Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.123 n.7 Johannesburg Jul. 2023
http://dx.doi.org/10.17159/2411-9717/2906/2023
COPPER COBALT EDITION
Copper solvent extraction on the African Copperbelt: From historic origins to world-leading status
O.S. TinklerI; K.C. SoleII
ISolvay, Phoenix, Arizona, USA. ORCID: O.S. Tinkler: http://orcid.org/0009-0004-4367-2234
IISole Consulting, Johannesburg, and University of Pretoria, Pretoria, South Africa. ORCID: K.C. Sole: http://orcid.org/0000-0003-4707-1060
SYNOPSIS
Approximately 20% of current world copper cathode output is produced using a hydrometallurgical process route, generally referred to as the leach-solvent extraction-electrowinning flowsheet. Since its commercialization in the late 1960s, steady improvements in the performance and efficiency of the solvent-extraction reagents and equipment, combined with significant developments in leaching and electrowinning, have made an ever-widening range of ore types amenable to this technology. Following successful implementation on all major continents, a large proportion of growth in recent years derives from the re-emergence of copper solvent extraction in the Central African Copperbelt. This review provides a brief history of the development and evolution of copper solvent extractants and mixer-settler contactors, and the significance of the Copperbelt region in achieving commercialization and acceptance of the technology. The opportunities and challenges presented by the abundant high-grade oxide ores of the Copperbelt are contrasted with the processing of solutions derived from the low-grade mixed oxide-sulfide ore bodies that are prevalent in other geological regions. The current status of hydrometallurgical copper production in the African Copperbelt, within a global context, and a medium-term outlook for the technology are discussed.
Keywords: copper, solvent extraction, review, history, African Copperbelt, Zambia, Democratic Republic of Congo.
Introduction
Prior to the development and commercialization of solvent extraction (SX) as a hydrometallurgical unit operation, copper was recovered from dilute acid leach solutions either by cementation using scrap iron or by direct electrowinning (EW). The leach-cementation process (Equations [1] and [2]) requires large amounts of acid. Other shortcomings include limited markets for the low-quality copper product and the relatively high cost of scrap iron.
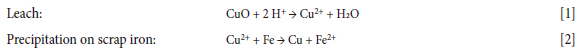
As an improvement to this process, recovery of copper by direct EW (Equation [3]) from an impure pregnant leach solution (PLS) has the advantage that acid generated in the spent electrolyte in the EW step can be recycled back to the leach.
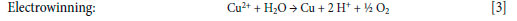
The downside is that, even if the copper tenor is high (> 40 g/L), impurities, such as Mn and Fe, build up in the PLS with each leach cycle, which reduces current efficiency and copper cathode quality. To control these elements, a portion of the copper-stripped solution (spent electrolyte) has to be bled out of the process, making the process less economical and introducing potential environmental risks.
The development of copper SX in the mid-1960s changed everything. Direct production of London Metal Exchange (LME) Grade A/AA copper cathode became possible, and the hydrometallurgical flowsheet now known as leach-SX-EW steadily became the default process for copper recovery from oxide ore bodies and existing stockpiles (Schlesinger et al., 2021). At the heart of the process lie the copper-selective extraction reagents that make it all possible.
Development and commercialization of copper solvent-extraction reagents
By the late 1950s, the new technology of liquid-liquid extraction or SX had been commercialized for uranium recovery at Buffelsfontein in South Africa (Sole et al., 2011) using tertiary amine extractants developed by General Mills1. General Mills had also developed amine systems for extraction of V, Mo, and W from alkaline systems, so there was interest in extending this new technology to other metals. Joe House, who later rose to the position of Vice President of General Mills Chemicals, can be regarded as the 'father' of copper SX. General Mills had the foresight and generous policy of allowing its scientists to work one day per week on anything that they were interested in (so-called 'bootleg projects'), and so it was that Joe House (Figure 1), together with colleagues Don Agers and Ronald Swanson, took on the task of developing an extractant that would be able to recover copper from leach liquors generated from the leaching of low-grade oxide ores and that could not be profitably treated using existing technologies (House, 1985; House, 1989).

The first reagent was LIX 63, an aliphatic hydroxyoxime (Figure 2a), so-called because it was developed in 1963, where LIX stood for liquid ion-exchanger2. Although this extractant was very selective for Cu, it only operated above pH 5, so was unsuitable for treating acidic leach liquors, which typically have 0.7 < pH < 2.2. KELEX 100 (Figure 2b), an 8-hydroxyquinoline structure developed by Shering Berlin, extracted copper below pH 2, but co-extracted considerable amounts of acid. In 1965, LIX 65 became available (Figure 2c): incorporation of aromatic rings into the oxime structure provided an electron-withdrawing effect that allowed copper to be extracted at lower pH values. This reagent operated at about pH 2.5, but it occurred in two isomeric structures: only the anti-isomer could form a complex with copper. However, when used in a continuous extraction-stripping process, complexation with Cu would isomerize all the syn-isomers to the desired anti configuration. Shortening the alkyl sidechain from C12 to C9 (LIX 65N; N for nonyl) (Figure 2d) improved the kinetics. LIX 64 was the first extractant to be commercially employed, comprising a mixture of LIX 65 (40% by mass) with LIX 63 (2%), which acted as a catalyst for copper loading.
