Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Journal of the Southern African Institute of Mining and Metallurgy
versão On-line ISSN 2411-9717
versão impressa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.123 no.6 Johannesburg Jun. 2023
http://dx.doi.org/10.17159/2411-9717/2806/2023
PROFESSIONAL TECHNICAL AND SCIENTIFIC PAPERS
Spontaneous combustion of carbonaceous shale at an iron ore mine, South Africa
C. Gous; B. Genc
School of Mining Engineering, University of the Witwatersrand, South Africa. ORCID: B. Genc: http://orcid.org/0000-0002-3943-5103
SYNOPSIS
Spontaneous combustion during coal mining operations is a major problem that affects the health and safety of workers and causes environmental problems. The phenomenon is associated with the presence of coal, coal shale, and pyrite. In 2020, a premature detonation incident occurred at an iron ore mine where the waste material contains black carbonaceous shale units known to be associated with pyrite. The spontaneous combustion propensity and properties of samples of the black carbonaceous shales from the mine were examined and compared with samples from the Witbank Coalfield. The spontaneous combustion liability indexes of these samples were correlated with X-ray fluorescence (XRF) and proximate and ultimate analyses using linear regression. The Wits-Ehac Index classification results show that the samples were between medium and high risk. The linear regression analysis showed very poor correlations between the Wits-Ehac Index results and the XRF and proximate and ultimate results. The most valuable relationship found is between the presence of relatively high sulphur (greater than 3%) and ground reactivity with nitrate-bearing explosive emulsion.
Keywords: coal, spontaneous combustion, carbonaceous shale, linear regression, premature detonation, iron ore mine.
Introduction
An unplanned detonation incident occurred in April 2020 at an iron ore mine ('Mine A') in South Africa. The incident was classified as a high potential incident (HPI) that could have caused a fatality. The post-incident investigation confirmed the presence of carbonaceous shale near the detonation site. The carbonaceous shale was known to be present in the mine, but was neither separately modelled nor predicted to occur in the specific mining block (Scott and Gous, 2020). The chemical properties of the carbonaceous shale were not studied extensively as the mine's drilling operations focused on intersecting the iron ore and were not concerned with the overlying waste domains. The incident could have occurred due to self-heating of the carbonaceous shale, leading to spontaneous combustion, or reaction between the carbonaceous shale and the bulk explosive emulsion.
Coal-shale, or carbonaceous shale, is a sedimentary rock containing less than 50% organic material (Schopf, 1966), and according to the ECE-UN coal classification (1998), a rock with 80-50% ash content. These shales are believed to be formed in depositional environments such as nondeltaic coastal salt-marshes from peats preserved in the stratigraphy. The non-carbonaceous and inorganic mineral matter content in carbonaceous shale is non-combustible and is represented by ash and sulphur-forming compounds (Jones and Cameron, 1988). The organic constituents are lower in carbonaceous shales than in coal itself and are represented by microscopic macerals.
The risk of spontaneous combustion in coal and coal-shale are commonly known and managed in the mining of coal deposits. Spontaneous combustion occurs due to the self-heating characteristic of coal and coal-shale (Onifade and Genc, 2018a). However, the risk of spontaneous combustion occurring in the iron ore environment is not well known or widely documented. Spontaneous combustion will result in lost working time which adversely impacts the mine performance, and the safety of the mine personnel (Phillips, Uludag, and Chabedi, 2011). These incidents occur commonly in underground coal mines as well as surface mines (Genc and Cook, 2015). Such incidents also occur in sulphide mines, where ground reactivity incidents were recorded as recent as April 2021 (Pieterse, 2021).
The major risk of blasting in ground conditions that have elevated temperatures is the unplanned premature detonation of blast-holes, which has the potential to cause loss of life (Australasian Explosives Industry Safety Group (AEISG), 2017). This risk exists due to the potential of the ground to react with the explosives used (Pieterse, 2021). Elevated ground temperatures may be caused by the inherent rock chemistry, such as the case with coal seams, and when sulphides are present (AEISG, 2017). Other causes of elevated ground temperatures are related to the geothermal gradient, which is the rate of increase in temperature per unit depth due to heat flow from the Earth's core (Kanana and Matveyev, 1989). Sulphide oxidation is a special case of elevated temperature ground conditions where the addition of nitrates causes an oxidation reaction that releases heat and may lead to the premature detonation of blast blocks (AEISG, 2017).
