Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.122 n.12 Johannesburg Dec. 2022
http://dx.doi.org/10.17159/2411-9717/2117/2022
PROFESSIONAL TECHNNICAL AND SCIENTIFIC PAPERS
Purification of titanium sponge produced by lithiothermic reduction of titanium tetrachloride: Effect of leaching conditions
M.R. SerwaleI, II; T. CoetseeI; K.C. SoleI; S. FazluddinII
IDepartment of Materials Science and Metallurgical Engineering, University of Pretoria, Pretoria, South Africa. ORCID: M.R. Serwale: https://orcid.org/0000-0002-3183-5196; T. Coetsee: https://orcid.org/0000-0003-2028-5755; K.C. Sole: https://orcid.org/0000-0003-4707-1060
IIAdvanced Materials Engineering, Manufacturing Cluster, Council for Industrial and Scientific Research, Pretoria, South Africa. ORCID: S. Fazluddin: https://orcid.org/0000-0001-8364-7974
SYNOPSIS
The CSIR-Ti process employs lithiothermic reduction of titanium tetrachloride feedstock to produce titanium sponge. The product is therefore contaminated by a range of lithium and chloride species. In this study we examine the effects of particle size, temperature, and HCl concentration as input leaching variables on the removal of chlorides from the crude titanium sponge. A review of the aqueous chloride chemistry of Li and Ti provided initial conditions for leaching of impurity species from the sponge. Experimental results confirm that the effectiveness of leaching and removal of dissolved impurities from the sponge are dependent on leaching kinetics, which are influenced by temperature, particle size, and morphology. Of the variables tested, reaction temperature had the strongest influence on the oxygen content of the leached product. The HCl lixiviant concentration had a negligible effect under the conditions tested. Leaching of crude titanium sponge (-10 mm size fraction after crushing) at 14°C in either 1 M or 0.032 M HCl yielded a titanium sponge product that met the ASTM standard specification for commercially pure Grade 1 titanium, i.e., oxygen content < 0.18 mass% and chloride content < 0.15 mass%.
Keywords: titanium sponge, titanium tetrachloride, lithiothermic reduction, purification, leaching, CSIR-Ti process.
Introduction
A low-cost titanium manufacturing process, which is aimed at producing particulate titanium by the metallothermic reduction of titanium tetrachloride (TiCl4) with lithium, is under development at the Council for Industrial and Scientific Research (CSIR), South Africa. The process yields a crude product encapsulated in LiCl and, depending on whether the metallothermic reduction is completed with a stoichiometric excess of Li or TiCl4, may also contain traces of excess Li, TiCl3, and TiCl2. These residual reaction byproducts are detrimental to the mechanical characteristics of manufactured products (Peter et al., 2012): chlorides are volatile at high temperature, resulting in macro-porosity, which degrades fatigue properties (Yan, Tang, and Qian, 2015), while oxygen changes phase selection and microstructure, which increases the elastic modulus and yield strength, but reduces ductility (Baril, Lefebvre, and Thomas, 2011). The challenge encountered in most alternative methods to produce titanium powder is the economic removal of byproducts to achieve the target titanium specification (Liang et al., 2018; Peter et al., 2012).
Leaching is successfully used for impurity removal in the Hunter process (Gambogi and Gerdemann, 1999) and has the advantages of low energy intensity and removal of large quantities of salt at relatively low cost due to the simple equipment required (McKinley, 1955). Drawbacks include the low solution concentrations of byproducts for recycle due to water dilution and product contamination by the leach liquor (Hansen and Gerdemann, 1998). Initial experiments (van Vuuren, Oosthuizen, and Heydenrych, 2011; Serwale, Coetsee, and Fazluddin, 2020) showed promising results for use of this approach in treating the product of the CSIR-Ti process.
This follow-up study investigated the aqueous chemistry of titanium using the selected lixiviant to remove impurities from the sponge, followed by evaluation of selected experimental leaching conditions aimed at achieving a commercially pure (CP) Grade 1 product with respect to oxygen (0.18 mass%) and chloride (0.15 mass%) (ASTM, 2002). The aqueous chemistry behaviour of titanium and lithium salt compounds in the HCl leaching of the crude Ti product were explored. Experimental leaching testwork results were used to identify optimum process conditions.
Aqueous chemistry of titanium
Leaching of titanium sponge is complicated by a series of side reactions between the byproducts and water, which result in the formation of insoluble hydrolysis products. These precipitate and concentrate on the metal surface and in pores, contaminating the product with oxides and oxychlorides (Garmata et al., 1970; Jamrack, 1963). Dissolution reactions of Li and sub-chlorides (TiCl2, TiCl3) in water are exothermic; the resulting heat generation favours rapid precipitation of hydrolysis products and oxidation of titanium (Garmata et al., 1970).
The boundary conditions should be selected such that the titanium metal sponge or powder product is not dissolved or contaminated during acid leaching of the byproducts. In contrast to the high chemical stability of compact titanium, the sponge and powder forms are soluble in HCl solution due to their high specific surface areas (Garmata et al., 1970). Titanium solubility is more prominent in HCl concentrations above 1 M, where the titanous (Ti3+; violet) ion predominates, as demonstrated in the Pourbaix diagram of Figure 1 (Sole, 1999; Zhu, Zhang, and Cheng, 2011); however, Straumanis and Chen (1951) stated that dissolution of titanium metal in dilute HCl occurs with difficulty, solubility is extremely low, and is affected by impurities. Garmata et al. (1970) reported that only 0.5% titanium sponge dissolved in 0.16-0.33 M HCl at 100°C.