Several different blends and other novel copper extractants were tested, but it was the breakthroughs of the C9 ketoxime and C9 aldoxime that ultimately formed the basis of almost all modern extractants. The C9 ketoxime was invented by Shell and marketed as SME 529 (2-hydroxy-5-nonyl-acetophenone oxime (Figure 2(e)) (van der Zeeuw, 1972). The SME 529 technology was purchased by Henkel in 1984 (Kordosky, 2002) and its properties were greatly improved using a new manufacturing process: the resulting product was marketed as LIX 84I. The C9 aldoxime was ACORGA's P-1 (5-nonyl salicylaldoxime (Figure 2f), later known as P-503. A C12 variant of ACORGA P-1 was later developed by Henkel following successful commercialization of C9 aldoxime-based formulations.
Today's copper SX extractants include blends of C9 ketoxime with C9 aldoxime (e.g., LIX 984N); blends of C9 aldoxime with an equilibrium modifier (e.g., ACORGA M5774), and blends of C9 ketoxime, C9 aldoxime, and an equilibrium modifier (e.g., ACORGA OPT5540). Interestingly, the first generation of copper extractants cost around US$ 5.80 per kilogram in 1972 (Price and Tumilty, 1972), which, adjusted for inflation, is about US$ 42 per kilogram today: for context, modern extractants are priced from US$ 15 per kilogram.
The first copper leach-SX-EW operation
If Joe House is regarded as the father of copper SX, every newborn needs a midwife to assist its passage to an independent life: Maxie Anderson can then be regarded as the 'doula' of copper SX. Max Leroy Anderson (Figure 3) was an extremely wealthy, larger-than-life character, who is more well known for making the first crossing of the Atlantic Ocean and North America by hot-air balloon, for which he was awarded the US Congressional Gold Medal (Wikipedia, n.d.). In addition to a wine farm, Maxie Anderson owned a small copper mine in Arizona, which was producing low-grade solution by heap leaching with copper recovery by iron cementation. Not having to account to a board of directors-and always up for a challenge, he took the risky step of introducing a radically different technology into an industry in which the basic methods of making copper had not changed significantly since the Bronze Age (Monhemius, 2014), installing the first commercial copper SX-EW plant at Ranchers Exploration and Development Corporation's Bluebird Mine in 1968, using LIX 64 as the extractant4. Ken Power, a strong proponent of liquid-liquid extraction of copper, was hired as Rancher's General Manager, and the first solution was run through the plant in March 1968. Design production of 30 000 pounds per day (13.6 t/d or 4500 t/a) was reached a few months later (miningfoundationsw.org). The SX plant had three extraction stages and two strip stages, and processed a leach solution containing approximately 1 g/L Cu (Flett, 1974). The plant was originally planned to have a eleven-year life, but, owing to the lower-than-expected cost and better-than-expected product quality, continued to operate until 1982, producing in total almost 80 000 t Cu by SX-EW (Anon., n.d.).

Joe House was elected to the US National Academy of Engineers in 1997 for 'developing and applying solvent-extraction processes for copper recovery from low-grade ores.' Tragically, Maxie Anderson was killed at age 49 while ballooning in the Alps, but not before he was inducted into the US National Mining Hall of Fame.
The African Copperbelt and the rise of copper leach-solvent extraction-electrowinning
After commercialization of leach-solvent extraction-electrowinning (L-SX-EW) at Bluebird, several new acid-leach SX-EW operations were built in quick succession, notably at Bagdad Mining Company in Arizona in 1970, at Nchanga Consolidated Copper Mines Tailings Leach Plant (TLP) in Chingola, Zambia, in 1973 (Holmes and Fisher, 1972; Holmes et al., 1976), and at Anaconda Co. Twin Buttes in Arizona in 1975 (Flett, 1974). Steady adoption continued through the 1970s and began to accelerate in the 1980s with successful implementation at Sociedad Minera Pudahuel's Lo Aguirre plant in Chile (Lynch et al., 1995), at Miami and ASARCO Ray in Arizona, and at several Phelps Dodge properties in the USA (Dresher, 2001). Through the 1990s, implementation dominated in Chile, where production grew more than tenfold from 1991 to 2001 (Bartos, 2002).
Wide adoption of L-SX-EW in Central Africa could easily have pre-dated that in Chile, but for political upheaval in the region in the 1970s (Declercq, 2022). Tenke Fungurume (TFM) epitomises early potential challenges and rewards of operating in this region. One of the world's largest and richest known copper reserves, flowsheet development started in the 1970s following demonstration of SX-EW technology at Rancher's Bluebird Mine. In 1974, La Société Minière de Tenke Fungurume (SMTF) awarded the contract to General Mills to supply LIX 65N as the extractant. In 1976, however, after SMTF had invested more than US$ 240 million in the project and General Mills had produced more than 50% of the first-fill extractant requirement, civil war broke out in (then) Zaire and the entire project was abandoned. General Mills eventually sold the extractant stock to the Nchanga TLP project. In 2009, Freeport McMoRan Copper and Gold reopened the Tenke plant and-33 years later-General Mills' successor, Cognis, eventually supplied LIX 984N for the first fill of the new SX-EW plant (Cognis, 2008).