Occurrences of elevated ground conditions leading to spontaneous combustion in coal environments are common and well documented. The Witbank Coalfield is one of these localities and work has been done in this area to evaluate the propensity for spontaneous combustion using an index (Wits-Ehac Index). Studies by Onifade et al. (2019 , 2021) and, Onifade and Genc (2018a, 2018b, 2018c) aimed to relate the intrinsic properties of coal-shale to the Wits-Ehac Index. Analyses performed on samples from the Witbank Coalfield by Onifade et al. (2019) included X-ray diffractometry (XRD) and X-ray fluorescence (XRF) to geochemically characterize the coal-shale, as well as proximate, ultimate, and total sulphur analyses. The relationship between the inherent properties and risk of spontaneous combustion as determined via the Wits-Ehac Index was investigated by Onifade and Genc (2018a). Onifade et al. (2019) reported that the carbonaceous shale of the Witbank Coalfield is enriched in silica, aluminium, and iron, with kaolinite and quartz the dominant minerals.
Spontaneous combustion is described by Kim and Chaiken (1990) in terms of the rate at which energy is released and transferred to the surrounding rock mass. The rate of combustion depends on the concentration of reactants (carbon and oxygen), the surface area and particle size, ambient/initial temperature, and the activation energy (Kim and Chaiken, 1990). During the oxidation of coal or carbonaceous shale, CO2, CO, and heat are generated (Chaiken, 1977). With enough organic carbon as fuel, no external heat source may be required for spontaneous combustion to occur (Kim and Chaiken, 1990). In the case of carbonaceous shale, the presence of pyrite, and potentially water, increases the risk of spontaneous combustion due to the exothermic oxidation reaction of pyrite (Kim and Chaiken, 1990).
This exothermic oxidation reaction of carbon-rich material (coal and related shales) with pyrite is a three-stage self-heating process that may lead to spontaneous combustion (Beamish and Theiler, 2016; Kim and Chaiken, 1990). This process can occur either on waste dumps or in in-situ material into which blasting drill-holes have been drilled. Spontaneous combustion can also occur in places where there is air flow due to natural or non-natural fracturing (Potgieter, 2018; Restuccia, Ptak, and Rein, 2017). This reaction leads to elevated ground temperatures of varying degrees of intensity (Beamish and Theiler, 2016).
Due to the heterogeneous nature of coal and coal-shales and the many physical and chemical interactions at play during oxidation, it is very difficult to identify one variable to explain the propensity of coal or coal-shales to self-heat (Carras and Young, 1994; Singh and Demirbilek, 1987). No single property can be identified to predict spontaneous combustion (Carras and Young, 1994; Singh and Demirbilek, 1987). Many methods exist to assess the propensity of coal to heat and spontaneously combust and to analyse the factors influencing this event. These methods can be grouped into mathematical models, experimental models, and statistical methods. Onifade and Genc (2020) provide a summary of global methods based on this grouping. However, the most readily available testing method for spontaneous combustion liability in the Africa continent is the Wits-Ehac Index. The current study aims to quantify the risk of spontaneous combustion of carbonaceous shale at Mine A and compare the results with those for the Witbank coal-shale available from the literature. This will aid in understanding the risk of spontaneous combustion that informs the drill-and-blast activities at the mine.
Materials and methods
Location of the study area
Mine A is located on the southern extent of the Maremane Dome in the Northern Cape Province of South Africa, within the Palaeoproterozoic Transvaal Supergroup (Beukes, 1980). The Gamagara Formation of the Olifiantshoek Supergroup, in which the carbonaceous shale of the mine occurs, unconformably overlies the iron ore (Alchin and Botha, 2006, Cousins, 2016). The shale is classified geochemically as a 'poor' carbonaceous shale, falling on the limit of being classified as a 'rock, due to its low carbon content, low calorific value, and high ash content. The unit is characterized by a low iron content, except when siderite is present, and a low but variable sulphur content. Higher sulphur values may be ascribed to the presence of pyrite, which increases the risk of reactive ground. According to the classification of Wagner (2021), the carbonaceous shale at Mine A can be referred to as 'shale' and not 'carbonaceous shale' due to it containing less than 10% carbonaceous matter.
Visual inspection of the pit area was conducted following the detonation incident. The presence of carbonaceous shale was confirmed through visual identification based on the black colour in the pit. The location of the unit is indicated in Figure 1.
