Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.122 n.11 Johannesburg Nov. 2022
http://dx.doi.org/10.17159/2411-9717/241/2022
PROFESSIONAL TECHNICAL AND SCIENTIFIC PAPERS
Effect of zinc process water recycling on galena flotation from a complex sulphide ore
K. BoujounouiI; A. AbidiII; A. BaçaouiI; K. El AmariIII; D. HeIV; A. YaacoubiI
IFaculty of Sciences Semlalia, Department of Chemistry, Marrakech, Morocco. ORCID: A. Baçaoui: https://orcid.org/0000-0002-3867-2836
IIMining Institute of Marrakech (IMM), Marrakech, Morocco
IIIGeoresources Laboratory, Faculty of Sciences and Technologies Marrakech, Marrakech, Morocco
IVSchool of Resources & Safety Engineering, Wuhan Institute of Technology, Wuhan, P.R. China. https://orcid.org/0000-0002-6810-9171
SYNOPSIS
The effects of utilizing recycled tailings pond water from the flotation plant at the Mining Company of Guemassa (MCG) on galena recovery and selectivity towards chalcopyrite (RPb-Cu) , sphalerite (RPb-Zn), and pyrrhotite (RPb-Fe) were studied at bench scale. The results showed that recycling the tailings pond water in the lead circuit without addition of fresh water gave a good flotation performance in terms of lead recovery (Rpb) (75%) and selectivity towards the other metals: RPb-Cu (54%), RPb-Zn (60%) and RPb-Fe(65%). This allows the water to be recycled at least four times. However, increasing the d80 from 100 um to 160 um currently used at the MCG plant had a negative effect on the lead flotation performance.
Keywords: flotation, complex sulphide ore, process water recycling.
Introduction
In countries like Morocco that have a semi-arid climate, maximizing the recycling of process water, which could be both economically and environmentally beneficial, is a major challenge. Water recycling is an important aspect of sustainable management of the environment and water resources (Zeman, /Rich, and Rose, 2006; Orona et al. 2007; Hochstrat, Wintgens, and Melin, 2008; Mudd 2008). Especially as regards froth flotation, the mining industry is one of the most water-intensive industries, and this encourages greater use of recycled water in place of fresh water (McIntyre 2006; van der Bruggen 2010; Liu, Moran, and Fink, 2013; Molina et al. 2013). Recycling tailings pond water will clearly have a positive impact on the economics of industrial processes, because it reduces water cost and at the same time facilitates the recovery of unconsumed reagents retained in the tailings (Nedved and Jansz 2006; Slatter et al. 2009; Liu, Moran, and Fink, 2013; Molina et al. 2013). However, due to the accumulation of impurities in the pulp, suspended solids, the occurrence of adverse side reactions, bacterial oxidation of sulphide minerals, and decreased pH, recycling the water has an effect on its quality and disrupts flotation performance (Rao and Finch, 1989; Levay, Smart, and Skinner, 2001; N'gandu, 2001; Seke and Pistorius, 2006; Slatter et al., 2009; Muzenda 2010; Ikumapayi et al., 2012; Jing Xu et al., 2012; Deng, Liu, and Xu, 2013; Molina et al., 2013; Wang and Peng, 2014; Boujounoui et al., 2015, 2018, 2019; Wang et al., 2015. Some impurities present in the recycled water cause uncontrolled variations in the redox potential of the pulp, which has an adverse effect on the chemistry of the reagents and the flotation performance (Chadwick, 2007). These impurities also induce undesirable variations in the pulp properties, leading to alterations to the surface of the minerals and their floatability (Biçak et al., 2012; Dávila-Pulido et al., 2015).
The Mining Company of Guemassa (MCG) concentrator, located 30 km southwest of Marrakech (Morocco), uses selective flotation to successively produce concentrates of galena (using Aerophine A3418 at pH 11.3), chalcopyrite (using Aerophine A3418 at pH 8.9), and sphalerite (using potassium amyl xanthate, at pH about 12). The process water used consists mainly of fresh water from the mine site and the Lalla Takerkoust dam, which is located a few kilometres away from the plant. Owing to the complexity of ore processing, the only possible way to maintain the flotation plant performance is to re-use part of the zinc process water in the zinc circuit, the lead process water in the lead circuit, and the copper process water in the copper circuit. Production of zinc, lead, and copper concentrates at the MCG flotation plant in 2014 was 72 970, 13 812 and 16 755 t respectively (Managem Annual Report, 2014).
Previous work on sulphide ore flotation at MCG (Boujounoui et al., 2015, 2018) showed the need to control Cu2+, Zn2+, Mg2+, Ca2+, SO42-, and potassium amyl xanthate concentrations in the process water to maintain acceptable galena recovery in the presence of chalcopyrite, sphalerite, and pyrrhotite. These results, considering the scarcity of water in and around Marrakech, make process water recycling an alternative way of overcoming the problems of water management at the MCG plant. Some mining plants recycle up to 80% of their water (Atmacaand Kuyumcu, 2003), although the recycle rate does not exceed 34% for MCG.