After a troubled 40-year period from the early 1970s to the early 2000s, peace finally came to the Democratic Republic of Congo (DRC). The first L-SX-EW operations were not far behind: the first copper cathode was produced in 2008 at Metorex's Ruashi plant (Metorex, 2008) and Chemaf's Usoki plant (Shalina Resources, n.d.). As critical road, power, and water infrastructure steadily improved, new operations began to spring up around the towns of Lubumbashi, Likasi, and Kolwezi: the DRC's growth spurt had finally begun. In Zambia, SX-EW adoption was re-ignited after privatization of Zambia Consolidated Copper Mines (ZCCM) in 1996, peaking at 260 kt in 2008 (Solvay records), although total Zambian copper output (with concentrate and smelter production) reached 765 kt in 2012 (Sikamo et al., 2015).
As SX equipment design advanced and increasingly more efficient extractants were developed, operations became larger and more cost effective. An excellent illustration of this is a comparison between the Nchanga TLP plant, commissioned in 1973 (Figure 4), First Quantum Minerals' Kansanshi SX3 Plant, commissioned in 2004 (Figure 5), and TFM Phase I, commissioned in 2009. As shown in Table I, modern SX settlers are wider and shorter than early designs to accommodate higher flows, which results in higher settler flux but reduced organic velocity.
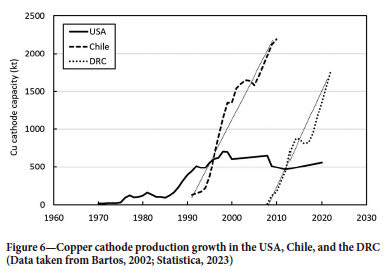
A comparison of the US, Chile, and DRC cathode growth periods (Figure 6) shows just how rapidly a mature technology can be implemented, under the right conditions. L-SX-EW technology was well established in the USA before adoption began to accelerate in Chile in the early 1990s as the full economic benefits over the traditional cementation process became apparent (Bartos, 2002). The growth rate in the DRC over the last 15 years closely mirrors that of Chile in the 1990s. SX-EW production of copper cathode in the DRC has recently surpassed that of Chile, where production has declined over the last decade as oxide ores have been depleted. Chilean copper cathode production in 2022 was just 1.5 Mt (Chilean Copper Commission, 2018), compared with 1.7 Mt in the DRC for the same period (Anon., 2023).
Leach-solvent extraction-electrowinning process overview
A high-level L-SX-EW flowsheet is shown in Figure 7. Extraction of Cu(II) ions from the PLS generates acid in the SX raffinate, which is recycled back to the leach to dissolve more copper; the stripping reaction consumes acid, which is provided by recycle of the spent electrolyte from EW to the SX circuit as the strip liquor. This flowsheet represents an almost-perfect closed-loop hydrometallurgical process: essentially acid generated by the decomposition of water is used as the lixiviant for copper. The only additional acid required is for the co-leaching of non-copper minerals.
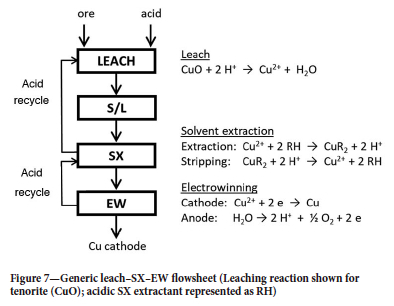
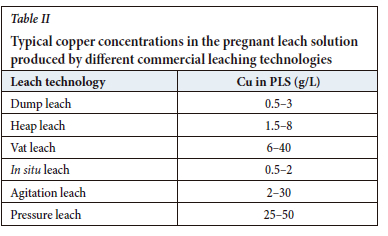
The variety of leach technologies employed today produce PLS with wide ranges of copper concentration, pH, and impurity content, as illustrated in Table II. To ensure consistent production of LME Grade A/AA copper cathode, copper EW has stringent specifications for the advance electrolyte: 45-50 g/L Cu, approx. 150 g/L H2SO4, < 2 g/L Fe, < 0.1 g/L Mn, and approx. 30 mg/L Cl. The versatility of modern copper extractants is such that the input stream to SX can vary widely in composition, but a consistent output stream, suitable for production of LME Grade A cathode, is nevertheless generated.
Flexibility and global characteristics of leach-solvent extraction-electrowinning process
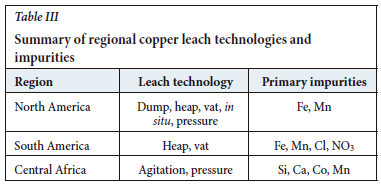
To appreciate the remarkable versatility of the L-SX-EW process, it is useful to examine a range of regional characteristics in the main centres where the technology is practised. These include mineralogy, leach technologies, impurities, and climate. A summary is shown in Table III.
