Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.123 n.7 Johannesburg Jul. 2023
http://dx.doi.org/10.17159/2411-9717/2445/2023
COPPER COBALT EDITION
The use of seawater in copper hydrometallurgical processing in Chile: A review
Á. AstudilloI; M. GarciaI; V. QuezadaI; L. ValásquezII
IDepartmento de Ingeniería Metalúrgica y Minas, Universidad Católica del Norte, Antofagasta, Chile
IIDepartment of Mining Engineering, Pontifícia Universidad Católica de Chile
SYNOPSIS
Seawater has become a viable alternative for different uses in copper hydrometallurgy. In this paper we review the main physical and chemical characteristics of seawater and how these influence copper production. Reliable data on the use of continental water are reported, and the current use and consumption of seawater in the Chilean mining industry is analysed, indicating the main areas of use and the main problems encountered. Additionally, the influence of the elements in seawaterthat have the most influence on the extractive metallurgy of copper are considered. The Chilean copper mining industry currently consumes approximately 4.1 m3/s of seawater, which corresponds to 25% of the total water used. The use of seawater for the leaching of copper sulphide minerals, such as chalcopyrite, is beneficial because it provides 20 g/L of chloride, thereby improving copper dissolution kinetics.
Keywords: seawater, copper, hydrometallurgy, Chile, leaching, chloride.
Introduction
Owing to the scarcity of water resources required by the mining industry (Kinnunen et al., 2021), it is becoming increasingly difficult to develop new projects. Water is used for a variety of applications, from dust control on roads to metal recovery processes such as leaching and flotation (Gunson et al., 2012). Most of these processes require large volumes of water, which compels the industry to recycle as much as possible and to explore new sources of water (Herrera-León et al., 2019).
In Chile, the most important mining area is in the extreme north, which is characterized by arid and semi-arid conditions (Atacama Desert). Even in the coastal areas, the meteorological stations record annual precipitation levels of less than 3 mm (García and Osses, 2017; Romero, Mendonca, and Catarina, 2012; Sarricolea, Ruiz, and Aravena, 2017). Water scarcity a growing problem for mining companies. Searching for an alternative to replace the use of inland waters is becoming increasingly important, with seawater being one such alternative.
Seawater contains various elements, many of which are provided by erosion of the Earth's crust. Seawater has high salinity (around 3.5%), comprising mainly Na+ and Cl ions, with lesser quantities of Ca2+, Mg2+, SO42-, and HCO3-, among others (Qiu et al., 2016). In addition, the pH of seawater is moderately alkaline, reaching values ranging from 7.2 to 8.2, depending on the amounts of dissolved salts (San Martín et al., 2020). The presence of ions contained in seawater varies depending on the local environmental characteristics. Another component of seawater is organic material, which is contributed by the ecosystem of organisms that proliferate throughout maritime environments (Morales, 2017).
To manage the challenge of water scarcities, there has been a considerable increase in the uses of non-conventional water sources and renewable energies in the mining industry. In Chile, since 2019, 14 companies have used seawater in their processes, including Minera Escondida, Centinela, Antucoya, and Sierra Gorda. Furthermore, in 2019, the use of seawater in copper mining reached 4.06 m3/s, which represents 25% of the water used in mining. Of the water utilized, 1.84 m3/s corresponds to seawater directly used in processes, while 2.22 m3/s is desalinated water (COCHILCO, 2020a). The rise in water consumption in Chilean mining is due to an increase in the production of sulphide copper minerals, which are treated by flotation. It is estimated that the production of concentrates to be processed will reach 890 Mt by 2027 (COCHILCO, 2016), an increase of 89.9% since 2017 (COCHILCO, 2017).
Seawater
Humans have in the past regarded the ocean as an unlimited resource. It is used as a food source and a means of transport. Approximately 70% of the Earth is covered by water, of which 97.5% is salt water and 2.5% is fresh water, which corresponds to about 35 million km3. Only 0.007% of that amount is suitable for human consumption (Carrión, 2020). According to the Food and Agriculture Organization (FAO), in 2006 Chile recorded withdrawals comprising 92% surface water, 7.8% groundwater, and only 0.2% saline water (FAO, 2015).
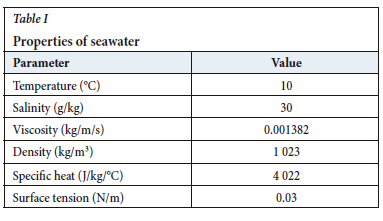
Seawater possesses unique characteristics with respect to the distributions of temperature, pressure, and density. Seawater is an effective solvent that dissolves many sediments that come from land. In addition, it has many conservative and non-conservative properties, such as viscosity, density, thermal expansion, and turbidity (Balasubramanian, 2011). Temperature and salinity, which determine the variability of other properties such as density and surface tension, are among the most important. According to Chang et al., (2012), if it assumed that the temperature of seawater is a constant 10°C with salinity of 30 g/kg, the other properties will be as given in Table I.
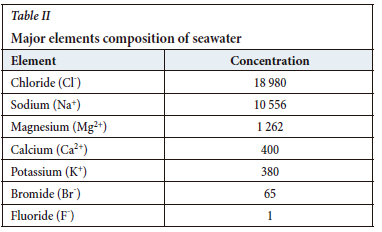
Seawater contains dissolved ions that represent approximately 3.5% of the composition, while the remaining 96.5% is water (Qiu et al., 2016). The most abundant elements in seawater, in cationic forms, are sodium, magnesium, potassium, and calcium. The most abundant anions are chlorine, sulphate, and bicarbonate. The chemical composition of seawater remains almost constant in the ocean due to thermodynamics. Some salts precipitate when an excess of these ions is created and are incorporated as sediments. Circulation of the oceanic masses allows for constant agitation and mixing of water. Marine biota fix certain salts, such as carbonates and silicates. These transform into insoluble materials, such as shells, which are added to the sediments in the sea when the animals die (Cisternas and Moreno,2014). The chemical composition of seawater is shown in Table II (Balasubramanian, 2011).
The mass fraction of material dissolved in seawater corresponds to absolute salinity: precisely determining this parameter is practically impossible. Millero et al. (2008) developed a new measure of salinity termed reference salinity. This parameter is used to better represent absolute salinity, based on Knudsen salinity, chlorinity, and practical salinity.
Temperature usually varies according to latitude and depth; for example, at the poles, the temperature in summer is about 3 °C in the deeper areas of the oceans. In the Baltic Sea and North Sea, temperatures vary between 14 and 18°C; and in the Mediterranean between 22 and 27°C. In Cuba, the water temperature is 25°C on average (Flórez and Bernabé Calle, 2015). According to the navy's hydrographic and oceanographic service (SHOA), temperatures in the north of Chile vary between 15 and 20°C, and the annual average from the centre of the country to the city of Arica in the most northern region is 17°C (SHOA, 2022).