Three water sources were used to supply 4 900 m3daily for this production; 25% from the dam at Lalla Takerkouste, located a few kilometers from the plant, 50% from mine dewatering and groundwater, and 25% from tailings pond water (TPW) recycled in the zinc circuit (Figures 1 and 2) (Boujounoui, 2017).
The aim of this study was to assess the effects of using recycled TPW on galena recovery in the MCG flotation plant and the selectivity towards chalcopyrite, sphalerite, and pyrrhotite. Tests were carried out according to the results of Boujounoui et al., (2018), who used a synthetic solution to simulate the industrial process water at MCG. These results showed a water quality limit to not exceed the specification in Table IV.
Flotation tests were performed using mixtures of fresh water and TPW produced by the flotation plant. Further flotation experiments were performed on the optimal water mixture obtained by increasing the d80 particle size to 160 um, the size currently used in the lead circuit at the plant.
Climatology of the site
According to the Agency of Basin Haouz Tensift (ABHT), the data on climatic parameters collected at the meteorological station at the Lalla Takerkoust dam from 1962 to 2009, particularly the data on pluviometry, temperature, and evaporation, highlights the need to recycle industrial water at the MCG plant. The Lalla Takerkoust dam is mainly filled by snowmelt from the High Atlas Mountains of Morocco. Snowfall correlates positively with rainfall in the area, and therefore the use of dam water and underground water in the flotation process at the MCG plant has to be carefully managed to preserve water resources in the area. The climatological data (Figure 3) reveals the following.
> Generally, rainfall is low and irregular (about 250 mm/a). Inter-annual rainfall is also irregular, with a maximum of 424 mm in 1970 and a minimum of 106 mm in 1982. The mean monthly rainfall variation over the same period shows two distinct seasons: a rainy season (November to April) and a dry season (May to October), with average total rainfalls of 187 and 67 mm respectively.
> The variation in the average monthly temperature
recorded from 1985 to 2008 show three distinct periods: a very hot period (June to September), a temperate period (October to May), and a relatively cold period (December to February). The temperature can reach 48°C in August and fall below zero in December.
> The trend in evaporation correlates with the temperature: the highest evaporation rates are linked to the hottest season of the year, and consequently both water from the Lalla Takerkoust dam and the rainfall during the hot season are drastically affected by evaporation.
Experimental
Materials
Bench-scale flotation tests were carried out on a representative sample of the complex sulphide ore from the Draa Sfar mine (Morocco) processed by MCG. The sulphide ore used was composed of 6.43% sphalerite (ZnS), 2.22% galena (PbS), 0.95% chalcopyrite (CuFeS2), 41.57% pyrrhotite (Fe9S10), and 48.82% gangue, consisting mainly of quartz, talc, chlorite, calcite, siderite, and ankerite (Abidi et al., 2014; Boujounoui et al., 2015, 2018, and 2020).
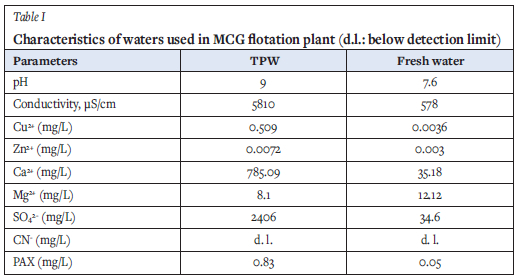
The industrial process water used in these tests consisted of tailings pond water (TPW) mixed with fresh water (Table I). TPW and the flotation reagents (sodium cyanide, Aerophine 3418A, and methyl isobutyl carbinol) were provided by MCG.
Methods
Solid sample preparation
A representative sample of 128 kg was taken from the feed belt to the primary ball mill at the MCG flotation plant and crushed down to 2 mm using a laboratory roll crusher. The sample was then divided into 1 kg batch samples for the flotation experiments. These batch samples were stored in vacuum-sealed bags to prevent the sulphide minerals from oxidizing.
Prior to each flotation test, a sample of 500 g was milled in 250 ml of process water using a Denver carbon steel ball mill with an internal volume of 9.5 l for 6 to 10 minutes, depending on whether the target grain size was 160 pm or 100 pm.
Water sample preparation
Four-litre samples of industrial water were prepared by mixing TPW with fresh water in proportions of 100, 90, 75, 65, 50, 40, 25, 15, and 0% TPW. Each test was repeated three times. The quality of the different mixtures was calculated from the individual analyses of the fresh water and TPW given in Tables I and II.