North America
North American production is concentrated in the US states of Arizona and New Mexico, and the Northern Mexico state of Sonora. The mines are located in dry, arid areas with summer temperatures as high as 45°C. This is the most mature region for this technology, so the higher-grade oxides ores have long been depleted: most operations now leach low-grade oxide along with low-grade secondary and primary sulfides. The sites are all heap and/or dump leach operations that utilize permanent heaps, rather than the on-off leach pads that are more typical in South America. A thin-layer acid cure is commonly employed to increase leach recovery. Several in-situ leach operations are also under development in Arizona (Seaman et al., 2019). The PLS are typified by low Cu (0.3-3 g/L), high Fe (3-15 g/L), high Mn (1-3 g/L), pH in the range of 1.0-2.5, and temperatures in the range of 5-35°C. Pressure leaching of copper concentrates is commercially practised at Morenci in the USA (Marsden et al., 2007a; Marsden et al., 2007b). The pressure leach PLS contains high levels of both copper and acid (Table II) that are diluted by blending with the heap leach PLS before processing by SX-EW (Green et al., 2018).
Most SX circuits employ a series-parallel flow configuration to allow for high PLS throughput to maintain copper production from the low-tenor feed. The raffinate stages may operate in aqueous-continuous mixing mode and there is emphasis on organic recovery, such as by increased retention time in the raffinate pond and use of equipment such as pace setters, Jameson cells, and pond skimmers. Typical extractant consumption ranges from 2-5 kg/t cathode.
South America
South American production is concentrated in Chile and Peru. The South American operations mainly process oxide deposits with an acid-soluble copper grade ranging from 0.4-0.8% by heap leaching. Many are located in the Atacama Desert, where atacamite (Cu2Cl(OH)3) is a common regional mineral, the leaching of which results in elevated levels (1-30 g/L) of chloride in the PLS. Owing to a lack of fresh water, as well as benefits in leaching, several operations now leach in sea water (approx. 20 g/L Cl) and there is a trend towards use of much higher salinity-as high as > 100 g/L Cl, as exemplified by Michilla and Zaldívar that use Cuprochlor-T technology. Chloride is particularly detrimental to copper EW, causing pitting corrosion of the cathode blanks, so one or more wash stages are used, in conjunction with coalescers, to control chloride transfer to the electrolyte.
Several operations also experience challenges with nitration of the extractant associated with high levels of nitrates dissolved in the PLS. This prompted the development of nitration-resistant reagents. These incorporate a more easily oxidized sacrificial component to reduce attack on the main extractant functionality (Virnig et al., 2003; Yanez et al., 2009). Mantos de la Luna and Pampa Camarones, for example, both contain close to 100 g/L chloride in addition to nitrates, and use ACORGA NR reagents to mitigate the detrimental effects.
Most mines are approaching the end of their oxide lifetime and the SX plants therefore operate at or below design flowrates using a conventional series configuration, such as 2E-2S or 2E-1S. The PLS have low levels of suspended solids owing to being 'filtered' through the heap, so extractant consumption ranges from 1-4 kg/t cathode.
Central Africa
The African Copperbelt is situated in the Lualaba and Haut-Katanga provinces of the DRC and the Copperbelt and NorthWestern provinces of Zambia. Copper ores are significantly richer than in other parts of the world, with grades of 1.5%-5% acid-soluble Cu. The main oxide resources are hosted in malachite (CuCO3-Cu(OH)2), azurite (Cu3(CO3)2(OH)2), and chrysocolla ((Cu,Al)2H2Si2O5(OH)4-nH2O). Cobalt, mainly present as heterogenite (Co3+O(OH)) at grades of 0.2%-0.4% Co, offers a byproduct credit. The high-grade ores and intense wet season (> 1000 mm of rain mainly occurring over a five-month period) favour agitated leaching over heap leaching. Agitated leaching is carried out in stirred tanks at 30-40°C, so the PLS contains high levels of suspended solids (100-250 mg/L). The high silica content of these ores contributes to significant crud formation and a range of other adverse physical effects throughout the hydrometallurgical circuit (Alexander et al., 2018; Kashala et al., 2018; Sole et al., 2018). The PLS is also often characterized by calcium saturation, owing to hosting of the valuable minerals in high-acid-consuming dolomite and limestone deposits.
Extractant consumption in this region has reduced significantly over the years as processing techniques and operational expertise have improved; while once 5-7 kg/t of cathode was common, these days most operations fall in the range of 2-4 kg/t (Sole et al., 2022). Several sites employ a 'split circuit' configuration, which produces a high-grade and a low-grade PLS, and has been shown to reduce overall acid consumption (Nisbett et al., 2009). Pressure leaching of copper concentrates is practised at First Quantum Minerals' Kansanshi operation in Zambia: as at Morenci, the PLS generated is diluted by blending with the leach PLS before processing by SX-EW.