Density depends on temperature only if a material is in its pure state; that of seawater also depends on salinity (Cisternas and Moreno, 2014). Fresh water reaches its maximum density at a temperature of 4°C, whereas the maximum density of seawater occurs at its freezing point, which is -1.9°C (Anil et al., 2016). The density of seawater varies between 1020 and 1050 kg/m3, depending on the depth and pressure, because it has a higher density as depth increases (Tenzer, Novák, and Gladkikh, 2011).
Current use of seawater in Chilean copper mining
In the Chilean copper mining industry, the current total water consumption rate is approximately 18.4 m3/s, of which 12.8 m3/s corresponds to continental waters and 5.6 m3/s is seawater. The use of seawater is projected to increase to 11.0 m3/s by 2030, representing approximately 47% of the water required by copper mining (COCHILCO, 2019). The use of seawater in Chilean copper mining has been driven by over-exploitation of water resources in arid or semi-arid regions, because 76% of the country's surface is affected by desertification, droughts, and degraded soils (Santoro et al., 2021). Average annual availability of fresh water in the north of Chile (Antofagasta region) reaches 53 m3 per capita per year, and values as high as 2 993 535 m3 per capita in the southern zone (Aysén) (Alvez et al., 2020).
Areas of seawater use
The Chilean Copper Commission identifies two types of seawater used in mining: water produced by desalination plants and by seawater impulsion systems. Desalination is carried out to eliminate the elements contained in seawater, thereby obtaining salt-free water. Seawater impulsion systems apply only minor pretreatments to reduce the amounts of impurities that may interfere with the metallurgical processes.
The use of untreated seawater is an unconventional method in the metallurgical extraction of copper. The contained ions can favour or harm processes. For example, the chloride ions in unprocessed seawater can benefit the leaching of certain copper sulphides (Lu and Dreisinger, 2013; Ruiz, Montes, and Padilla, 2011). This positive effect is due to an increase in the porosity of the passivating layer of elemental sulphur that is generated when copper sulphides are leached, allowing for better leaching kinetics (Hashemzadehm Dixon, and Liu, 2019).
Problems with untreated seawater
Desalination of seawater in Chile has been carried out since the 19th century (Hirschmann, 1975), with the installation of the first solar distillation plant by Charles Wilson in 1872. This plant, called Las Salinas, was located near the city of Antofagasta and produced about 20 kL/d of water for human and animal consumption (Arellano-Escudero, 2015). By 2017 there were 11 operating desalination plants and 10 under evaluation in Chile. In 2020 there were 23 desalination plants, of which 14 were associated with industrial mining and 9 were for urban use (MOP, 2020). It is estimated that 15 new desalination plants for mining will be added by 2028, almost equalizing the consumption of seawater and fresh water in mining processes (COCHILCO, 2020b).
Different methods can be used to desalinate seawater, such as electrodialysis (Jiang et al., 2015), solar evaporation (Olive, 2018), distillation (Arellano-Escudero, 2015), and reverse osmosis, the latter being most used due to continuous improvements in membrane technology and energy consumption, which increase performance and efficiency (Ncube and Inambao, 2019). One of the problems of this process is that the discarded brines, which have a high concentration of salts, are usually returned to the sea. Desalination removes approximately 99% of the salts, resulting in a a highly concentrated waste brine with about Tw.ce the salinity of raw seawater. This poses a disposal problem, since it can harm the marine environment if returned to the sea (Ordonez, 2015). There are also certain problems with using seawater, either raw or desalinated, that affect not only the processes but also cover more general issues, such as transportation to the mine sites, the energy required, and equipment corrosion.
These problems have been studied by researchers and mining companies over the years in efforts to make the use of seawater more economically feasible. The following paragraphs set out the most critical problems in more detail.
Corrosion
Corrosion is damage to metals and alloys through chemical or electrochemical interaction with their environment (Maafi, 2011). Raw seawater is oarticularly corrosive, owing to its high salt content, but corrosion can also occur with desalinated seawater due to its pH values (Schorr et al., 2010). Corrosion reactions can be classified as wet or dry. Depending on the nature of the structural damage, corrosion can be classified as general corrosion, pitting corrosion, crevice corrosion, intergranular corrosion, environmentally induced fracture, de-alloying, galvanic corrosion, and erosion (Kadhim et al, 2021). Pitting and galvanic corrosion are the most common types when using seawater. The corrosion rate of metals depends on their composition and the environment. The concentration of chloride in seawater is the most influential parameter because it accelerates the rate of corrosion (Cisternas and Moreno, 2014).
Pitting is a type of localized corrosion, characterized by formation of irregular cavities on the surface of a metal. This occurs when a metal is in permanent or intermittent contact with liquid, be it ordinary water, seawater, rain, or even humidity (Garita, Rivolta, and Vega, 2013). However, when working with seawater, resistance to pitting corrosion decreases notably, due to the breakdown of the passive protective layer on the surface of the metal (Antony et al, 2010). In the case of copper, pitting corrosion is known as nodular pitting, because pitting piles up in very localized places, forming nodules or small mounds (Garcia, Uruchurtu, and Genescá, 1995). In contrast, galvanic corrosion occurs when two metals immersed in an electrolyte are in electrical contact, and are characterized by different practical nobilities, that is, by different potentials for free corrosion. When the electrolyte is seawater, a galvanic cell is formed (Palmer, 2012). Seawater facilitates the formation of galvanic cells because it makes the migration of ions possible (Cisternas and Moreno, 2014).
Energy consumption and costs
Desalination plants and seawater transport systems entail significant investment and operating costs because the vast majority of mining operations in Chile are located at great distances from the coast and at high altitudes above sea level (EDITEC, 2015). In most cases, the most important costs associated with the use of seawater are transportation of the water to the mine, which often exceed the costs of desalination itself (Herrera, Cisternas, and Gálvez, 2015). Therefore, the energy consumption of seawater impulsion systems is high, requiring the use of fuels that have an impact on the environment.
According to Gálvez and Cisternas (2017), electricity generated for desalination in the north of Chile is based on fossil fuels, with associated environmental effects. To counteract this, solar plants have been implemented as a clean energy source, but not in sufficient numbers. The reverse osmosis process typically uses 3.78 kWh/m3. In addition, the higher the sodium chloride content in seawater, the more energy is required for desalination (Ncube and Inambao, 2019). According to Garcia (2017), the average cost for desalinated seawater in Chilean mining is approximately US$5.1 per compared with US$1.6 per m3 for fresh water. This high cost is mainly due to the costs associated with transportation and the desalination process. The great difference in prices between countries is mainly due to fifferences in the cost of electrical energy, height above sea level at which the mine is located, and the distance from the mine to the coast. Approximately 80% of Chilean mining operations are located at an altitude above 3000 m (Carrasco and Vega, 2011).