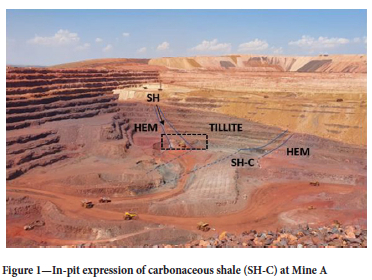
Existing boreholes were identified that had the potential to be twin-drilled. The boreholes were chosen to cover the expanse of the carbonaceous shale, to better delineate the risk area, as well as to confirm the modelled contacts. Due to the boreholes extending into the deposit, coverage of the carbonaceous shale was targeted at depth as well. The boreholes were planned at a spacing of 100-200 m. The extensive testing was done to describe risk levels for reactivity and spontaneous combustion and to identify potential proxies from geological features, such as chemistry and mineralogical content. This would aid in future risk identification and delineation per mining bench.
Sample collection and preparation
Ten core-drilled samples were identified for testing from the targeted drill-holes. The samples were collected from each hole as soon as it was drilled and stored in airtight plastic bags, clearly labelled, and recorded, to prevent oxidation and to retain sample integrity. The samples were crushed to 3 mm and then split into fractions for XRF and XRD analysis. These analytical splits were milled to 75 μm. The samples identified for spontaneous combustion liability via the Wits-Ehac Index were crushed to -212 μm. For the XRF analyses, the sample loss on ignition was determined by thermogravimetric analysis (ISO 9516-1 and ISO 11536), after which a fused glass-bead was prepared for analysis using Axios and Axios Advanced instruments. The percentage of total sulphur was measured using an ELTRA sulphur analyser according to ASTM 4239 . The proximate and ultimate analyses were done according to accredited and validated methods under ISO/IEC 17025 with practices outlined in ISO 17246 and ASTM 3176, for the proximate and ultimate analysis respectively. Proximate analyses were done as per ISO 1171 for the ash (A), ISO 562 for volatile matter (VM), and ISO 11722 for moisture (M). Total sulphur (TS) and calorific value (CV) were determined according to ASTM D4239 and ISO 1928 respectively. Ultimate analyses were done according to ASTM D3176-15. The XRD analyses were done on a Bruker X-ray diffractometer and identification of minerals was conducted using the Bruker AXS Topas software, based on the best matched peaks according to accredited and validated methods under ISO/IEC 17025. Lastly, the mineral phases were identified and quantified via the Rietveld refinement method.
The propensity for spontaneous combustion of the coal-shale samples was evaluated using the Wits-Ehac test apparatus at the University of the Witwatersrand (Wits University). The Wits-Ehac test apparatus was developed by Wits University in the late 1980s and the Wits-Ehac Index is a nationally accepted standard for determining the spontaneous combustion liability of coal. A detailed description of the apparatus and the test method is documented by Onifade et al. (2021). In summary, the test uses a combination of crossing-point temperature (XPT) and differential thermal analysis (DTA) to determine the likely point of spontaneous combustion, calculated via Equation [1]:
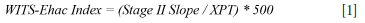
The XPT is measured by heating the coal sample and an inert sample at a constant rate in an oil bath. The point at which the temperature of the coal sample equals that of the inert sample is taken as the XPT. The DTA test measures and plots the temperature difference between the coal sample and the inert sample, and consists of three stages (Gouws and Wade, 1989; Wade, Gouws, and Phillips, 1987). Stage II is reached once all the moisture is evaporated and the coal sample is heating up to reach the bath-medium temperature (Wade, Gouws, and Phillips, 1987).
Results and discussion
Ten samples of carbonaceous shale from three successfully completed drill-holes were submitted for Wits-Ehac testing (Table I). The samples were taken at different intervals within each hole so as to be representative of the vertical extent of the unit, with a minimum of three samples per hole. The spomtaneous combustion liability of all samples is between medium and high, with variation between holes as well as variation with depth in each hole. These results correspond with the Genc and Cook (2015) results for Witbank Coalfield samples. The XPTs are on average lower per liability category for the Mine A carbonaceous shale compared to those of the Witbank Coalfield.
The Wits-Ehac Index results were further analysed by correlating them with the intrinsic factors and chemical composition to determine the linear relationship with the spomtaneous combustion propensity. This correlation can then be compared with the correlations determined from the results of studies of the Witbank Coalfields. The correlation was done by grouping the results according to the categories defined by Onifade and Genc (2018b) as presented in Table II.
Table II shows the categories in which results of statistical analysis can be grouped based on the determined R2 values. The closer to unity the R2 value is, the more perfect the correlation between the two variables plotted (Onifade and Genc, 2018b). The categories are numbered 1-6, which corresponds to ranges of R2 intervals between unity and zero. The interval ranges in turn correspond to a 'very strong to perfect relationship' to 'no linear relationship' at zero.