The African Copperbelt Growth Story
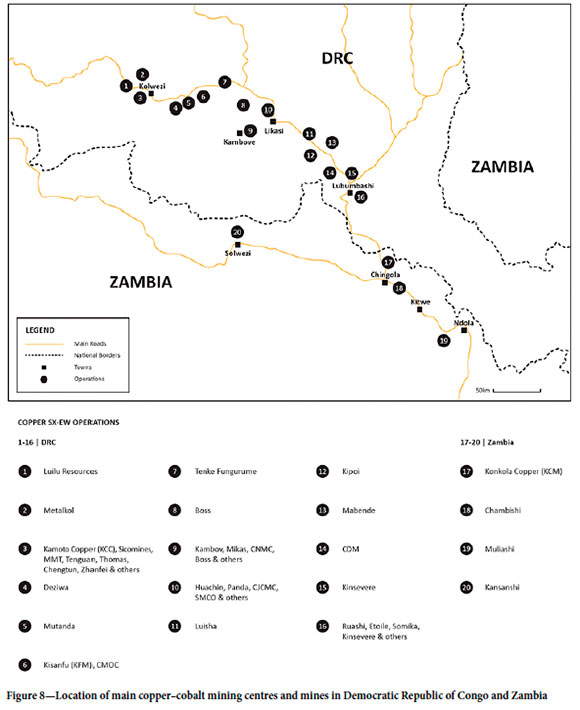
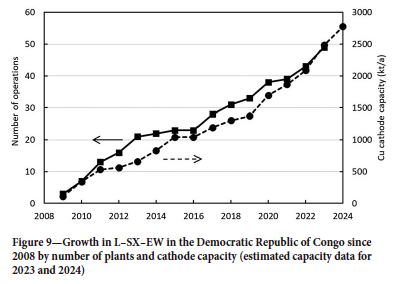
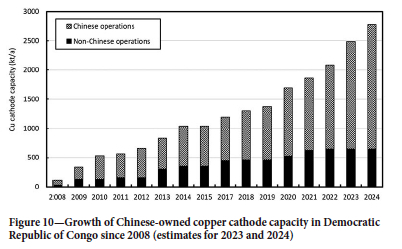
The main centres on the African Copperbelt are shown in Figure 8. Most L-SX-EW operations are in the DRC, where there are now nearly 50 production sites, of which 20 produce more than 40 kt/a Cu as cathode. The remarkable growth in DRC output is illustrated in Figure 9, from almost zero in 2008 to 1.77 Mt/a in 2022, wth an anticipated output of over 2.50 Mt/a by 2024/25. It is significant that some 80% of this production is from Chinese-owned operations (Figure 10).
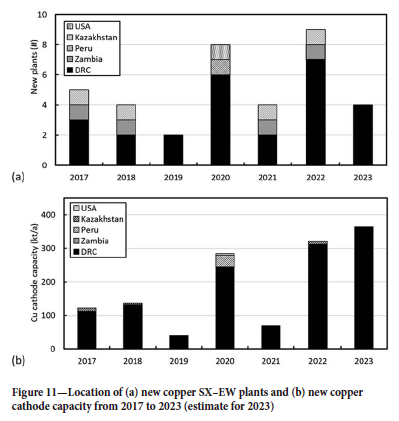
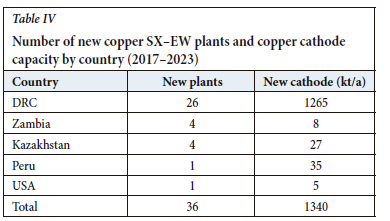
As further evidence of the enormous growth in this region, Figure 11 and Table IV depict the location and capacity of new L-SX-EW plants commissioned since 2017 and expected to come online within the next two years. These data show that more than 70% of all global recent new projects are in the DRC, accounting for more than 90% of new copper cathode capacity. Despite the well-documented logistics and power challenges-and the ever-present political uncertainly and instability-the enormous resource potential of this region is outweighing these considerations for those companies with an appetite for risk and the incentive to meet the burgeoning demand for the red metal.
Future directions
Hydrometallurgical processing of copper by SX-EW was originally developed for the treatment of solutions produced by heap leaching of low-grade oxide materials. As demonstrated, the remarkable versatility of modern SX process chemistry has enabled a much wider variety of PLS characteristics to be treated. Oxides are, however, surface resources and have a finite mine lifetime. As these easily accessible minerals are depleted and mining becomes deeper, the necessity arises to treat mixed ore containing low-grade oxides together with low-grade secondary and primary sulfides, which are progressively more refractory towards acid leaching.
Several new leaching technologies have been in development in recent years to exploit the vast tonnages of copper tied up in low-grade sulfide ore bodies (Baxter, 2016; Steiper, 2018, Voigt et al., 2019, for example). Jetti Resources's catalyst technology has been deployed in the USA for several years and the first South American trial will begin in mid-2023 (Jetti Resources, 2023). Rio Tinto's Nuton comprises a portfolio of proprietary copper leach-related technologies and capability that offer the potential to economically unlock copper sulfide resources, copper-bearing waste, and tailings, and achieve higher copper recoveries from oxide and transitional material, allowing for significantly increased copper production (Rio Tinto, 2023).