Use of seawater in copper hydrometallurgical processes
With the steady depletion of oxidized copper ores and increasing production from sulphide ores, the need arises to find more economical alternatives to process these types of ores. In general, sulphide copper ores are treated by flotation, which requires large amounts of water. Thus, alternatives for hydrometallurgical processes, such as leaching using seawater, are being studied for both oxidized and sulphide copper ores. The latter are most studied, due to their complex behaviour during leaching. The chloride ion, present in seawater, is beneficial for the leaching of copper sulphides because it promotes faster dissolution kinetics (Lu and Dreisinger, 2013). The influence of chloride ions contained in seawater on the solvent extraction (SX) process has also been studied, with the results being similar to use of fresh water, and sometimes even better (Shakibania et al., 2020). Additionally, in the copper electrowinning (EW) process, the presence of chloride ions at a concentration of 0.02 g/L can improve the compaction and smoothness of cathodes (Nkuna and Popoola, 2019).
Leaching of oxidized copper ores using seawater
In Chile, leaching of oxidized copper ores with raw seawater has been carried out for more than 60 years. In 1960, the Diana plant, owned by Compania Minera de Tocopilla, obtained copper precipitates from agitation leaching. Michilla also used seawater for its leaching processes in 1970. In both plants, copper was precipitated with iron scrap to obtain copper cement (Cisternas and Moreno, 2014).
Copper oxide minerals generally have a high acid solubility, therefore the most widely used extraction method is leaching. These minerals are usually leached in an acidic medium, mainly using sulphuric acid (Deng et al., 2017). The effect of using different acids, such as HNO3 and HCl, has also been studied. Habbache et al., (2009) evaluated different acidic media (H2SO4, HNO3, and HCl) were and compared to determine which gave better copper extraction: HCl achieved the highest dissolution kinetics and copper extraction (99.95%). Senanayake (2007) asserted that high-stability chloro-complexes are formed during dissolution, which increase the adsorption kinetics and, therefore, the dissolution rate.
The effect of chloride ion concentration has also been studied. Nicol (2018) leached malachite in an acidic medium with H2SO4 and chloride ions. The author observed that faster leaching kinetics were achieved by increasing the concentration of chloride ions. A low (10 g/L) chloride concentration was sufficient to improve the leaching kinetics.
In a study by Velásquez and Quezada-Reyes (2018), various leaching tests were carried out on an oxidized copper mineral in solutions of seawater, discarded brine, and distilled water, which had chloride concentrations of 20, 32 and 20 g/L, respectively. In the first experiments, stirred-flask leaching was performed. The seawater test gave a copper recovery of 96% after 90 minutes of leaching. Kinetics of the process were high and extraction exceeded 90% in all the tests within 30 minutes. The second leaching method was carried out in stirred reactors under ambient conditions and 40 g/L H2SO4 was added to each chloride solution. The results indicated that seawater leaching gave a copper extraction greater than 90% after 20 minutes' leaching. However, the authors indicated that chloride ions did not have a strong effect on copper extraction from copper oxides.
Leaching of sulfide copper ores using seawater
The leaching of sulphide copper ores has been extensively studied. The dominant process for recovery of copper from chalcopyrite is by froth flotation to produce a copper-rich concentrate followed by pyrometallurgical treatment. This is associated with significant disadvantages, including the release of harmful emissions, being capital- and energy-intensive, and uneconomical for low-grade finely disseminated complex sulphide ores (Li et al., 2013). Hydrometallurgical methods for copper sulphide ores arise as a necessity to lower production costs, especially because chalcopyrite (CuFeS2) accounts for 70% of the world's copper reserves and is the main future feed (Aguirre et al., 2016; Gonzalez et al., 2020; Rodriguez et al., 2020; Toro et al., 2020).
One of the main problems when leaching copper sulphides is their slow dissolution rate (Bogdanovic et al., 2020) owing to overly slow dissolution kinetics. The slow dissolution kinetics is attributed to passivation, which involves formation of a layer of elemental sulpsur on the mineral surface (Beiza et al., 2019; Hernandez et al, 2020; Quezada et al, 2020). However, the nature of this passivating layer has not yet been established with certainty. The layer inhibits contact between the mineral and the oxidizing agents, which reduces the dissolution rate (Córdoba et al., 2008).
Figure 1 represents an example of passivation of chalcopyrite, mainly associated with the presence of elemental sulphur.
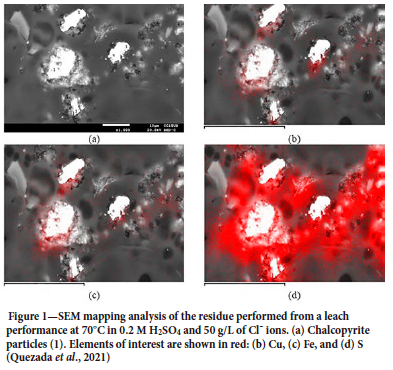
According to Velásquez-Yévenes-, Malverde, and Quezada (2022), sulphide mineral dissolution, particularly the passivation of chalcopyrite, is governed by redox potential-dependent reactions. Many studies have been devoted to determining the redox potentials at which dissolution is possible and passivation occurs (Beiza et al., 2019). Methods to reverse passivation to increase the effectiveness of copper sulphide (mainly chalcopyrite) dissolution are being studied. Although encouraging results have been obtained in the laboratory/pilot scale, these are still very expensive treatments to use on an industrial scale. However, some studies indicate that better results can be obtained in chloride media with the addition of cupric ions, because chloride solutions are more aggressive and cuprous ions are stabilized by the formation of chlorine complex ions (Torres et al., 2019). The influence of seawater on this process has been studied since the chloride ions that it contains can contribute to leaching, as discussed below.
Effect of chloride in copper ore leaching
Chloride media generate faster dissolution kinetics in the leaching Chloride media promote faster dissolution kinetics in the leaching of copper sulphide ores. Hernandez et al., (2015) studied the effect of Cl- and Cu2+ ions on the leaching of chalcopyrite in seawater and pure water containing 0.32 and 1 M H2SO4. The results indicated that the presence of seawater has a positive effect on the extraction of copper. This can be attributed to the formation of chloride-copper complexes that increase leaching kinetics. However, if extra sodium chloride is added to the seawater to ingrease the concentration above 37 g/L, the high ionic strength decreases the mobility of individual ions from solution to solid and impairs extraction rates.