Linear regression of Wits-Ehac Index and XRF data
In Table III, the XRF data is compared with the measured Wits-Ehac Index. At Mine A, Fe, Si, Al2O3, K2O, P, and S are the common analytes used to classify the quality of the ore. Therefore, this suite of analyses is commonly understood by mine personnel and was used to investigate a possible link with the Wits-Ehac Index. If a proxy could be picked up between any of these analytes with the potential for spontaneous combustion, it would be useful to manage the risk going forward.
The Fe values fall within 0-3%, SiO2 above 60%, Al2O3 above 15%, K2O below 2.5%, P ranging from 0.010 to 0.014%, and S values are low, all below the 1% limit defined as a risk in AEISG (2017). The highest Fe sample is 0028, which has an outlying Fe content greater than 3%, compared to the rest of the samples at less than 1.7% Fe. The Wits-Ehac Index for this outlying sample is 4.75, falling within the medium risk range for spontaneous combustion. The highest S values are for samples 0017 and 0026, which fall within the high and medium ranges of liability respectively, based on their Wits-Ehac Index results. To aid in this correlation determination between the chemical analytes and Wits-Ehac Index, the XRF results and the Wits-Ehac Index values were subjected to linear regression as illustrated in Figure 2.
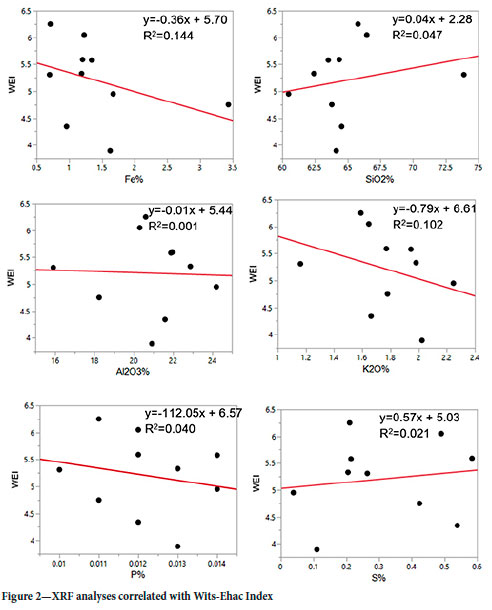
Based on Figure 2, the Fe, K2O, and P contents show a weak negative relationship with the WEI, and S shows a weak positive relationship when using the categories set out in Table II, according to R2 values. The SiO2 and Al2O3 contents have a very weak positive and negative relationship with WEI respectively. Although still weak, the strongest correlation of WEI is with the Fe and K2O contents since their R2 values are the highest. The correlation between Fe and the Wits Ehac Index might make sense if the Fe is from pyrite, which is known to increase spomtaneous combustion risk.
Linear regression with proximate and ultimate analyses
Coal and coal-shales are known to undergo spomtaneous combustion due to the interaction of the organic carbon with atmospheric conditions (Onifade and Genc, 2018a, 2018b, 2018c). The carbonaceous shale at Mine A is not related to any coal deposit, and spomtaneous combustion in situ is highly unlikely considering that such incidents typically occur in the coal environment (Kim and Chaiken, 1990). However, the carbonaceous shale does pose a risk of elevated ground temperatures, especially where fractures are present due to blasting activities (Potgieter, 2018; Restuccia, Ptak, and Rein, 2017), as was the case in the area in which the incident occurred.
In order to compare the carbonaceous shale of Mine A to the coal-shale of the Witbank area, proximate and ultimate analyses were conducted. The analyses were carried out on 9 out of the 10 samples sent for Wits-Ehac Index tests (Table IV). Sample 0016 is excluded from the linear regression analyses due to no results being available, but for reference, it is included since it has a Wits-Ehac Index value.
High ash content is related to high mineral matter content (Snyman, 1989). The Witbank coal-shale described by Onifade and Genc (2018a) has a lower ash content than the Mine A carbonaceous shale, thus the Mine A carbonaceous shale poses a lower risk with respect to spontaneous combustion.
The low calorific values are expected since calorific value determines the coal grade, which is not applicable to a carbonaceous shale. However, the low calorific value may be an indication that the material may be more inert since more energy will be consumed during heating/combustion than is released (Alpern and de Sousa, 2002). The higher the volatile matter content, the higher the propensity for spontaneous combustion (Onifade and Genc, 2018a; Banerjee, 2000), and since the volatile matter content of the shale at Mine A is much lower than that of the Witbank coal-shales, the shale poses less of a risk.