In Chile, many large operations are moving towards addition of chloride to the heap leach operations to enable sulfide processing under ambient conditions, particularly for chalcopyrite: concentrations as high as 100 g/L Cl are employed. The main issue with treating this tuype of PLS is the risk of chloride transfer to the electrolyte. A focus on engineering developments, particularly with respect to SX wash stages, efficiency of removal of entrained aqueous phase in the loaded organic, and corrosion control, is expected.
In the DRC, with the tremendous growth in L-SX-EW, depletion of high-grade oxide ore is well under way. To maximize the use of existing SX-EW capacity, several projects are considering roasting of sulfide concentrates. Sulfides can be converted to oxides by roasting at approximately 800°C. The resulting calcine can then be fed to the oxide processing circuit. This technology also enables SO2 off-gases to be converted to sulfuric acid for use in leaching.
Owing to the low capital requirements and ease of implementation and use, continued worldwide proliferation of small copper SX-EW operations (< 10 kt/a Cu) is predicted. In line with global sustainability targets, a trend to lower-carbon-footprint diluents is anticipated, as well as a trend to lower-footprint and 'recyclable' equipment. An example of the latter is the modular mixer-settlers supplied by Metso Outotec (Hursi et al., 2018), which can be relocated to a new site at end of mine life. Improved dynamic predictive process control using artificial intelligence techniques is also in advanced stages of development and trials.
Conclusion
From its very modest beginnings in the 1960s, supported by visionary scientists and entrepreneurs, copper SX technology has evolved and adapted to currently produce some 4.0 Mt/a cathode, corresponding to approximately 18% of global primary production. The Nchanga Tailings Leach Plant in Zambia, commissioned in 1973, was the first full-scale copper SX-EW plant in the world. Today, the tremendous increase in production in the African Copperbelt over the last 15 years is driving innovation to solve challenges associated with the unique operating conditions in that region. The DRC has now overtaken Chile as the leading producer of SX-EW copper.
In conclusion, it is fitting to return to the words of Joe House on his election to the National Academy of Engineers, who described copper solvent extraction as 'a multimillion-dollar global business built from an idea.'
Acknowledgements
The authors thank the following colleagues for useful discussions during the preparation of this review: Andrew Nisbett (BASF, USA), Professor John Monhemius (retired, Imperial College, London), and Rod Whyte (retired, Anglo American, South Africa). The contents of this paper are based on a Keynote Address given at the International Solvent Extraction Conference ISEC 2022, Sweden, on 30 September 2022 by KCS and a presentation at Copper Cobalt Africa 2023, Zambia, on 13 June 2023 by OST.
Credit author statement
KCS: Investigation, data analysis, writing - original draft preparation; writing - review and editing; OST: Investigation, data analysis, writing - review and editing
References
Alexander, D., van der Merwe, O., Lümbüle, R., and Kgomo, J. 2018. Innovative process design for oxide ores in the Democratic Republic of the Congo. Proceedings of Copper Cobalt Africa 2018. Southern African Institute of Mining and Metallurgy, Johannesburg. pp. 225-238. [ Links ]
Anon. Not dated. Bluebird Copper Mine, Miami, Arizona. https://thediggings.com/mines/28798 [ Links ]
Anon. 2023. Leading copper cathode exporters in DRC: SICOMINES, Koe, and TFM in 2022. Copperbelt Katanga Mining. https://copperbeltkatangamining.com/leading-copper-cathode-exporters-in-drc-sicomines-kcc-and-tfm-in-2022/ [ Links ]
Bartos, P.J. 2002. SX-EW copper and the technology cycle. Resources Policy, vol. 28, no. 3-4. pp. 85-94. [ Links ]
Baxter, K. 2016. Are we any closer to hydromet overtaking smelting for copper sulfide concentrates? Proceedings of ALTA 2016 Nickel Cobalt Copper Conference. ALTA Metallurgical Services, Melbourne. pp. 1-32. [ Links ]
Chilean Copper Commission. 2018. Chile copper production: SX-EW cathodes 1990-2018. https://www.ceicdata.com/en/chile/copper-production-chilean-copper-commission/copper-production-sxew-cathodes [ Links ]
Cognis. 2008. Cognis wins Tenke Fungurume contract. .again! TechNews (August). p. 2. [ Links ]
Declercq, R. 2022. Katanga and the American world of copper: mechanization, vertical integration, and territorialization of colonial capitalism, 1900-30. Born with a Copper Spoon: A Global History of Copper, 1830-1980. [ Links ]
Declercq, R., Money, D., and Froland, H.O. (eds). UBC Press, Vancouver. pp. 253-273. [ Links ]