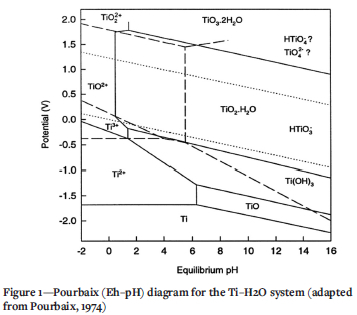
The slow dissolution is attributed to stable passivated TiO2-H2O that forms in aqueous media in the absence of a complexing agent (Figure 1). The stability range of this dominant aqueous species can be expanded in the presence of reducing acidic media (pH 6.0-2.5) (Pourbaix, 1974). Titanium metal dissolution and TiO2-H2O formation are minimized in the range of 0 < pH < 1.5, which is consistent with the recommendation by Seon and Nataf (1988) to use pH 1.5 for leaching of metallothermically produced crude titanium.
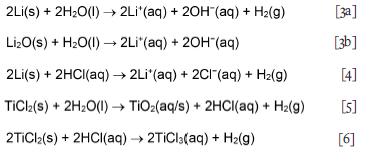
Although titanium sub-chlorides in the crude product are likely present as complex salts, Garmata et al. (1970) reported that these react with aqueous solutions just like the individual sub-chlorides; in contrast, however, the reaction is perceived to be slow. The aqueous chemistry of TiCl2 present in the CSIR-Ti crude product is reviewed from that perspective. The aqueous titanium(II) cation (Ti2+(aq)) oxidizes rapidly, even at low pH (Gould, 2011), so its chemistry is not well known. It is reported to exist for some time in ice-cold HCl solution; further support for its existence is provided by the electrode potential of Ti2+/ Ti3+ = -0.37 V vs. standard hydrogen electrode, which appears in most resources and the Pourbaix diagram in Figure 1 (Kölle and Kölle, 2003). What is known about Ti2+(aq) was notably reported by Kölle and Kölle (2003), Park et al. (2012), and Yang and Gould (2005). Gould (2011) concluded that in all reports confirming the existence of Ti2+(aq), the samples contained both HF and Ti4+ in highly acidic conditions, indicating that these contaminants might have stabilized the Ti2+ ion. The implication is that, under different conditions, the transients might be short-lived. However, it must be noted that Sekimoto et al. (2010) based their H2 volumetric analysis and titration procedure on the premise that TiCl2 reacts with 1 M HCl solution in a standard redox reaction to form Ti3+ ions and evolve H2 in the absence of an oxidizing agent, according to Equation [1] (Richens, 1997; Sekimoto et al., 2010; Wang et al., 2013):
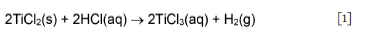
TiCl3 impurity has more severe consequences for final product properties, so the speciation and stability of Ti3+ in HCl have direct implications for the probability of recovering the ion in its soluble state, and consequently the selection of the leaching parameters and scheme. According to Figure 1, Ti3+ predominates under reducing conditions and pH < 1. It can, however, be easily oxidized in air to form tetravalent titanium(IV) TiO2+, as shown by Equation [2]:
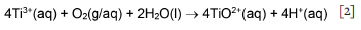
The rate of autoxidation in HCl is dependent on the volume of absorbed oxygen and solution pH (Mackenzie and Tompkins, 1942; Yakovleva et al., 1973), so autoxidation is significantly retarded in highly concentrated acidic solutions (Ashton, 1977).
The existence of the free Ti3+ ion in aqueous solutions remains controversial, with some postulates that it cannot exist due to its relatively high ionic potential: instead, it either oxidizes, hydrolyses, or forms hydrolysed polymeric species or complexes with various ligands, such as Cl", OH", CN", SO42-, and F- (Ashton, 1977; Nabivanets, 1965). Some reports suggest that Ti3+ ion dominates only in HCl solution at pH < 0.5 and that the main Ti3+ ionic species in dilute HCl solution (pH > 1) is the hexaquotitanium ion [Ti(H2O)6]3+ (pale reddish-purple) (Cassaignon, Koelsch, and Jolivet, 2007; Pecsok and Fletcher, 1962). [Ti(H2O)6]3+ has a measured pKa value between 1.8 and 2.5 (Cassaignon, Koelsch, and Jolivet, 2007; Clark, 1973; Richens, 1997). Ti3+ is appreciably hydrolysed at pH > 0.7 (Sole, 1999).
Hydrolysis reactions can be minimized or inhibited in acidified solution because the precipitation reactions are slow under these conditions (Jamrack, 1963; Kelly, 1963).
Methodology
Selection of experimental input variables
Based on the literature and the results of preliminary experimental work (Serwale, Coetsee, and Fazluddin, 2020), the leaching variables selected for experimental investigation were pH, feed material particle size, and temperature. The variable ranges were based on a two-level factorial experimental design, which only considers extremities (minima and maxima) in the test parameters (Free, 2022; Montgomery, 2017). This approach has the advantage that several variables, their interactions, and the effect of each factor on the response output are simultaneously investigated, thereby minimizing the number of experiments without sacrificing precision (Montgomery, 2017). Table I illustrates the experimental design matrix and interactions investigated in each test.
