.149166. https://doi.org/10.1016/0021-9797(86)90247-X [ Links ]
 Correspondence:
Correspondence:
K. Esmeli
Email: kesmeli@ktun.edu.tr
Received: 19 Apr. 2021
Revised: 29 Oct. 2022
Accepted: 26 Jul. 2023
Published: July 2023