Dresher, W.H. 2001. Phelps Dodge Morenci has converted all copper production to mine-for-leach. Copper Development Association, Inc. https://www.copper.org/publications/newsletters/innovations/2001/08/phelpsdodge.html [ Links ]
Flett, D.S. 1974. Solvent extraction in copper hydrometallurgy: a review. Transactions of the Institute of Mining and Metallurgy, vol. C83. pp. C30-C38. [ Links ]
Green, C., Roberson, J., and Marsden, J.O. 2018. Pressure leaching of copper concentrates at Morenci, Arizona - 10 years of experience. Minerals and Metallurgical Processing, vol. 35. pp. 109-116. https://link.springer.com/article/10.19150/mmp.8459 [ Links ]
Holmes, J.A. and Fisher, J.F.C. 1972. Development of a process for the extraction of copper from tailings and low-grade materials at the Chingola Division of Nchanga Consolidated Copper Mines, Zambia. Advances in Extractive Metallurgy and Refining. Jones, M.J. (ed.). Institution of Mining and Metallurgy, London. pp. 169-188. [ Links ]
Holmes, J.A., Deuchar, A.D., Stewart, L.N., and Parker, J.D. 1976. Design, construction and commissioning of the Nchanga Tailings Leach Plant. Extraction Metallurgy of Copper, Vol. II. American Institute of Mining and Metallurgical Engineers, New York. pp. 907-925. [ Links ]
House, J.E. 1985. Success stories in speciality chemicals. SRI Newsletter (June). pp. 1-8. [ Links ]
House, J.E. 1989. The development of the LIX reagents. Minerals and Metallurgical Processing, vol. 6, no. 2. pp. 1-6. [ Links ]
Hursi, T., Saario, R., and Weatherseed, M. 2018. Development, design and implementation of Outotec's VSF*X solvent extraction technology. Proceedings of ALTA Nickel Cobalt Copper 2018. ALTA Metallurgical Services, Melbourne. pp. 275-282. [ Links ]
Lynch, A.J., Taylor, A., and Avendano Varas, C. 1994. Solvent extraction boom in Latin America. Engineering and Mining Journal, no. 12. pp. 18-21. https://www.altamet.com.au/wp-content/uploads/2014/11/Solvent-Extraction-Boom-in-Latin-America-EMJ-December-1995.pdf [ Links ]
Jetti Resources. 2023. First Chilean deployment of Jetti's novel leaching technology at El Abra Copper Mine, majority-owned and operated by Freeport-McMoRan Inc. https://www.jettiresources.com/news-reports/press-releases/first-chilean-deployment-jettis-novel-leaching-technology-el-abra-copper-mine-majority-owned-and-operated-freeport-mcmoran-inc/ [ Links ]
Kashala, A., Mitshabu, G., Cheng, Y., Bradford, L., Modi, A., and Tshisand, P. 2018. Management of mixing continuity in a solvent-extraction plant with a leach solution of high silica at Ruashi Mining. Proceedings of Copper Cobalt Africa 2018, Southern African Institute of Mining and Metallurgy, Johannesburg, pp. 303-310. [ Links ]
Kordosky, G.A. 2002. Copper recovery using leach/solvent extraction/electrowinning technology: forty years of innovation, 2.2 million tonnes of copper annually. Proceedings of the International Solvent Extraction Conference ISEC 2002, vol. 2. Sole, K.C., Cole, P.M., Preston, J.S., and Robinson. D.J. (eds.). South African Institute of Mining and Metallurgy, Johannesburg. pp. 853-862. [ Links ]
Marsden, J.O., Wilmot, J.C., and Mathern, D.R. 2007. Medium-temperature pressure leaching of copper concentrates-Part III: Commercial demonstration at Bagdad, Arizona. Minerals and Metallurgical Processing, vol. 24, no. 6. pp. 218-225. [ Links ]
Marsden, J.O., Wilmot, J.C., and Smith, R.J. 2007. Medium-temperature pressure leaching of copper concentrates-Part IV: Application at Morenci, Arizona. Minerals and Metallurgical Processing, vol. 24, no. 6. pp. 226-236. [ Links ]
Metorex Limited. 2008. Annual Report. https://www.sharedata.co.za/Data/000886/pdfs/METOREX_ar_08.pdf [ Links ]
Monhemius, J. 2014. A changing environment: Reflections on 50 years of hydrometallurgy. Plenary address: 7th International Symposium on Hydrometallurgy 2014 (Hydro2014), Victoria, British Columbia, Canada, 22-25 June 2014. [ Links ]
National Academy of Engineers. 1998. Memorial tribute to Joe E. House. https://www.nae.edu/19579/19581/20412/30099/Mr-Joe-E-House [ Links ]
Nisbett, A., Baxter, K., Marte, K., and Urbani, M. 2009. Flowsheet considerations for copper-cobalt projects. Proceedings of Base Metals 2009. Southern African Institute of Mining and Metallurgy, Johannesburg. pp. 139-152. https://www.saimm.co.za/Conferences/BM2009/139-152_Nisbett.pdf [ Links ]
Price, R. and Tumilty, J.A. 1972. An interpretation of some aspects of solvent extraction as related to the extraction of copper using o-hydroxyoximes. Institution of Chemical Engineers Symposium Series, no. 42, paper 18. [ Links ]
Rio Tinto. 2023. Nuton acquires common shares of Regulus Resources. https://www.riotinto.com/en/news/releases/2023/nuton-acquires-common-shares-of-regulus-resources [ Links ]