Torres et al. (2015) compared the effect of using seawater with a concentration of 18 g/L Cl- and synthetic seawater with 30 g/L of Cl-. The results indicated that the extraction of copper increased for both cases, reaching 70% and 75%, respectively. Similar results were shown in a study by Toro et al. (2020), where chalcopyrite was leached with H2SO4 in wastewater and seawater with Cl- concentrations of 39.19 g/L and 19.35 g/L, respectively, as well as only H2SO4. Manganese nodules were used as oxidizing agents for all solutions. The authors indicated that wastewater gave slightly higher copper extraction than seawater, reaching almost 80%. However, it should be noted that the seawater solution had a chloride ion concentration of approximately half that of the wastewater.
Hernández et al. (2020a), using seawater and distilled water at Cl- concentrations of 20 and 40 g/L, and brine containing approximately 36 g/L Cl-, obtained the best copper extraction, approximately 95%, with seawater and distilled water at 20 g/L Cl-.
In the case of distilled water with 40 g/L Cl-, a lower extraction (< 80%) was obtained. These results are in contrast to that of Toro et al. (2020), who obtained better extractions at higher chloride concentrations. However, Lu, Jeffrey, and Lawson (2000) indicated that a chloride ion concentration greater than 17 g/L does not increase the kinetics of leaching. Nevertheless, it is important to have enough chloride ions in solution, because these ions promote the oxidation of copper (I) and iron (II) by dissolved oxygen. However, leaching depends on the operating conditions, because high chloride concentrations can extend the potential window to higher values (Velásquez-Yévenes, Miki, and Nicol, 2010).
Effect of temperature on leaching of copper sulfide ores using seawater
Aguirre et al. (2016) and Ruiz, Montes, and Padilla (2011) showed that copper extraction from sukphides increases with increasing temperature.
The leaching of copper sulphide minerals, especially chalcopyrite, requires approximately 75 to 85 kJ/mol activation energy (Munoz, Miller, and Wadsworth, 1979). By increasing the temperature the required activation energy is generated to increase the reaction kinetics (Rodriguez et al., 2020).
Hernández et al. (2020b) obtained a copper extraction of 90% from chalcopyrite at a maximum temperature of 65°C using seawater, and 40% extraction at 25°C (Hernandez et al. 2020a). These authors also showed that a lower temperature was required to achieve copper extraction close to 90%, a result that differed from that of Rodriguez et al. (2020). This is explained by Bogdanovic et al. (2020), who indicated that chalcopyrite leaching in a chloride medium requires a lower activation energy, approximately 42 kJ/ mol. Therefore, a lower temperature is required when leaching in chloride media to achieve high copper extractions.
Effect of pretreatment
It has been shown that agglomeration followed by curing improves the extraction of copper from sulphide ores (Hernandez et al., 2020a; 2019). The agglomeration process aids permeability of the heap, causing the fine and coarse particles to bind together. Agglomeration improves contact between the mineral and solution, thus achieving a better copper extraction (Quezada et al., 2020). A curing or resting step can make minerals easier to dissolve in the leaching stage, which increases leaching efficiency and decreases processing times. The curing period results in a homogeneous distribution of the acid, and also promotes the inhibition of aluminum silicate minerals (acid consumwes) (Dhawan et al., 2013; Quezada et al., 2018). Velásquez-Yévenes, Torres, and Toro (2018) leached agglomerated chalcopyrite ore with different concentrations of chloride. The resukte suggested that at least 20 kg/t of Cl- ions delivers higher extractions (approximately 20%) compared with no Cl- (approximately 10%). All the tests were performed in column leaching mode. In a more recent study by Quezada et al. (2021), agglomeration and curing were carried out with the addition of NaCl. They obtained 94% copper extraction at 90°C with a concentration of 50 g/l Cl-. The effect of pretreatment of copper sulphides with the addition of seawater has also been studied by Cerda et al. (2018), who used seawater to pretreat a chalcopyrite and bornite ore. The results showed 93% dissolution of copper using a concentration of 90 kg Cl-/t of mineral with 40 days curing at 50°C. In a study by Quezada et al. (2018), agglomeration and curing were carried out using seawater and discarded brine, with Cl- concentrations of 20 and 32 g/L respectively. A curing time of 50 days resulted in a copper extraction of 72% for the discarded brine, and 68% for the seawater. Hernández et al. (2020b) evaluated the effect of seawater as a source of chloride ions on acid curing. The procedure consisted of creating a mineral paste with NaCl (solid), NaNÜ3, H2SO4, and seawater, with a moisture content of 15%. The paste was allowed to stand for 3 days before leaching. A copper extraction of 60% was obtained, compared with 25% without the pretreatment step.
Seawater in the solvent extraction process
Acid leaching in solutions with seawater show better copper extraction kinetics; however, the presence of ions such as Cl-, Na+, Mg2+, K+, and Ca2+ could affect the efficiency of subsequent processes such as SX. Mahmoudi et al. (2020) conducted SX testwork with seawater, using LIX 984N and Acorga M5774 as extractants. Between pH values of 0.5 and 1.5, LIX 984N achieved a greater extraction of copper from solutions that contained at least 20 g/L chloride ion. However, at pH values exceeded 1.5, a higher extraction was obtained from chloride-free solutions. This effect was also observed with Acorga M5774, with the difference being that better extractions from chloride-free solutions ere obtained above pH 1.7. This difference in behaviour can be attributed to the interaction between chloride ions and copper ions, which form CuCl+ and CuCl2 complexes (Puigdomenech and Taxén, 2000). However, for both extractants, when maximum copper extraction was reached, at pH 2.5, the effect of chloride ions was not significant.
To better understand the effect of pH in SX in chloride-containing solutions, Shakibania et al. (2020) used LIX 984 at different chloride concentrations. The authors reported that copper extraction at pH < 2 was higher at concentrations of at least 20 g/L of chloride ions. Furthermore, in the range from 0-40 g/L chloride, the predominant species were Cu2+, CuCl+ and CuCl3. When the pH was increased to above 2, there did not appear to be a significant difference in copper extraction capacity between systems with and without chloride. Two probable reactions governing copper extraction in chloride systems were proposed where HRorg represents the organic solvent molecules:
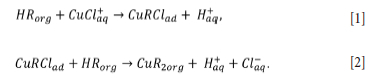
It has also been suggested that chloride ions can have an adverse influence on copper SX when low extractant concentrations are used, because a lower concentration gradient is generated between the aqueous and organic phases. In addition, the use of salt water in SX systems requires in changes in the circuit configuration, because an additional washing stage may be required to avoid excessive transfer of chloride and iron to the electrolyte (Shakibania et al., 2020).
Effect of chloride on copper electrowinning
EW of copper using seawater has not been studied in depth, but the effect of chloride on the process is known. The electrolyte used in electrowinning typically contains 0.01-0.06 g/L chloride ions.