Material preparation and analyses
The crude titanium product for this study was produced at the CSIR titanium pilot plant (Pretoria, South Africa) using a batch process. The metallothermic reduction batch was run with 2% stoichiometric excess of TiCl4 Samples for this testwork were prepared from a 1500 g sample of crude titanium by crushing under argon gas at 20 mm jaw-crusher gap setting then screening at sieve sizes of 20 mm, 10 mm, and 630 μm. The materials retained on the 10 mm and 630 μm sieves were used in this work and are labelled as the +10 mm and -10 mm size fractions, respectively (Table I). Each size fraction was blended and split into 20 g samples using the cone-and-quartering method. Each 20 g sample was transferred to a glass bottle with a screw-on lid and stored prior to use in a sealed grade 304 stainless-steel vessel flushed with argon.
A composite head sample was analysed for total chloride content by gravimetry at the Nuclear Energy Corporation of South Africa (NECSA), following the ASTM E-120 standard test method for chemical analysis of Ti and Ti alloys. Oxygen content was determined by combustion (ASTM E-1409-97) using an Eltra ONH-2000 instrument. Lithium content was measured by inductively coupled plasma optical emission spectrometry (Optima 5300DV, Perkin Elmer, USA).
Experimental procedure
Batch leaching experiments were conducted in a 250 mL cylindrical glass reactor equipped with four ports on the glass lid, as illustrated in Figure 2. The central port was fitted with a stirrer shaft. One port was used for pH measurements; the other two accommodated an argon gas sparge pipe, a thermocouple inlet, and an argon vent pipe that was connected to a gas trap.
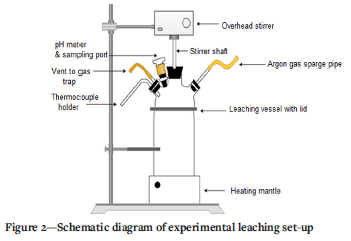
The experimental procedure was initiated by transferring 200 mL of the required dilute acid solution (1 M or 0.032 M HCl) into the leaching vessel, clamping the lid in position, then heating the vessel on a heating mantle to an initial solution temperature of 30°C. The temperature was continuously measured for the duration of the experiment with a K-type thermocouple that was submerged in the solution and recorded using a multi-channel data logger. The solution was agitated at 400 r/min with a polytetrafluoroethylene-coated anchor propeller impeller fitted to an overhead stirrer. Argon gas was sparged through a pipe with an internal diameter of 25 mm at a flow rate of 0.05 L/min, for approximately 10 minutes before leaching commenced, to ensure a non-oxidative environment. The argon flow was controlled and measured with a rotameter.
The heating mantle was switched off when the solution reached the set temperature because the leaching reactions are exothermic (Garmata et al., 1970). A 20 g solid sample was then slowly added into the leaching vessel while agitating vigorously (400 r/min) to dissipate the heat into the bulk solution and prevent the temperature from exceeding 60°C. The solution pH was measured and recorded with a portable pH meter fitted with a glass body pH electrode (Orion 8172 Ross, Thermo Scientific, USA) and stainless-steel temperature-compensation electrode (Orion 917004) to achieve automatic temperature compensation. The measurements intervals were 5, 10, 15, 30, and 60 minutes.
On completion of leaching, the sample was filtered (Munktell no. 1 filter paper, Ahlstrom, Sweden). The solid residue was subjected to four cycles of agitated washing in 200 mL of deionized water at 30°C to neutralize the acid and dissolve the salt from the metal. The deionized water was heated following the same process steps as described for heating the dilute HCl solution. The durations of the four sequential washing cycles were 120, 180, 120, and 60 minutes. The initial and final conductivities of the wash waters were measured with a portable conductivity meter (EC60 Waterproof EC/TDS/C, Martini Instruments, USA). On completing the washing cycles (i.e., when the conductivity reached 14-16 us), the residue was filtered then dried in a vacuum oven (VO400, Memmert, Germany) at 60°C for 6 hours.
To mitigate the safety risks posed by the pyrophoric behaviour of fine titanium (Bolivar and Friedrich, 2019), the solid residue was not washed with alcohol or acetone to evaporate soluble phases, despite the advantages associated with this process step with regard to the total oxygen content in the final product.
The same experimental procedure was used for the 14-30°C temperature range, except that the leach solution was precooled to 14°C by submersion in a 1000 mL beaker filled with ice blocks before being transferred to the leaching vessel. No external heating was provided. The deionized water washing steps were conducted at room temperature.
Results and discussion
Impurity content of crude titanium sponge
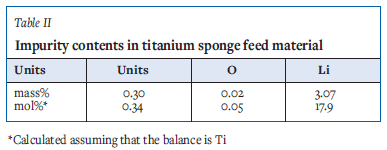
Analyses of the major impurities in a composite sample of the titanium sponge feed are shown in Table II. It was assumed that the impurity concentrations were consistent across both size fractions employed in this testwork. Owing to the low molecular mass of lithium, it is a very significant component when calculated on a molar basis. The chloride content is an order of magnitude lower. The results suggest that only a small proportion of the lithium was present as LiCl, and that most lithium occurred in the titanium sponge in metallic form. The lithiothermic reduction was carried out using excess TiCl4, so this result indicates that the reaction did not go to completion. Lithium is also present in the feed material as Li2O. Chlorides in this material were identified as LiCl, TiCl2, Ti(OH)Cl2, and Ti(OH)Cl.