The carbon and sulphur contents are very low. The carbon, hydrogen, and nitrogen levels of the Mine A carbonaceous shale are significantly lower than ths values for the Witbank coal-shales reported by Onifade and Genc (2018a), resulting in it posing a relatively lower risk.
The sulphur content is significant for drill-and-blast activities due to the potential for reactive ground. The sulphur levels are low, well below the risk limit of 1% as supplied in the AEISG (2017) guidelines. Carbonaceous shales with the following properties have a higher spontaneous combustion liability index (Onifade and Genc, 2018a):
> Lower ash content (less risk with between 51.5% and 68.4% ash, and above)
> Higher moisture content (lower risk with moisture content less than 1.5%)
> High volatile matter (Lower risk with VM less than 15.9%)
> High sulphide (low risk with less than 0.1% pyrite, medium with 0.1% to 4%).
Based on the observations, the carbonaceous shale samples from Mine A pose a low risk for spontaneous combustion compared to that of the Witbank coal-shale (Onifade and Genc 2018a).
The volatile matter is below 10% for the carbonaceous shale samples listed in Table IV, while the ash content is greater than 90%. The high ash content indicates that the Mine A carbonaceous shale has a high mineral (inorganic) matter content and low organic matter (Falcon and Ham, 1988). The low volatile matter content is indicative of a low maceral (organic) composition (Snyman, 1989). The total carbon content for the samples is very low, below 0.5% in all the samples. The Wits-Ehac Index results indicate a medium to high propensity for spontaneous combustion.
The relationship between ultimate and proximate results was investigated further by linear regression analysis to correlate the results with the Wits-Ehac Index results shown in Figure 3. The correlation is compared to results previously reported for the Witbank coal-shales (Onifade and Genc, 2018a).
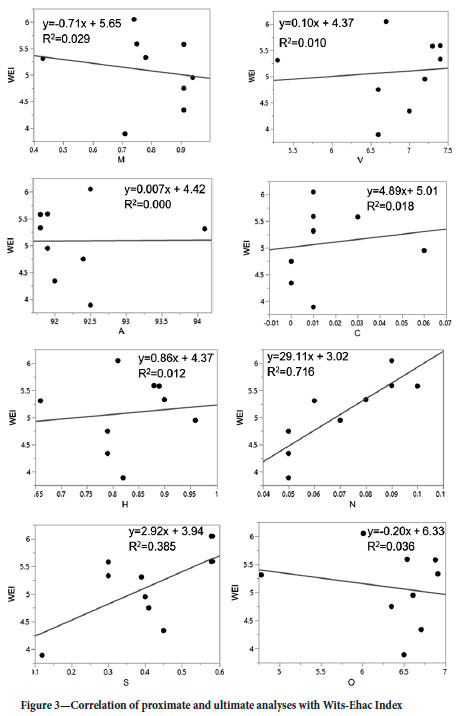
Based on the R2 values in Figure 2, the strongest positive linear relationship is between nitrogen and the Wits-Ehac Index. Total sulphur has a moderate positive relationship, while the rest of the variables (volatile matter, hydrogen, oxygen, moisture, total carbon, and ash) have very weak relationships with the Wits-Ehac Index. Nitrogen occurs in the organic carbonaceous material itself (Kim and Cheiken, 1990); none of the minerals in the XRD analysis contain nitrogen. This may indicate a relationship between the carbonaceous material content (albeit low) and the propensity for spontaneous combustion.
Categorical comparison
Table V presents the categorical results for the Witbank coal-shale samples as reported by Onifade and Genc (2018a), along with the Mine A categorical results from this study.
The categorical results of the Mine A carbonaceous shale are different to those of the Witbank coal-shales. No proximate or ultimate result reports to category 1 or category 4 for either the Witbank coalfields or Mine A. The only analytes of both mines reporting to the same category (category 5) are moisture and oxygen. Sulphur and nitrogen are the chemical constituents with the strongest relationship to the Wits-Ehac Index results for Mine A, where volatile matter, ash, carbon, hydrogen, and nitrogen have the strongest relationship for the Witbank coal-shale. Therefore, nitrogen is the only common analyte with a relatively strong relationship with the Wits-Ehac Index for both the Witbank coal-shale and the Mine A shale. Sulphur does not have a common relationship with the Wits-Ehac Index for either the Mine A shale or the Witbank carbonaceous shale. Contrasting the Witbank coal-shale results, the ash content of Mine A shale has no relationship with the Wits-Ehac Index.