Chloride adversely affects the EW process. When using stainless-steel cathodes, the chloride concentration must be kept below 0.030 g/L to avoid pitting corrosion, therefore a washing stage is incorporated in the upstream SX process (Schlesinger et al., 2021). Coalescers are used in the presence of high concentrations of chloride in the advance electrolyte, which reduce aqueous carry-over in the organic phase to the SX strip circuit. However, Aragón and Camus (2011) found that better quality copper cathode was obtained in the presence of chloride ions, because chloride reduces the grain size and improves the mechanical properties of the cathode. This is corroborated by a more recent study (Nkuna and Popoola, 2019), which indicates that a chloride ion concentration of 0.02 g/L gave a smoother and more compact cathode. However, nodules and irregularities formed on the cathode at a concentration above 0.025 g/L.
Conclusions
Today, seawater contributes approximately 25% of the main water requirements in the Chilean mining industry. The shortage of fresh water has motivated the mining sector to face the important challenge of looking for new ways and tools to supply its processes. The use of seawater is presented as a viable way to address water scarcity and reduce the use of continental waters.
For hydrometallurgical processes used for oxidized copper ores, seawater can be either beneficial or detrimental. Leaching kinetics are better with chloride, but in general, these ions do not significantly affect the process, as indicated by the successfuk leaching of chrysocolla and atacamite. The use of seawater for leaching copper sulphide minerals, such as chalcopyrite, is beneficial because it provides 20 g/L of chloride. However, the leaching of copper sulphides is, as yet, not economically viable on an industrial scale.
In the SX process, chloride can increase copper extraction at pH values below 2 using LIX 984N and Acorga M5774 at 15% v/v. However, this effect does not occur at pH > 2.5. It should also be borne in mind that with a greater amount of salts in the system an increased number of washing stages may be required to avoid excessive transfer of chloride to the electrolyte.
The chloride concentration in the advance electrolyte must be carefully controlled in copper electrowinning, because pitting corrosion of the stainless-steel cathode ocures at chloride concentrations above 0.03 g/L.
References
Aguirre, C.L., Toro, N., Carvajal, N., Watling, H., and Agüirre, C. 2016. Leaching of chalcopyrite (CuFeS2) with an imidazolium-based ionic liquid in the presence of chloride. Minerals Engineering, vol. 99. pp. 60-66. doi.org/10.1016/j.mineng.2016.09.016 [ Links ]
Alvez, a., Aitken, D., Rivera, D., Vergara, M., McIntyre, N., and Concha, F. 2020. At the crossroads: Can desalination be a suitable public policy solution to address water scarcity in Chile's mining zones? Journal of Environmental Management, vol. 258. p. 110039. doi.org/10.1016/j.jenvman.2019.110039 [ Links ]
Anil, M., Reddy, D., Medidi, R., and Genanü, M. 2016. Special properties of water and sea water. International Journal of Science and Research, vol. 3, no. 40. pp. 1-22. [ Links ]
Antony, P.J., Raman, R.K.S., Raman, r., and Kumar, P. 2010. Role of microstructure on corrosion of duplex stainless steel in presence of bacterial activity. Corrosion Science, vol. 52, no. 4. pp. 1404-1412. doi.org/10.1016/j.corsci.2009.12.003 [ Links ]
Aragón M.J. and Camus A.J. 2011. Efecto de la concentracion de ion cloruro en la estructura de electrodepósitos de cobre. Revista Latinoamericana de Metalurgia y Materiales, vol. 31, no. 2. pp. 128-133. [ Links ]
Arellano-Escudero, N. 2015. La ingeniería y el descarte artefactual de la desalación solar de agua. Las industrias de Las Salinas, Sierra Gorda y Oficina Domeyko (1872-1907). PhD thesis, Universitat Politécnica de Catalunya, Spain. [ Links ]
Balasubramanian, A. 2011. Properties of seawater. 11 pp. https://www.researchgate.net/publication/309785723_Properties_of_Seawater-Documentary?channel=doi&linkId=582363e208ae7ea5be71fa4b&showFulltext=true [accessed 19 May 2022]. [ Links ]
Beiza, L., Quezada, v., Melo, E., and Valenzuela, G. 2019. Electrochemical behaviour of chalcopyrite in chloride solutions. Metals, vol. 9, no. 1. pp. 1-12. doi.org/10.3390/met9010067 [ Links ]
Bogdanovic, G.D., Petrovic, S., Sokic, M., and Antonijevic, M.M. 2020. Chalcopyrite leaching in acid media: A review. Metallurgical and Materials Engineering, vol. 26, no. 2. pp. 177-198. doi.org/10.30544/526 [ Links ]
Carrasco, C. and Vega, P. 2011. Una aproximación a las condiciones de trabajo en la gran minería de altura. Cuaderno de investigación N°40. https://www.dt.gob.cl/portal/1629/w3-article-100032.html [accessed 10 January 2022]. [ Links ]
Carrión, M. 2020. ¿Cuánta agua hay en el planeta? El Ágora Report, March 20, 2020. https://www.elagoradiario.com/agorapedia/cuanta-agua-planeta/ [ Links ]
Cerda, C.P., Taboada, M.E., Jamett, N.E., Ghorbani, Y., and Hernandez, P. C. 2018. Effect of pretreatment on leaching primary copper sulfide in acid-chloride media. Minerals, vol. 8, no. 1. pp. 1-14. doi.org/10.3390/min8010001 [ Links ]
Chang, Daejun; Han, Sang Heon; Yang, and Kyung-won. 2012. Modeling of non-isothermal CO2 particle leaked from pressurized source: I. Behavior of single bubble. Ocean Systems Engineering, vol. 2. pp. 17-31. doi.org/10.12989/ose.2012.2.1.017 [ Links ]
Cisternas, L. and Moreno, L. 2014. El agua de mar en la minería: Fundamentos y Aplicaciones. RIL editores. [ Links ]