Variation in solution pH with reaction duration
Typical pH behaviour observed during tests 1 to 8 is shown in Figure 3. For both initial acid concentrations (Table I), the pH increased rapidly within the first 5 minutes in all experiments, then dropped and stabilized at a constant value after 10 minutes.
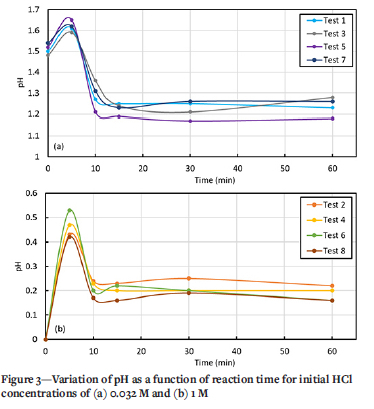
These observations are attributed to the increase in OH-concentration, as represented by the Li and Li2O neutralization reaction steps that take place near or on the exposed titanium surface, in which the weakly basic LiOH(aq) ion pair is formed via Equation [3]. Li also reacts with HCl(aq) to form LiCl(aq) according to Equation [4]. TiCl2 dissolves in water and HCl according to Equations [5] and [6].
The thermodynamic values for Equations [3] to [6]) were calculated using HSC Chemistry 7 (Chemistry Software, Finland). These reactions all exhibit negative Gibbs free energies (∆G) in the applied temperature range, indicating that there are no thermodynamic restrictions to these reactions. Enthalpy of reaction (∆H) was negative, confirming their exothermic nature.
Figure 3 shows that the cumulative acid consumption declined significantly after 5 minutes, suggesting that all exposed excess Li metal and Li2O on the particle surfaces were consumed and that hydrolysis of Ti(III) occurred in the presence of Cl". The pH therefore decreased due to the resulting H3O+ (Equation [7]). The presence of HCl retards hydrolysis by converting the pH-dependent hydrolysis product Ti(OH)2+ to TiCl2+, in proportion to the acid concentration (Mackenzie and Tompkins, 1942). These authors postulated that the reaction proceeds via two mechanisms that involve pH-dependent hydrolysis (Equation [7]) and complex formation (Equation [8]). If these reversible reactions were simultaneously established, the resulting equilibria would correspond to Equation [9] (Mackenzie and Tompkins, 1942; Shuvalov, Solov'ev, and Lebedev, 1978):
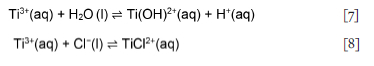
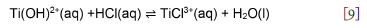
Chloride complexes of Ti3+ do not form to any appreciable extent; chloro-complex formation depends on the acid concentration (Nicholls, 2017). Acidic solutions of Ti(III) salts in inert atmospheres (nitrogen and argon) are relatively stable (Ashton, 1977). Therefore, the recovery of pH to lower levels, at 5 to 10 minutes (Figure 3), can be explained by the dominance of Equation [7].
The various aqueous titanium hydroxide and water-coordinated species are thermodynamically unstable at low pH. Hence, it is postulated that the ions continue to react by a bridging reaction or co-ordination re-arrangement to form stable species and water. For instance, it is postulated that a [Ti(H2O)6]3+ bridging reaction forms [Ti(H2O)5Cl]2+ according to Equations [10]-[13], which accounts for the stabilization in pH observed after 10 minutes. Despite an order of magnitude difference in initial pH, all test conditions showed similar trends, indicating that the first 5 minutes of the experiments are the most critical. It can be concluded that the concentration of the complexing ligand and the pH of the media are key to stabilizing the ions in solution.
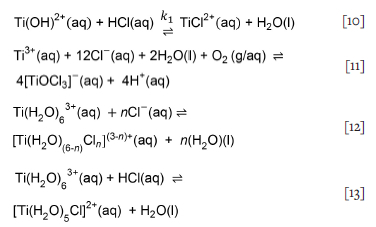
Variation in solution temperature with reaction duration
Examination of the temperature profile as a function of reaction time (Figure 4) shows that the temperature increased sharply within 5 minutes of adding the crude sample into the leaching vessel and then decreased, suggesting that most exposed and accessible by-products were dissolved during the initial temperature increase. This phenomenon was observed in all tests, despite the precautionary measures implemented to prevent localized overheating at the reaction-mass-solution interface. This effect was also reported in the leaching of sponge produced in the Hunter process, which uses Na as reducing agent (Garmata et al., 1970). Metallic Li, like metallic Na, has a low standard reduction potential and reacts with water to release heat and hydrogen gas, as displayed in Equation [3a], and thereby increases the solution pH (Schweitzer and Pesterfield, 2010).
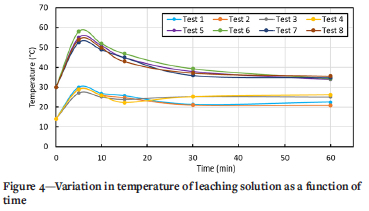
The temperature profiles in Figure 4 suggest that after the initial consumption of the excess reagents on the exposed titanium surface, the reaction changed from activation to mass-transfer control due to the formation of a TiH2 layer on the surface of the titanium particles owing to the incorporation of released hydrogen gas. At this point, the reaction was dependent on the stirring rate, which governs the diffusion rate through the TiH2 layer.
The observed temperature response indicates that reactivity is related to the rate at which heat is liberated during the initial chemical reaction, rather than the total amount of heat evolved over a prolonged period.