Mineralogical composition of the Mine A carbonaceous shale
The XRD results for the Wits-Ehac samples are presented in Figure 4. The mineralogical composition of the Mine A carbonaceous shale is typical of shales occurring in a coal environment, namely clays, pyrite, and carbonates (Wagner, 2021). The dominant minerals in the Mine A samples are kaolinite, quartz, and siderite, whereas the Witbank coal-shale reported by Onifade et al. (2019) is dominated by kaolinite and quartz. Kaolinite is a common clay mineral, and quartz is a common silicate mineral. Pyrite can have a catalytic effect on the oxidation reaction that may lead to an increased spontaneous combustion propensity (Kim and Chaiken, 1990).
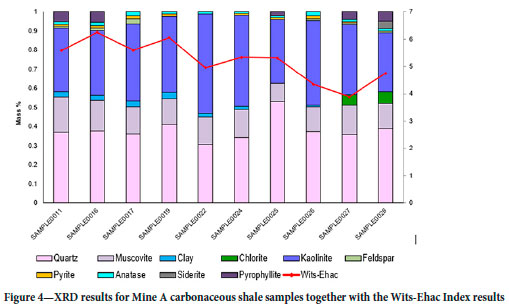
The Witbank coal-shale results studied by Onifade, et al. (2019) suggest that a higher pyrite and muscovite content poses a higher risk for spontaneous combustion, and higher kaolinite, quartz, plagioclase feldspar, and siderite a lower risk. The significance of siderite (iron carbonate) is that the carbonate acts as a buffer (inhibitor) to the exothermic oxidation reaction (Descotes et al., 2002). Siderite is present in low amounts, and the greater abundance of it in sample 0028 might contribute to the sample's lower Wits-Ehac Index (Descotes et al., 2002). The pyrite content is the highest in sample 0026, but this sample has the second lowest Wits-Ehac Index classification. Pyrite is present in almost all of the samples, ranging from the highest to the lowest Wits-Ehac Index, therefore no direct relationship is apparent to link the spontaneous combustion risk with the presence of pyrite. Apart from the presence of pyrite and siderite, the mineralogical composition of the Mine A carbonaceous shale does not exhibit the same trend. The high Wits-Ehac Index samples (0011, 0016, 0019, 0024, 0025) do not exhibit any significant correlation with a specific mineral. The medium liability samples (WEI 3-5) have a higher siderite content, and high liability samples (WEI>5) a higher pyrite content.
Conclusion
The properties of the carbonaceous shale at iron ore Mine A are of importance regarding the propensity for spontaneous combustion and the potential for ground reactivity. Based on the proximate and ultimate analyses, the carbonaceous shale in question falls between being classified as a 'rock' and as a carbonaceous shale. The proximate and ultimate analysis results bring into question the probability of a pure spontaneous combustion reaction taking place at the mine. Reaction between the explosive emulsion and the sulphide and carbonaceous materials in the shale might be a plausible explanation as opposed to pure spontaneous combustion.
With respect to the XRF results, all analytes have a weak to poor correlation with the Wits-Ehac Index, the strongest relationship being for iron. As regards the proximate and ultimate analyses, the strongest relationship is with nitrogen, followed by sulphur. Sulphur has a moderate linear relationship with the Wits-Ehac Index and this may indicate a risk for spontaneous combustion in areas where the sulphur content in the carbonaceous shale is elevated. The risk for spontaneous combustion according to the Wits-Ehac Index is between medium and high, with no spatial trends. No correlation between the Wits-Ehac Index and the presence of pyrite could be made. However, a negative correlation is observed between the siderite content and the Wits-Ehac Index analysis results.
In comparison with the Witbank coal-shale, the Mine A carbonaceous shale shows little to no correlation between chemistry and spontaneous combustion propensity, apart from nitrogen. The validity of the Wits-Ehac test as a measure of spontaneous combustion risk in the Mine A carbonaceous shale may be low due to the inconsistency between the response variables in comparison to the Witbank coal-shale. The reason for the inconsistency may be the inherent differences in composition and genesis of the lithologies between the two localities.
Acknowledgements
The work reported in this paper is part of an MSc research report in the School of Mining Engineering at the University of the Witwatersrand, Johannesburg, South Africa.