Cochilco. 2016. Proyección de la producción de cobre en Chile 2016 - 2027. Dirección de Estudios y Políticas Públicas. Comisión Chilena del Cobre, Santiago, Chile. pp. 1-32. [ Links ]
Cochilco. 2017. Sulfuros primarios : desafíos y oportunidades. Dirección de Estudios y Políticas Públicas. Comisión Chilena del Cobre, Santiago, Chile. pp. 1-40. [ Links ]
Cochilco. 2019. Proyección de consumo de agua en la minería del cobre 20192030. Dirección de Estudios y Políticas Públicas. Comisión Chilena del Cobre, Santiago, Chile. pp. 1-32. [ Links ]
Cochilco. 2020a. Consumo de agua en la minería del cobre al 2019. Dirección de Estudios y Políticas Públicas. Comisión Chilena del Cobre, Santiago, Chile. pp. 1-52. [ Links ]
Cochilco. 2020b. Proyección de consumo de agua en la minería del cobre 20202031. Dirección de Estudios y Políticas Públicas. Comisión Chilena del Cobre, Santiago, Chile. pp. 1-36. [ Links ]
Córdoba, E.M., Munoz, J.A., Blázquez, M.L., Gonzalez, F., and Ballester, A. 2008. Leaching of chalcopyrite with ferric ion. Part IV: The role of redox potential in the presence of mesophilic and thermophilic bacteria. Hydrometallurgy, vol. 93, no. 3-4. pp. 106-115. doi.org/10.1016/j.hydromet.2007.11.005 [ Links ]
Deng, J., Wen, S., Yin, Q., Wu, D., and Sun, Q. 2017. Leaching of malachite using 5-sulfosalicylic acid. Journal of the Taiwan Institute of Chemical Engineers, vol. 71. pp. 20-27. doi.org/10.1016/j.jtice.2016.11.013 [ Links ]
Dhawan, N., Safarzadeh, M.S., Miller, J.D., Moats, M.S., and Rajamani, R.K. 2013. Crushed ore agglomeration and its control for heap leach operations. Minerals Engineering, vol. 41. pp. 53-70. doi.org/10.1016/j.mieng.2012.08.013 [ Links ]
EDITEC. 2015. Catastro de plantas desalinizadoras y sistemas de impulsión de agua de mar. Compendio de La Minería Chilena. Santiago, Chile. [ Links ]
FAO. 2015. AQUASTAT Perfil de País - Chile. Organización de Las Naciones Unidas Para La Alimentación y La Agricultura. Rome, Italy. [ Links ]
FLÒREZ, D.A. and Bernabé Calle, B.V. 2015. El agua de mar en la alimentación y en la terapéutica. Boletin Sociedad Espanola Hidrologia Medica, vol. 30, no. 1. pp. 37-55. doi.org/10.23853/bsehm.2017.0378 [ Links ]
GÁLVEZ, E.D., and Cisternas, L.A. 2017. Innovative solutions for seawater use in mining operations. Case Study of Innovative Projects - Successful Real Cases. doi.org/10.5772/intechopen.68191 [ Links ]
García, E., Urüchürtü, J., and Genescá, J. 1995. Efecto de los componentes del agua de mar durante el fenómeno de corrosión por picaduras del cobre. Revista de Metalurgia, vo. 31, no. 5. pp. 307-313. doi.org/10.3989/revmetalm.1995.v31.i5.946 [ Links ]
García, J.L. and Osses, P. 2017. Investigaciones en el desierto de Atacama Centro UC Desierto de Atacama - Estación Atacama UC, Oasis de Niebla Alto Patache. Revista de Geografia Norte Grande. pp. 5-10. doi.org/10.4067/S0718-34022017000300005 [ Links ]
García, N. 2017. Costo econômico del uso de agua desalada en la minería chilena. Biblioteca Del Congreso Nacional de Chile. pp. 1-4. Santiago de Chile, Chile. [ Links ]
Garita, L., Rivolta, L., and Vega, M. 2013. Evaluación de la corrosion por picadura en aleaciones de aluminio. Ingeniería, Revista de La Universidad de Costa Rica, vol. 23, no. 1. pp. 13-25. doi.org/10.15517/ring.v23i1.11690 [ Links ]
Gonzalez, Y., Ayala, L., Escobar, C., Hernandez, P., Sepulveda, R., and Toro, N. 2020. Chalcopyrite leaching with ionic liquid based on idimazolium, chloride and pyrite in an oxygenated medium. AIP Conference Proceedings, vol. 2281. pp. 1-6. doi.org/10.1063/5.0026186 [ Links ]
Günson, A.J., Klein, B., Veiga, M., and Dunbar, S. 2012. Reducing mine water requirements. Journal of Cleaner Production, vol. 21, no.1. pp. 71-82. doi.org/10.1016/j.jclepro.2011.08.020 [ Links ]
Habbache, N., Alane, N., Djerad, S., and Tifouti, L. 2009. Leaching of copper oxide with different acid solutions. Chemical Engineering Journal, vol. 152, no. 2-3. pp. 503-508. doi.org/10.1016/j.cej.2009.05.020 [ Links ]
Hashemzadeh, M., Dixon, D.G., and Liu, W. 2019. Modelling the kinetics of chalcocite leaching in acidified cupric chloride media under fully controlled pH and potential. Hydrometallurgy, vol. 189. pp. 105-114. doi.org/10.1016/j.hydromet.2019.105114 [ Links ]
Hernandez, P.C., Taboada, M.E., Herreros, O.O., Torres, C. M., and Ghorbani, Y. 2015. Chalcopyrite dissolution using seawater-based acidic media in the presence of oxidants. Hydrometallurgy, vol. 157. pp. 325-332. doi.org/10.1016/j.hydromet.2015.09.007 [ Links ]
Hernandez, P., Dorador, A., Martínez, M., Toro, N., Castillo, J., and Ghorbani, Y. 2020. Use of seawater/brine and caliche's salts as clean and environmentally friendly sources of chloride and nitrate ions for chalcopyrite concentrate leaching. Minerals, vol. 10, no. 5. p. 477. doi.org/10.3390/min10050477 [ Links ]
Hernandez, P., Gahona, G., Martínez, M., Toro, N., and Castillo, J. 2020a. Caliche and seawater, sources of nitrate and chloride ions to chalcopyrite leaching in acid media. Metals, vol. 10, no. 4. p. 551. doi.org/10.3390/met1004055 [ Links ]
Hernandez, Pía C., Dupont, J., Herreros, O.O., Jimenez, Y.P., and Torres, CM. 2020b. Accelerating copper leaching from sulfide ores in acid-nitrate-chloride media using agglomeration and curing as pretreatment. Minerals, vol. 9, no. 4. pp. 1-13. doi.org/10.3390/MIN9040250 [ Links ]
Herrera-León, S., Lücay, F.A., Cisternas, L.A., and Kraslawski, A. 2019. Applying a multi-objective optimization approach in designing water supply systems for mining industries. The case of Chile. Journal of Cleaner Production, vol. 210. pp. 994-1004. doi.org/10.1016/j.jclepro.2018.11.081 [ Links ]