Product quality
Oxygen content
The initial oxygen content of the crude titanium was 0.02 mass%. Assays of the final products (Figure 5) show that the oxygen content increased as a consequence of the leaching process. Nevertheless, the oxygen content was within ASTM standard specifications of 0.18 mass% for CP Grade 1 to 0.4 mass % for CP Grade 4 titanium for tests 1-4 and 7-8. The markedly higher oxygen contents recorded for tests 5 and 6 correspond to the smaller particle size and higher reaction temperature. The oxygen contents for tests 7 and 8 approached the upper limit of the specification, which suggests that the higher reaction temperature was responsible for this effect, rather than the particle size (see Table I).
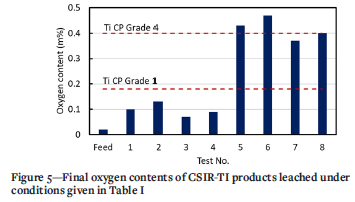
Statistical analysis of the experimental data with respect to oxygen content of the product is presented in Table IV. Inspection of the P-values shows that the experimental parameters with a significant effect at the 0.05 validity boundary level were particle size, temperature, and concentration. The F-values reveal that temperature and particle size had the most significant effect on the final oxygen content.
The strong effect of temperature on the oxygen content is in reasonable agreement with the prediction that the rate of oxidation of titanium in water increases with increasing temperature. No interaction between temperature and pH (HCl concentration) was detected, although these two factors cannot be considered in isolation in actual practice. Hydrolysis of Ti(III) ions occurs at approximately pH 0.6, so any localized overheating may lead to the formation of TiO2.
As noted in Table I, the two temperature variables considered both involved a wide temperature range, up to 30°C, rather than a defined temperature, owing to the high exothermicity of the leaching reactions and despite efforts to experimentally limit this temperature range. This wide experimental temperature range limits data interpretation. This effect will likely be exacerbated at larger- and commercial-scale operations, so future work should consider how to improve control of this parameter, particularly as a higher temperature is shown to exert the strongest detrimental influence on oxygen content of the product.
The increase in oxygen content during leaching is attributed to an increase in surface area owing to particle attrition by the vigorous agitation, combined with the inherent tendency of titanium to form an oxide monolayer (TiO2) on its surface in both air and water. Hansen and Gerdemann (1998) demonstrated the strong effect of particle size on final oxygen content during leaching of a titanium powder that initially assayed 0.82 mass%. Individual assays of various size fractions, ranging from 0.014760 μm, showed that the finest fraction contained 8.38 mass% O, but the coarsest fraction contained only 0.00002 mass% O. Oxygen contamination may also be attributed to the presence of water, which can increase passivation of oxygen on titanium particles (Kelly, 1982; Liang et al., 2020), from precipitation of hydrolysis products due to localized overheating on particle-particle surface contact owing to the exothermicity of leaching, or from surface oxidation of the titanium metal.
Chloride content
The maximum chloride specification for CP Grade 1 titanium sponge is 0.12-0.15 mass% (Nechaev and Polezhaev, 2016; Yan, Tang, and Qian, 2015; Yu and Jones, 2013). The efficiency of chloride removal (ne) was calculated using Equation [14] (Nechaev and Polezhaev, 2016):

where C0 and C are the initial and final total chloride contents in the titanium sponge, respectively. The data are summarized in Table V and Figure 6. Statistical analysis of the experimental data is presented in Table VI.
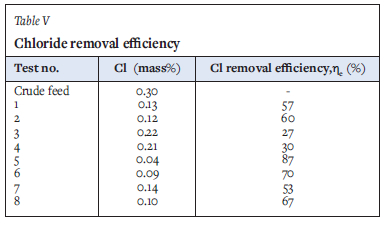
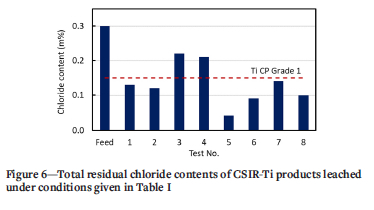
Table V and Figure 6 show that the total residual chloride content in the purified product decreased with increasing temperature and particle size. This trend is opposite to that of the oxygen content. Residual chlorides were significantly lower in the samples washed with deionized water at an average temperature of 30°C (tests 5-8) than those washed at 14°C (tests 3 and 4), which could be attributed to the correlation of higher solubility (and hence driving force for dissolution) and higher diffusion coefficient with higher temperature (Richardson et al., 2002). The results for tests 1 and 2 were similar to that of test 7, although the samples were leached at lower temperature. This is attributed to the smaller particle size (-10 mm) of the former and associated larger surface area, resulting in an increased extent of leaching. Test 8 also presented lower chloride content, despite the larger particle size, due to the higher leaching temperature. The combination of small particle size (-10 mm), high exothermic temperature, and deionized water washing at 30°C (tests 5 and 6) enhanced chloride removal. These observations suggest that both particle size and temperature exhibit considerable influence on chloride removal.
The results in Table VI show that the individual effects of both temperature and particle size on the extent of chloride removal were statistically significant at the 0.05 boundary level; however, their interaction was not statistically significant, as evidenced by the large P-value (0.3459 > 0.05). The effect of temperature was more significant than all factors and interactions.