Flotation experiments
Flotation tests of galena were carried out in a Denver flotation cell of 1.5 l capacity. Solid concentration was about 27% by weight, using mixtures of TPW and fresh water at different proportions.
The natural pH was about 7. NaOH was used in all tests to adjust the PH value to 11.3. Sodium cyanide (NaCN) was used as a depressant for sphalerite, chalcopyrite, and pyrrhotite for all tests at a specific dosage of 350 g/t. Diisobutyl phosphinate (Aerophine 3418A) (40 g/t) and methyl isobutyl carbinol (MIBC) (40 g/t) were used as galena collector and frother respectively. The impeller rotation speed was a constant 1000 r/min. The level of the pulp was constantly adjusted by the addition of water at the required quality. The flotation time was 10 minutes for each test, and the concentrates were recovered by automatic scraping every 30 seconds
All concentrates and tails were filtered, dried, weighed and then analysed by atomic absorption spectroscopy (AGILENT 280FS) for Cu, Pb, Zn, and Feat in the laboratory at the Reminex Center (Morocco). Metal recoveries to the concentrates were calculated from the following equation:

where R (%) is the metal recovery, tc (%) is the grade of the concentrate metal, f (%) is the grade of the feed metal, C is the concentrate weight, and A is the feed weight. The proportions of iron combined with chalcopyrite were taken into account in the calculations of iron sulphide recoveries. Lead selectivity was calculated as the difference between lead recovery and the recoveries of the other metals.
Results and discussion
The optimal results of water quality, for galena recovery, were obtained with a synthesized water simulating industrial TPW (Boujounoui et al., 2018). These results indicate that fresh water usage could be reduced in the lead circuit at the MCG plant by substitution with water from the tailings pond.
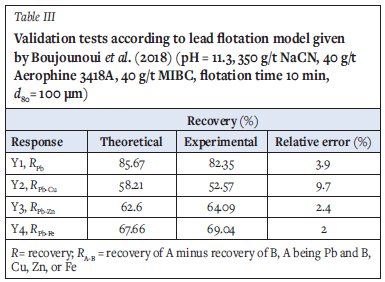
Prior to considering these results as a reference (limit of water quality to not exceed) for the lead flotation circuit, in which the process water contained 5 mg/L Cu2+, 13 mg/L Zn2+, 1390 mg/L Ca2+, 140 mg/L Mg2+, 4130 mg/L SO42-, and 13 mg/L PAX, three validation tests were performed using these optimal operating conditions. The results given in Table III verified the mathematical model proposed for lead recovery by Boujounoui et al., (2018) and showed that as long as the process water quality is close to the reference, the lead flotation performance was maintained.
Flotation tests using tailings pond water
Bench-scale flotation tests were conducted on galena with various proportion of TPW from zero to 100% to optimize its proportion in the lead circuit. The results would help in assessing the recycling ratio without affecting the lead flotation performance. Table II presents the water qualities used and their relationship with the reference water quality obtained by Boujounoui et al., (2018). It can be deduced from the data that 100% TPW is below the maximum limits for all constituents and could be successfully used as process water.
The results presented in Table IV show that the variation in water quality had no significant effect on the recovery of Pb and selectivity over Zn and Fe. However, the selectivity towards copper was adversely affected due to chalcopyrite activation by copper ions (Deng et al., 2014) and their interactions with calcium and PAX (Boujounoui et al., 2018). The best recovery of lead was 82% obtained at 15% TPW and the best selectivities towards copper (54%), zinc (61%) and iron (70%) were obtained at 100%, 90%, and 75% TPW respectively. Nonetheless, according to the objective of best flotation performance and maximum water process recyclability, the optimal proportion of TPW that can be recycled to the lead circuit is 100%.This proportion allowed 75% of Pb to be recovered, with galena retaining good selectivity over the other minerals: RPb-Cu (54%), RPb-Zn (60%) and RPb-Fe (65%). This result confirmed the robustness of the mathematical model used, which states that the closer the water process to the reference quality (limit to not exceed), the better the performance.
Based on the robustness of this model, four successive recycling stages using 100% TPW were performed, with the focus on the evolution of the process water quality after each stage with no need to determine the flotation performances. The results given in Table V show that most of the process water was recycled; the best performance was for galena flotation, except for selectivity over chalcopyrite which remained relatively constant. After four recycling stages with 100% TPW, the water quality was still within the quality limits required for the lead circuit (water quality reference). This means that tailings pond water can be recycled at least four times, as long as chalcopyrite activation is controlled.