Herrera, S., Cisternas, L.A., and Gálvez, E.D. 2015. Simultaneous design of desalination plants and distribution water network. Computer Aided Chemical Engineering, vol. 37. pp. 1193-1198. doi.org/10.1016/B978-0-444-63577-8.50044-9 [ Links ]
Hirschmann, J. 1975. Solar distillation in Chile. Desalination, vol. 17. pp. 17-30. [ Links ]
Jiang, Q., Han, Y., Tang, W., Zhu, H., Gao, C., Chen, S., Willander, M., Cao, X., and Lin Wang, Z. 2015. Self-powered seawater desalination and electrolysis using flowing kinetic energy. Nano Energy, vol. 15. pp. 266-274. doi.org/10.1016/j.nanoen.2015.04.036 [ Links ]
Kadhim, A., Al-Amiery, A.A., Alazawi, R., Al-Ghezi, M.K.S., and Abass, R.H. 2021. Corrosion inhibitors. A review. International Journal of Corrosion and Scale Inhibition, vol. 10, no. 1. pp. 54-67. doi.org/10.17675/2305-6894-2021-10-1-3 [ Links ]
Kinnunen, P., Obenaus-Emler, R., Raatikainen, J., Guignot, S., Guimerà, J., Ciroth, A., and Heiskanen, K. 2021. Review of closed water loops with ore sorting and tailings valorisation for a more sustainable mining industry. Journal of Cleaner Production, vol. 278. p. 123237. doi.org/10.1016/j.jclepro.2020.123237 [ Links ]
Li, Y., Kawashima, N., Li, J., Chandra, A.P., and Gerson, A.R. 2013. A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite. Advances in Colloid and Interface Science, vol. 197. pp. 1-32. doi.org/10.1016/j.cis.2013.03.004 [ Links ]
Lu, J. and Dreisinger, D. 2013. Copper chloride leaching from chalcopyrite and bornite concentrates containing high levels of impurities and minor elements. Hydrometallurgy, vol. 138. pp. 40-47. doi.org/10.1016/j.hydromet.2013.06.001 [ Links ]
Lu, Z.Y., Jeffrey, M.I., and Lawson, F. 2000. Effect of chloride ions on the dissolution of chalcopyrite in acidic solutions. Hydrometallurgy, vol. 56, no. 2. pp. 189-202. doi.org/10.1016/S0304-386X(00)00075-X [ Links ]
MAAp, P. 2011. Corrosion and corrosion protection. Handbook of Hot Dip Galvanization. Maafi, P and Peifiker, P. (eds). Chapter 1. Wiley-VCH Berlin. doi.org/10.1002/9783527636884.ch1 [ Links ]
Mahmoudi, A., Shakibania, S., Rezaee, S., and Mokmeli, M. 2020. Effect of the chloride content of seawater on the copper solvent extraction using Acorga M5774 and LIX 984N extradants. Separation and Purification Technology, vol. 251. p. 117394. doi.org/10.1016/j.seppur.2020.117394 [ Links ]
Millero, F.J., Feistel, R., Wright, D.G., and McDougall, T.J. 2008. The composition of Standard Seawater and the definition of the Reference-Composition Salinity Scale. Deep-Sea Research Part I: Oceanographic Research Papers, vol. 55, no. 1. pp. 50-72. doi.org/10.1016/j.dsr.2007.10.001 [ Links ]
MOP. 2020. Informe de prensa DGA-MOP. Dirección General de Aguas, pp. 1-7. https://dga.mop.gob.cl/estudiospublicaciones/Documents/Informe_de_PrensaDGAMOP.pdf [ Links ]
Morales, F. 2017. Estudio del efecto de las interacciones del sistema "Agua de marcal" en procesamiento de minerales. Master thesis, Universidad de Chile, Chile. [ Links ]
Münoz, P.B., Miller, J.D., and Wadsworth, M.E. 1979. Reaction mechanism for the acid ferric sulfate leaching of chalcopyrite. Metallurgical Transactions B, vol. 10, no. 2. pp. 149-158. doi.org/10.1007/BF02652458 [ Links ]
Ncube, R. and Inambao, F. L. 2019. Sea water reverse osmosis desalination: Energy and economic analysis. International Journal of Mechanical Engineering and Technology (IJMET), vol. 10, no. 12. pp. 716-731. [ Links ]
Nicol, M.J. 2018. The kinetics of the dissolution of malachite in acid solutions. Hydrometallurgy, vol. 177. pp. 214-217. doi.org/10.1016/j.hydromet.2018.03.017 [ Links ]
Nkuna, E.H. and Popoola, A.P.I. 2019. Effect of chloride electrolyte additive on the quality of electrorefined copper cathode. Procedia Manufacturing, vol. 35. pp. 789-794. doi.org/10.1016/j.promfg.2019.06.024 [ Links ]
Nuorivaara, T., Bjõrkqvist, A., Bacher, J., and Serna-Guerrero, R. 2019. Environmental remediation of sulfidic tailings with froth flotation: Reducing the consumption of additional resources by optimization of conditioning parameters and water recycling. Journal of Environmental Management, vol. 236. pp. 125-133. doi.org/10.1016/j.jenvman.2019.01.107 [ Links ]
Olive, G. 2018. Desalted water production by a sea water solar desalter aqueduct : Experimental and numerical results. https://www.researchgate.net/profile/Gino-0live/publication/327369966_T0WARDS_LARGE-scale_sea_water_solar_desalination_the_sea_water_S0LAR_DESALTER_AQUEDUCT/links/5b8ac392299bf1d5a737e3b7/towards-large-scale-sea-water-solar-desalination-the-SEA-WATER-SOLAR-DESALTER-AQUEDUCT.pdf [ Links ]
Ordonez, J.I., Moreno, L., Gonzalez, J.F., and Cisternas, L.A. 2015. Use of discharged brine from reverse osmosis plant in heap leaching: Opportunity for caliche mining industry. Hydrometallurgy, vol. 155. pp. 61-68. doi.org/10.1016/j.hydromet.2015.04.008 [ Links ]
Palmer, D. 2012. Understanding galvanic corrosion. Design News, January 20, 2012 [ Links ]
Puigdomenech, I. and Taxén, C. 2000. Thermodynamic data for copper. Implications for the corrosion of copper under repository conditions. Report SKB-TR--00-13. SKB, Stockholm, Sweden. [ Links ]
Qiu, Z., Liu, G., Liu, Q., and Zhong, H. 2016. Understanding the roles of high salinity in inhibiting the molybdenite flotation. Colloids and Surfaces A: Physicochemical and Engineering Aspects, vol. 509. pp. 123-129. doi.org/10.1016/j.colsurfa.2016.08.059 [ Links ]