Effect of particle size
The interfacial area of a solid-liquid interface increases with decreasing particle size, which is beneficial to the leaching rate owing to the reduced distance within the porous structure of the solid through which the solute needs to diffuse. In this work, both particle-size variables considered covered a wide range: the +10 mm material ranged from 10-20 mm; the -10 mm class ranged from 0.63-10 mm, which exceeds one order of magnitude. Nevertheless, general conclusions can be drawn from the experimental results. As shown by the data in Table V and Figure 6, the lowest residual chloride contents were measured for the -10 mm size fractions. The combination of -10 mm size fraction and 30°C washing temperature gave the highest chloride removal efficiency (tests 5 and 6); oxygen contents of the product were, however, adversely affected by the higher temperature (Figure 5). Particle size selection is therefore a balance between achieving adequate leaching efficiency of chlorides from the crude titanium, favoured by smaller particles sizes, and limiting the amount of oxygen contamination due to leaching in aqueous solution, which is countered by using large particles with low surface area exposed to oxygen adsorption reactions.
Effect of particle morphology
In addition to temperature and particle size, morphology of the titanium sponge is also a key factor that determines the extent of chloride removal. Secondary-electron scanning electron microscopy analysis of the purified products (Figure 7a) showed that the CSIR product had an irregular coral-like porous structure, akin to a sponge or agglomerate. Higher magnification observation revealed that during crushing to -10 mm, in addition to cracks propagating and isolated pores opening, some particles were flattened (as seen at locations A and B marked on Figure 7b). Formation of sintered and flattened agglomerates with semi-closed voids or closed pores would impede LiCl and TiCl2 removal during leaching. Sintered grains and dendritic particulates were also detected (Figure 7c). The sintering is attributed to prolonged contact between titanium particles and overheating on the metal surface during metallothermic reduction. Salt that is entrapped in closed pores of the powder by sintering cannot be completely removed by this acid leaching purification process, even if the residence time is increased. Salt entrapment in the microstructure has also been reported in titanium powders produced via the hydride-dihydride and Armstrong processes (Peter et al., 2012).
Conclusions
This study examined the effects of particle size, temperature, and HCl concentration on the leaching removal of chloride from crude titanium sponge produced in the CSIR-Ti batch process, with the aim of achieving target specifications for oxygen and chlorides in the purified product. The experimental results confirm that the effectiveness of leaching and removal of dissolved impurities from the sponge is dependent on temperature, particle size, and morphology. Reaction temperature had the strongest influence on the oxygen content of the leached product. There was negligible difference in the results using HCl concentrations of 1 M and 0.032 M. The low final pH values of the leach solutions indicated that hydroxy/oxychloride formation was unlikely. The chloride impurities likely originated from undissolved LiCl and TiCl2 salts that were physically trapped in the crude sponge by sintering.
Leaching of -10 mm crude titanium sponge at an initial solution temperature of 14°C in 1 M and 0.032 M HCl (tests 1 and 2) followed by washing with deionized water yielded a product that met the ASTM standard specification for CP Grade 1 oxygen content (< 0.18 mass%) and the general specification for chloride content (< 0.15 mass%). These values nevertheless exceed the maximum specification of 0.08 mass% for hydride-dehydride (HDH) process powder (Yan, Tang, and Qian, 2015), the highly recommended value of 0.05 mass% Cl, and are significantly higher than the ideal 0.01 mass% Cl required for premium metal powders (van Vuuren, Oosthuizen, and Heydenrych, 2011; Withers et al., 2013). Unfortunately, the conditions that gave the lowest chloride content (tests 5 and 6) correlated with the highest oxygen content, which gave a product that did not even meet the Grade 4 specification. The original premise of this work should perhaps be questioned: whether leaching is, in fact, the best route to achieving product purity in this particular system. The average chloride removal for all tests was only 56%. Options for better chloride separation or improvement in reduction efficiency in the pyrometallurgical parts of the process could be considered.
Acknowledgements
The authors thank the CSIR and South African Department of Science and Innovation for funding, the CSIR-Titanium Centre of Competence production team for advice-specifically Mr Jabu Skosana, and the laboratory team for analysing samples, without whom this study would not have been possible. We are also most grateful for the insights and useful comments of anonymous reviewers of earlier drafts of this manuscript.
CRediT author statement
MRS: Methodology, Investigation, Data analysis, Writing - original draft preparation; TC: Supervision, Writing - review and editing; KCS: Supervision, Writing - review and editing; SF: Project administration, Funding, Supervision, Writing - review and editing.