References
Alchin, D.J. and Botha, W.J. 2006. The structural/stratigraphic development of the Sishen South iron ore deposit, South Africa, as deduced from ground gravity data modelling. Applied Earth Science, vol. 115, no. 4. pp. 174-186. [ Links ]
Alpern, B. and de Sousa, M.J.L. 2002. Documented international enquiry on solid sedimentary fossil fuels, coal: definitions, classifications, reserves-resources, and energy potential. International Journal of Coal Geology, vol. 50, no. 1-40. pp. 3-41. [ Links ]
ASTM 3176. 2017. Standard practice for ultimate analysis of coal and coke. ASTM International, West Conshohocken, PA. [ Links ]
ASTM D-4239. 2017. Standard test method for sulfur in the analysis sample of coal and coke using high-temperature tube furnace combustion. ASTM International, West Conshohocken, PA. [ Links ]
Australasian Explosives Industry Safety Group (AEISG). 2017. Code of Practice. Elevated temperature and reactive ground. https://www.aeisg.org.au/wp-content/uploads/ELEVATED-TEMPERATURE-AND-REACTIVE-GROUND-COP-EDITION-5-APRIL-2020-3.pdf [accessed: January 2022]. [ Links ]
Banerjee, S.C. 2000. Prevention and Combating Mine Fires. Oxford and IBH, New Delhi. [ Links ]
Beamish, B. and Theiler, J. 2016. Characterising the spontaneous combustion propensity of waste rock. Proceedings of the Life-of-Mine Conference, Brisbane, Queensland, 28-30 September 2016. Australasian Institute of Mining and Metallurgy, Melbourne. [ Links ]
Beukes, N.J. 1980. Lithofacies and stratigraphy of the Kuruman and Griquatown iron-formations, northern Cape Province, South Africa. South African Journal of Geology, vol. 83. pp. 69-86. [ Links ]
Carras, J.N. and Young, B.C. 1994. Self-heating of coal and related materials: Models, application, and test methods. Progress in Energy and Combustion Science, vol. 20, no. 1. pp.1-15. [ Links ]
Chaiken, R.F. 1977. Heat balance in in-situ combustion. Report of Investigations 8221. Department of the Interior, US Bureau of Mines. [ Links ]
Cousins, D.P. 2016. A stratigraphic, petrographic and geochemical study of the Gamagara Formation at the Maremane Dome, Northern Cape Province, South Africa. MSc dissertation, Rhodes University, Grahamstown, South Africa. [ Links ]
Descotes, M., Beaucaire, C., Mercier, F., Savoye, S., Sow, J., and Zuddas, P 2002. Effect of carbonate ions on pyrite (FeS2) dissolution. Bulletin de la Societe Geologique de France, vol. 173, no. 3. pp. 265-270. [ Links ]
ECE-UN. 1998. International classification of in-seam coals. Economic Commission for Europe Commitee on Sustainable Energy. https://digitallibrary.un.org/record/260911?ln=en [accessed September 2021] [ Links ]
Falcon, R. and Ham, A. 1988. The characteristics of Southern African coals. Journal of the South African Institute of Mining and Metallurgy, vol. 88, no. 5. pp. 145-161. [ Links ]
Genc, B. and Cook, A. 2015. Spontaneous combustion risk in South African coalfields. Journal of the South African Insitute of Mining and Metallurgy, vol. 115. pp. 563-568. [ Links ]
Gouws, M.J. and Wade, L. 1989. The self-heating liability of coal: Predictions based on composite indices. Mining Science and Technology, vol. 9. pp. 81-85. [ Links ]
ISO 1171. 2010. Solid mineral fuels - Determination of ash. International Organization for Standardization, Geneva. [ Links ]
ISO 562. 2010. Hard coal and coke - Determination of volatile matter. International Organization for Standardization, Geneva. [ Links ]
ISO 17246. 2010. Practice for the proximate analysis of coal. International Organization for Standardization, Geneva. [ Links ]
ISO 11722. 2013. Solid mineral fuels - Hard coal, method for determining moisture. International Organization for Standardization, Geneva. [ Links ]
ISO 11536. 2015. Iron ores - Determination of loss on ignition. International Organization for Standardization, Geneva. [ Links ]
ISO 1928. 2020. Determination of gross calorific value. International Organization for Standardization, Geneva. [ Links ]