Qüezada, V., Velasquez, L., Roca, A., Benavente, O., Melo, E., and Keith, B. 2018. Effect of curing time on the dissolution of a secondary copper sulphide ore using alternative water resources. IOP Conference Series: Materials Science and Engineering, vol. 427, no. 1. doi.org/10.1088/1757-899X/427/1/012030 [ Links ]
Qüezada, V., Roca, A., Benavente, O., Cruells, M., Keith, B., and Melo, E. 2020. Effect of pretreatment prior to leaching on a chalcopyrite mineral in acid media using NaCl and KNO3. Journal of Materials Research and Technology, vol. 9, no. 5. pp. 10316-10324. doi.org/10.1016/j.jmrt.2020.07.055 [ Links ]
Qüezada, V., Roca, A., Benavente, 0., Cruells, M., and Melo, E. 2021. The effects of sulphuric acid and sodium chloride agglomeration and curing on chalcopyrite leaching. Metals, vol. 11, no. 6. p. 873 doi.org/10.3390/met11060873 [ Links ]
Rodriguez, M., Ayala, L., Robles, P., Sepúlveda, R., Torres, D., Carrillo-Pedroza, F. R., Jeldres, R. I., and Toro, N. 2020. Leaching chalcopyrite with an imidazolium-based ionic liquid and bromide. Metals, vol. 10, no. 2. pp. 1-12. doi.org/10.3390/met10020183 [ Links ]
Romero, H., Mendonça, M., and Catarina, D.S. 2012. Socioclimas y glocalización en el Desierto de Atacama. IX Seminário Latino-Americano e V Seminário Ibero-Americano de Geografia Física, Guimaraes, Portugal. pp. 1-12. [ Links ]
Ruiz, M.C., Montes, K.S., and Padilla, R. 2011. Chalcopyrite leaching in sulfate-chloride media at ambient pressure. Hydrometallurgy, vol. 109, no. 12. pp. 37-42. doi.org/10.1016/j.hydromet.2011.05.007 [ Links ]
San Martín, F., Kracht, W., Vargas, T., and Rudolph, M. 2020. Mechanisms of pyrite biodepression with Acidithiobacillus ferrooxidans in seawater flotation. Minerals Engineering, vol. 145. p. 106067. doi.org/10.1016/j.mineng.2019.106067 [ Links ]
Santoro, S., Estay, H., Avci, A.H., Pügliese, L., Rüby-Figueroa, R., Garcia, A., Aquino, M., Nasirov, S., Straface, S., and CuRCio, E. 2021. Membrane technology for a sustainable copper mining industry: The Chilean paradigm. Cleaner Engineering and Technology, vol. 2. p. 100091. doi.org/10.1016/j.clet.2021.100091 [ Links ]
Sarricolea, P., Ruiz, Ó.M., and Aravena, H.R. 2017. Tendencias de la precipitación en el norte grande de Chile y su relación con las proyecciones de cambio climático. Dialogo Andino, vol. 54. pp. 41-50. doi.org/10.4067/S0719-26812017000300041 [ Links ]
Schlesinger, M.E., Sole, K.C., Davenport, W.G., and Flores, G.R.A. 2021. Extractive Metallurgy of Copper: 6th edn. Elsevier. [ Links ]
Schorr, M., Valdez, B., Ocampo, J., and Eliezer, A. 2010. Corrosion control in the desalination industry. Desalination, Trends and Technologies, vol. 95. pp. 29-32. doi.org/10.4028/www.scientific.net/amr.95.29 [ Links ]
Senanayake, G. 2007. Review of theory and practice of measuring proton activity and pH in concentrated chloride solutions and application to oxide leaching. Minerals Engineering, vol. 20, no. 7. pp. 634-645. doi.org/10.1016/j.mineng.2007.01.002 [ Links ]
Shakibania, S., Mahmoudi, A., Mokmeli, M., and Rashchi, F. 2020. The effect of chloride ions on copper solvent extraction from sulfate-chloride medium using LIX 984N. Minerals Engineering, vol. 156. p. 106498. doi.org/10.1016/j.mineng.2020.106498 [ Links ]
SHOA. 2020. Temperatura superficial del mar (TSM). http://www.shoa.cl/php/tsm.php [accessed 25 February 2022] [ Links ]
Tenzer, R., Novak, P., and Gladkikh, V 2011. On the accuracy of the bathymetry-generated gravitational field quantities for a depth-dependent seawater density distribution. Studia Geophysica et Geodaetica, vol. 55, no. 4. pp. 609-626. doi.org/10.1007/s11200-010-0074-y [ Links ]
Toro, N., Pérez, K., Saldana, M., Jeldres, R.I., Jeldres, M., and Canovas, M. 2020. Dissolution of pure chalcopyrite with manganese nodules and waste water. Journal of Materials Research and Technology, vol. 9, no. 1. pp. 798-805. doi.org/10.1016/j.jmrt.2019.11.020 [ Links ]
Torres, C.M., Taboada, M.E., Graber, T.A., Herreros, 0.0., Ghorbani, Y., and Watling, H.R. 2015. The effect of seawater based media on copper dissolution from low-grade copper ore. Minerals Engineering, vol. 71. pp. 139-145. doi.org/10.1016/j.mineng.2014.11.008 [ Links ]
Torres, C.M., Ghorbani, Y., Hernandez, P.C., Jüstel, F.J., Aravena, M.I., and Herreros, 0.0. 2019. Cupric and chloride ions: Leaching of chalcopyrite concentrate with low chloride concentration media. Minerals, vol. 9. p. 639. doi.org/10.3390/min9100639 [ Links ]
Velasquez -Yévenes, L., Miki, H., and Nicol, M. 2010. The dissolution of chalcopyrite in chloride solutions: Part 2: Effect of various parameters on the rate. Hydrometallurgy, vol. 103, no. 1-4. pp. 80-85. doi.org/10.1016/j.hydromet.2010.03.004 [ Links ]
Velasquez -Yévenes, L., Torres, D., and Toro, N. 2018. Leaching of chalcopyrite ore agglomerated with high chloride concentration and high curing periods. Hydrometallurgy, vol. 181. pp. 215-220. doi.org/10.1016/j.hydromet.2018.10.004 [ Links ]
Velasquez -Yévenes, L., and Qüezada-Reyes, V. 2018. Influence of seawater and discard brine on the dissolution of copper ore and copper concentrate. Hydrometallurgy, vol. 180. pp. 88-95. doi.org/10.1016/j.hydromet.2018.07.009 [ Links ]
Velasquez -Yévenes, L., Malverde, S., and Qüezada, V. 2022. A Sustainable Bioleaching of a Low-Grade Chalcopyrite Ore. Minerals, vol. 12, no. 4. p. 487. doi.org/10.3390/min12040487 [ Links ]
 Correspondence:
Correspondence:
V. Quezada
Email: vquezada@ucn.cl
Received: 9 Nov. 2022
Revised: 19 Jul. 2023
Accepted: 9 Aug. 2023
Published: July 2023