References
ASTM. 2002. Standard specification for titanium sponge. Designation B 299-01, American Society for Testing and Materials, West Conshohocken, PA. [ Links ]
Ashton, J.F. 1977. Some aspects of the solution chemistry of titanium (III). Master's thesis, University of Tasmania. https://eprints.utas.edu.au/19394/1/whole_AshtonJohnFrederick1977_thesis.pdf [ Links ]
Baril, E., Lefebvre, L.P., and Thomas, Y. 2011. Interstitial elements in titanium powder metallurgy: Sources and control. Powder Metallurgy, vol. 54, no. 3. pp. 183-186. https://doi.org/10.1179/174329011X13045076771759 [ Links ]
Bolivar, R. and Friedrich, B. 2019. Magnesiothermic reduction from titanium dioxide to produce titanium powder. Journal of Sustainable Metallurgy, vol. 5, no. 2. pp. 219-229. https://doi.org/10.1007/s40831-019-00215-z [ Links ]
Cassaignon, S., Koelsch, M., and Jolivet, J.P. 2007. From TiCl3 to TiO2 nanoparticles (anatase, brookite and rutile): Thermohydrolysis and oxidation in aqueous medium. Journal of Physics and Chemistry of Solids, vol. 68, no. 5-6. pp. 695-700. https://doi.org/10.1016/j.jpcs.2007.02.020 [ Links ]
Clark, R.J.H. 1973. The chemistry of titanium. Comprehensive Inorganic Chemistry. Bailer Jr., J.C., Emeleus., H.J., Nyholm. R., and Trotman-Dickenson, A.F. (eds), Pergamon Press, Oxford, UK. pp. 355-417. [ Links ]
Free, M.L. 2022. Hydrometallurgy: Fundamentals and Applications. 2nd edn. Wiley, Hoboken, NJ: [ Links ]
Gambogi, J. and Gerdemann, S.J. 1999. Titanium metal: extraction to application. Review of extraction, processing, properties & applications of reactive metals. Proceedings of the Minerals, Metals & Materials Society Annual Meeting, San Diego, CA. Mishra, B. (ed.), Wiley, New York. [ Links ]
Garmata, V.A., Gülyanitskii, B.S., Lipkes, Y.M., Seryakov, G.V., and Kramnik, V.Y. 1970. The Metallurgy of Titanium. National Technical Information Service (1st edn). Springfield, IL: Wright Patterson Air Force Base, OH. [ Links ]
Gould, E.S. 2011. Redox chemistry of aquatitanium(II), Ti2+(aq). Coordination Chemistry Reviews, vol. 255, no. 23-24. pp. 2882-2891. https://doi.org/10.1016/j.ccr.2011.06.006 [ Links ]
Hansen, D.A. and Gerdemann, S.J. 1998. Producing titanium powder by continuous vapor-phase reduction. JOM, vol. 50, no. 11. pp. 56-58. https://doi.org/10.1007/s11837-998-0289-3 [ Links ]
Jamrack, WD. 1963. Rare Metal Extraction by Chemical Engineering Techniques. Pergamon, Oxford, UK. [ Links ]
Kelly, E.J. 1982. Electrochemical behaviour of titanium. Modern Aspects of Electrochemistry: No. 14. Bockris, J., Conway, B., and White, R. (eds). Plenum Press, New York. pp. 319-417. https://doi.org/10.1136/bmj.1.3567.930-a [ Links ]
Kelly, J.T. 1963. Metal purification process. US patent 3085874. [ Links ]
Kölle, U. and Kölle, P. 2003. Aqueous chemistry of titanium(II) species. Angewandte Chemie International Edition, vol. 42, no. 37. pp. 4540-4542. https://oi.org/10.1002/anie.200351280 [ Links ]
Liang, L., Dachun, L., Heli, W., Kaihua, L., Juhai, D., and Wenlong, J. 2018. Removal of chloride impurities from titanium sponge by vacuum distillation. Vacuum, vol. 152. pp. 166-172. https://doi.org/10.1016/j.vacuum.2018.02.030 [ Links ]
Liang, L., Zhu, F., Deng, P., Jia, Y., Kong, L., Deng, B., Li, K., and Liu, D. 2020. Separation and recycling of chloride salts from electrolytic titanium powders by vacuum distillation. Separation and Purification Technology, vol. 236. 116282. https://doi.org/10.1016/j.seppur.2019.116282 [ Links ]
Mackenzie, H.A.E. and Tompkins, F.C. 1942. The kinetics of the autoxidation of inorganic reducing agents. Part I.-Titanous chloride. Transactions of the Faraday Society, vol 38. pp. 465-473. https://doi.org/10.1039/tf9423800465 [ Links ]
McKinley, T.D. 1955. Recovery of titanium metal. US patent 2707149. [ Links ]
Montgomery, D.C. 2017. Design and Analysis of Experiments. 9th edn.. Wiley, Hoboken, NJ. [ Links ]
Nabivanets, B.I. 1965. The use of ion-exchange chromatography for studying the state of ions of high-valency elements in solution. Russian Chemical Reviews, vol. 34, no. 5. pp. 392-402. https://doi.org/10.1070/rc1965v034n05abeh001452 [ Links ]
Nechaev, N.P. and Polezhaev, E.V. 2016. Effect of physicochemical treatment on titanium porous powder quality. Metallurgist, vol. 60, no. 3-4. pp. 339-341. https://doi.org/10.1007/s11015-016-0296-5 [ Links ]
Nicholls, D. 2017. Complexes and First-Row Transition Elements. Macmillan, London, UK. [ Links ]
Park, S.H., Batchelor, B., Lee, C., Han, D.S., and Abdel-Wahab, A. 2012. Perchlorate degradation using aqueous titanium ions produced by oxidative dissolution of zero-valent titanium. Chemical Engineering Journal, vol. 192. pp. 301-307. https://doi.org/10.1016/j.cej.2012.04.013 [ Links ]