ISO 9516. 2003. Iron ores - Determination of various elements by X-ray fluorescence spectrometry Part 1: Comprehensive procedure. International Organization for Standardization, Geneva. [ Links ]
Jones, J.R. and Cameron, B. 1988. Modern coastal back-barrier environment: Analog for coal basin or carbonaceous black shale? Geology, vol. 16, no. 4. pp. 345-348. [ Links ]
Kanana, YE and Matveyev, A.K. 1989. Temperature and geologic time in the regional metamorphism of coal. International Geology Review, vol. 31, no. 3. pp. 258-261. [ Links ]
Kataka, M.O., Matiane, A.R., and Odhiambo, B.D.O. 2018. Chemical and mineralogical characteristics of reactive coal from Northern Natal and Venda-Pafuri coalfields in South Africa. Journal of African Earth Sciences, vol. 137. pp.278-285. [ Links ]
Kim, A.G. and Chaiken, F. 1990. Relative self-heating tendencies of coal, carbonaceous shales and coal refuse. Proceedings of the American Society of Mining and Reclamation. pp. 535-544. [ Links ]
Onifade, M. and Genc, B. 2018a. Prediction of the spontaneous combustion liability of coals and coal-shales using statistical analysis. Journal of the Southern African Institute of Mining and Metallurgy, vol. 118, no. 8. pp. 779-808. [ Links ]
Onifade, M. and Genc, B. 2018b. Spontaneous combustion of coals and coal-shales. International Journal of Mining Science and Technology, vol. 28, no. 6. pp. 933-940. [ Links ]
Onifade, M. and Genc, B. 2018c. Modelling spontaneous combustion liability of carbonaceous materials. International Journal of Coal Science and Technology, vol. 5, no. 2. pp. 191-212. [ Links ]
Onifade, M. and Genc, B. 2020. A review of research on spontaneous combustion of coal. International Journal of Mining Science and Technology, vol. 30, no. 3. pp. 303-311. [ Links ]
Onifade, M., Genc, B., Gbadamosi, A.R., Morgan, A., and Ngoepe, T. 2021. Influence of antioxidants on SPONCOM and coal properties. Process Safety and Environmental Protection, vol. 148. pp. 1019-1032. [ Links ]
Phillips, H., Uludag, S., and Chabedi, K. 2011. Prevention and control of spontaneous combustion. Best practice guidelines for surface coal mines in South Africa. Coaltech Research Association. https://miningandblasting.files.wordpress.com/2009/09/spontaneous_combustion_guidelines.pdf [accessed January 2022]. [ Links ]
Pieterse, D. 2021. Reactive ground - April 2021. Internal BME report. Unpublished. [ Links ]
Potgieter, N. 2018. Analysis and investigation of drill-core samples of characteristics of reactive ground. EnviroSim Consulting, Pretoria. [ Links ]
Priyananda, P., Djerdjev, A., Gore, J., Neto, C., Beattie, J., and Hawkett, B. 2015. Premature detonation of an NH4NO3 emulsion in reactive ground. Journal of Hazardous Materials, vol. 283. pp. 314-320. [ Links ]
Restuccia, F., Ptak, N., and Rein, G. 2017. Self-heating behaviour and ignition of shale rock. Combustion and Flame, vol. 176. pp. 213-219. [ Links ]
Schopf, J.M. 1966. Definitions of peat and coal and of graphite that terminates the coal series (graphocite). International Journal of Coal Geology, vol. 74, no. 5, part 1. pp. 584-592. [ Links ]
Scott, T. and Gous, C. 2020. Geological input for reactive ground identification. Kumba Iron Ore. [ Links ]
Singh, R.N. and Demirbilek, S. 1987. Statistical appraisal of intrinsic factors affecting spontaneous combustion of coal. Mining Science and Technology, vol. 4, no. 2. pp.155-165. [ Links ]
Snyman, C.P. 1989. The role of coal petrography in understanding the properties of South African coal. International Journal of Coal Geology, vol. 14. pp.83-101. [ Links ]
Wade, L., Gouws, M.J., and Phillips, H.R. 1987. An apparatus to establish the spontaneous combustion propensity of South African coals. Proceedings of the Symposium on Safety in Coal Mines, CSIR, Pretoria. pp. 7.1-7.2. [ Links ]
Wagner, N.J. 2021. Geology of coal. Encyclopedia of Geology. Alderton, D. and Elias, S.A. (eds). : Academic Press, Lindon. pp. 745-761. [ Links ]
 Correspondence:
Correspondence:
B. Genc
Email: bekir.genc@wits.ac.za
Received: 12 May 2023
Revised: 22 May 2023
Accepted: 12 Jun. 2023
Published: June 2023