Seaman, B., Vollert, L., and O'Callaghan, J. 2019. In-situ recovery in hard rock applications: Idealistic notion or realistic future processing option? Proceedings of Copper 2019. Canadian Institute of Mining, Metallurgy and Petroleum, Montreal. Paper no. 590358. [ Links ]
Schlesinger, M.E., Sole, K.C., Davenport, W.G., and Alvear Flores, G.R.F. 2021. Extractive Metallurgy of Copper, 6th edn, Elsevier, Oxford. 573 pp. [ Links ]
Shalina Resources. Not dated. https://www.shalinaresources.com/chemafcommissions16000tons.html [ Links ]
Sikamo, J., Mwanza, A. and Mweemba, C. 2015. Copper mining in Zambia - history and future. Proceedings of Copper Cobalt Africa 2015. Southern African Institute of Mining and Metallurgy, Johannesburg. pp. 1-10. [ Links ]
Sole, K.C., Cole, P.M., Feather, A.M., and Kotze, M.H. 2011. Solvent-extraction and ion-exchange applications in Africa's resurging uranium industry: A review, Solvent Extraction and Ion Exchange, vol. 25, no. 5-6. pp. 869-900. [ Links ]
Sole, K.C., Zaraté, G., Steeples, J., Tinkler, O., and Robinson T.G. 2013. Global survey of copper solvent extraction operations and practices. Proceedings of Copper-Cobre 2013, Vol. IV, Gecamin, Santiago, Chile. pp. 137-148. [ Links ]
Sole, K.C., Crundwell, F.K., Dlamini, N., and Kruger, G. 2018. Silica mitigation in copper solvent-extraction circuits. Proceedings of Copper Cobalt Africa 2018. Southern African Institute of Mining and Metallurgy, Johannesburg, pp. 331-342. [ Links ]
Sole, K.C., Taute, J.J., Tinkler, O.S., Steeples, J., and Zaraté, G. 2019. Global survey of copper solvent extraction: 2018 Operating data and practice. Proceedings of Copper-Cobre 2019. Canadian Institute of Mining, Metallurgy and Petroleum, Montreal. Paper 581100. [ Links ]
Sole, K.C., Steeples, J., Taute, J.J., Zhou, Y., and Parker, J. 2022. Copper solvent extraction: 2022 global survey of operating practice and performance. Proceedings Copper 2022 International Conference. Gecamin, Santiago. pp. 119-132. [ Links ]
Statistica. 2023. Copper production in Democratic Republic of Congo. https://www.statista.com/statistics/1276790/copper-production-in-democratic-republic-of-the-congo/ [ Links ]
Stieper, G. 2018. First chalcopyrite copper concentrate leaching using Albion Process™ technology. Proceedings of Hydroprocess 2018, 10th International Seminar on Process Hydrometallurgy. Gecamin, Santiago. [ Links ]
Van der Zeeuw, A.J. 1972. Selective copper extractants of the 5-alkyl-2-hydroxyphenyl alkyl ketone oxime. Institution of Chemical Engineers Symposium Series, no 42. paper 16. [ Links ]
Virnig, M., Eyzaguirre, D., Jo. M., and Calderon, J. 2003. Effects of nitrates on copper SX circuits: a case study. Copper-Cobre 2003, Hydrometallurgy of Copper: Modelling, Impurity Control and Solvent Extraction, Vol. VI, Book 2. Rivieros, P.A., Dixon, D.G., Dreisinger, D.B., and Menacho, J.H. (eds.) Canadian Institute of Mining, Metallurgy and Petroleum, Montreal. pp. 795-810. [ Links ]
Voigt, P., Stieper, G., and Hourn, M. 2019. First commercialization of the Albion Process" for copper. Proceedings of Copper 2019. Canadian Institute of Mining, Metallurgy and Petroleum, Montreal. Paper no. 576516. [ Links ]
Wikipedia (undated). Maxie Anderson. Available from https://en.wikipedia.org/wiki/Maxie_Anderson [ Links ]
Yánez, H., Soto, A., Soderstrom, M., and Bednarski, T. 2009. Nitration in copper SX? Cytec Acorga provides a new reagent. Proceedings of HydroCopper 2009. Domic, E. and Casas, J. (Eds.), Gecamin, Santiago. pp. 332-341. [ Links ]
 Correspondence:
Correspondence:
K.C. Sole
Email: kathy@soleconsulting.co.za
Received: 3 Jul. 2023
Revised: 26 Jul. 2023
Accepted: 2 Aug. 2023
Published: July 2023
1 A question that may be asked is how a food company, General Mills, ended up producing chemicals for use in the mining industry? The processing of a variety of grains and oil seeds into food products resulted in plant fats and oils as byproducts, for which useful applications were sought. Some of the fatty alcohols were ideal for conversion to amines, from which flotation reagents were first developed and later liquid anion-exchangers for solvent extraction. The tertiary amine derived from a mixture of C8 and C10 fatty alcohols was found to be the ideal blend for extraction of uranium, and later became known as Alamine 336.
2 LIX is a registered trademark of BASF.
3 ACORGA is a registered trademark of Solvay.
4 As a quirky footnote to history, Kordosky (2002) reports that initial industry reception of this new technology was hostile: when the R&D director of a large US copper producer predicted at an AIME Annual Meeting that there would never be a pound of copper produced using SX, his comment prompted applause.