Pecsok, R.L. and Fletcher, A.N. 1962. Hydrolysis of titanium (III). Inorganic Chemistry, vol. 1, no. 1. pp. 155-159. https://doi.org/10.1021/ic50001a031 [ Links ]
Peter, W.H., Chen, W., Yamamoto, Y., Dehoff, R., Muth, T., Nunn, S.D., Kiggans, J.O., Clark, M.B., Sabau, A.S., Gorti, S., Blue, C.A., and Williams, J.C. 2012. Current status of Ti-PM: Progress, opportunities and challenges. Key Engineering Materials, vol. 520. pp. 1-7. https://doi.org/10.4028/www.scientific.net/kem.520.1 [ Links ]
Pourbaix, M.J.N. 1974. Atlas of Electrochemical Equilibria in Aqueous Solutions: Translated from the French except sections I, III 5, III 6 which were originally written in English. Franklin, J. (trans.). National Association of Corrosion Engineers, Houston, TX. [ Links ]
Richardson, J.F., Harker, J.H., Backhurst, J.R., and Coulson, J.M. 2002. Coulson and Richardson's Chemical Engineering. Vol 2. Butterworth-Heinemann, Oxford, UK. [ Links ]
Richens, D.T. 1997. The Chemistry of Aqua Ions. Wiley, Chichester, UK. [ Links ]
Sekimoto, H., Nose, Y., Uda, T., and Sugimura, H. 2010. Quantitative analysis of titanium ions in the equilibrium with metallic titanium in NaCl-KCl equimolar molten salt. Materials Transactions, vol. 51, no. 11. pp. 2121-2124. https://doi.org/10.2320/matertrans.M2010238 [ Links ]
Seon, F. and Nataf, P. 1988. Production of metals by metallothermia. US patent 4725312. [ Links ]
Serwale, M.R., Coetsee, T., and Fazluddin, S. 2020. Purification of crude titanium powder produced by metallothermic reduction by acid leaching. Journal of the Southern African Institute of Mining and Metallurgy, vol. 120, no. 5. pp. 349-354. [ Links ]
Shuvalov, V.F., SoloVev, S.L., and Lebedev, Y.S. 1978. ESR investigation of the hydrolysis of titanium(III) in aqueous hydrochloric acid solutions. Bulletin of the Academy of Sciences of the USSR Division of Chemical Science, vol. 27, no. 1. pp. 5-9. https://doi.org/10.1007/bf01153196 [ Links ]
Sole, K.C. 1999. Recovery of titanium from the leach liquors of titaniferous magnetites by solvent extraction: Part 1. Review ofthe literature and aqueous thermodynamics. Hydrometallurgy, vol. 51, no. 2. pp. 239-253. https://doi.org/10.1016/s0304-386x(98)00081-4 [ Links ]
Straumanis, M.E. and Chen, P.C. 1951. The corrosion of titanium in acids-The rate of dissolution in sulfuric, hydrochloric, hydrobromic and hydroiodic acids. Corrosion, vol. 7, no. 7. pp. 229-237. https://doi.org/10.5006/0010-9312-7.7.229 [ Links ]
Schweitzer, GK. and Pesterfield, L.L. 2010. The Aqueous Chemistry of the Elements. Oxford University Press, New York. [ Links ]
Van Vuuren, D.S., Oosthuizen, S.J., and Heydenrych, M.D. 2011. Titanium production via metallothermic reduction of TiCl4 in molten salt: Problems and products. Journal of the South African Institute of Mining and Metallurgy, vol. 111, no. 3. pp. 141-147. [ Links ]
Wang, Q., Song, J., Hu, G., Zhu, X., Hou, J., Jiao, S., and Zhu, H. 2013. The equilibrium between titanium ions and titanium metal in NaCl-KCl equimolar molten salt. Metallurgical and Materials Transactions B, vol. 44, no. 4. pp. 906-913. https://doi.org/10.1007/s11663-013-9853-5 [ Links ]
Withers, J.C., Shapovalov, V., Storm, R., and Loutfy, R.O. 2013. The production of titanium alloy powder. Key Engineering Materials, vol. 551. pp. 32-36. https://doi.org/10.4028/www.scientific.net/KEM.551.32 [ Links ]
Yakovleva, E.G., Pechurova, N.I., Martynenko, L.I., and Spitsyn, V.I. 1973. Study of the complex formation of Ti(III) with nitrilotriacetic and diethylenetriaminepentaacetic acids in aqueous solution. Bulletin of the Academy of Sciences of the USSR Division of Chemical Science, vol. 22, no. 8. pp. 1655-1657. https://doi.org/10.1007/bf00932086 [ Links ]
Yan, M., Tang, H.P., and Qian, M. 2015. Scavenging of oxygen and chlorine from powder metallurgy (PM) titanium and titanium alloys. Titanium Powder Metallurgy. Qian, M.A. and Froes, F.H. (eds). Butterworth-Heinemann, Oxford, UK. pp. 253-276. https://doi.org/10.1016/B978-0-12-800054-0.00015-0 [ Links ]
Yang, Z. and Gould, E.S. 2005. Reductions by aquatitanium(II). Dalton Transactions, vol. 10. pp. 1781-1784. https://doi.org/10.1039/b416975c [ Links ]
Yu, C.Z. and Jones, M.I. 2013. Investigation of chloride impurities in hydrogenated-dehydrogenated Kroll processed titanium powders. Powder Metallurgy, vol. 56, no. 4. pp. 304-309. https://doi.org/10.1179/1743290113y.0000000055 [ Links ]
Zhu, Z., Zhang, W., and Cheng, C.Y. 2011. A literature review of titanium solvent extraction in chloride media. Hydrometallurgy, vol. 105, no. 3-4. pp. 304-313. https://doi.org/10.1016/j.hydromet.2010.11.006 [ Links ]
 Correspondence:
Correspondence:
S. Fazluddin
Email: S.Fazluddin@csir.co.za
Received: 10 May 2022
Revised: 6 Nov. 2022
Accepted: 7 Nov. 2022
Published: December 2022