Lead flotation using industrial grain size
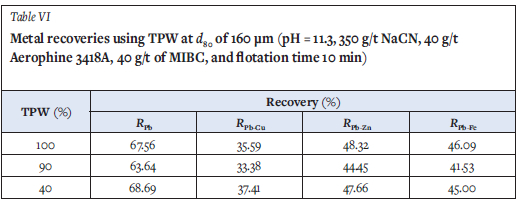
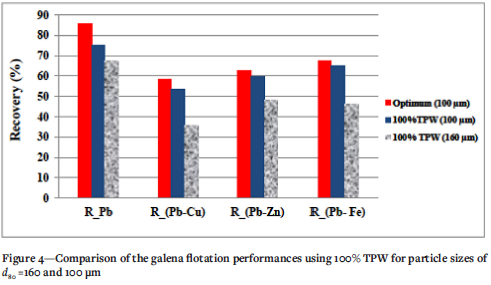
Because a d80 grain size of 160 μm is currently used in the lead circuit at the MCG flotation plant, further tests were carried out to assess the effect of increasing the particle-size from 100 to 160 μm on the lead flotation performance. Experiments were then carried out under MCG plant operating conditions using 100, 90 and 40% TPW. The results presented in Table VI and Figure 4 show that the flotation performance in terms of galena recovery and selectivity was not affected by the process water quality. However, comparison with the results in Table IV shows that, the increase in the ore grain size adversely affected the lead flotation performance. The lead recovery decreased from 75% to 68%, and its selectivity over Cu, Zn, and Fe, decreased from 54% to 36%, 60% to 48% and 65% to 46% respectively. These results confirmed those of Boujounoui et al., (2015) in which particle-size adversely affected galena selectivity.
Conclusion
Our study on the effects of recirculating MCG tailings pond water on galena recovery and its selectivity towards Cu, Zn, and Fe revealed the following.
> The variation in water quality had no significant effect on the lead recovery and its selectivity over zinc and iron, but adversely affected the Pb-Cu selectivity.
> Recycling tailings pond water without mixing with any fresh water is possible. At least four recycling stages can be used if a d80 particle size of 100 μm is adopted.
> Increasing the particle size from 100 to 160 μm had a negative effect on the performance of Pb flotation.
Supplementary work needs to be performed to compare the cost savings gained by the reduced use of fresh water with the increase in energy consumption on an industrial scale from reducing the particle size from 160 to 100 μm. Environmental concerns and water scarcity in the region of the MCG plant must also be considered.
Acknowledgments
The authors thank the Mining Company of Guemassa for providing the sulphide ore sample, flotation reagents, and tailings pond water, and Reminex Society for the chemical analyses.
References
Atmaca, T. and Kuyumcu, H.Z. 2003. Investigations on water consumption in mineral processing plants. Erzmetall, vol. 56, no. 9. pp. 558-562. [ Links ]
Abidi, A., Elamari, K., Bacaoui, A., and Yacoubi, A. 2014. Entrainment and true flotation of a natural complex ore sulphide. Journal of Mining Sciences, vol. 50. pp. 1061-1068. [ Links ]
Abidi, A., Boujounoui, K., Bacaoui, A., El Amari, and K., Yaacoubi, A. 2019. Contribution to improve water process recycling in the flotation plant of a complex Zn-Pb-Cu sulphide ore. Journal of Mining Sciences, vol. 55, no. 4. pp. 658-667. [ Links ]
Bicak, O., Ekmekci, Z., Can, M., and Ozturk, Y. 2012. The effect of water chemistry on froth stability and surface chemistry of the flotation of a Cu-Zn sulfide ore. International Journal of Mineral Processing, vol. 102-103. pp. 32-37. [ Links ]
BoujouNOui, K., Abidi, A., Bacaoüi, A., El Amari, K., and Yaacoubi, A. 2015. The influence of water quality on the flotation performance of complex sulphide ores: case study at Hajar Mine. Morocco. Journal of the Southern African Institute of Mining and Metallurgy, vol. 115. pp. 1243-1251. [ Links ]
BoujouNoui, K. 2017. Optimisation du potentiel de recyclage de l'eau de procédé dans les circuits de flottation des minerais sulfurés complexes : Cas de Draa Lasfar Sud (Maroc). Thesis, Université Caddi Ayyad, Faculté des Sciences Semlalia, Maroc. [ Links ]
BoujouNoui, K., Abidi, A., Bacaoui, A., El Amari, K., and Yaacoubi, A. 2018. Flotation process water recycling investigation for the complex Draa Sfar sulphide ore, Morocco. Mine Water and the Environment, vol. 37, no. 1. pp. 75-87. https://doi.org/10.1007/s10230-017-0471-3 [ Links ]
BoujouNoui, K., Abidi, A., Bacaoui, A., El Amari, K., and Yaacoubi, A. 2019. Effect of water quality on the performance of the galena and blend flotation: Case of Draa Sfar complex sulphide ore. Moroccan Journal of Chemistry, vol. 7, no. 2. pp. 337-345. [ Links ]
Boujounoui, K., Abidi, A., Bacaoui, A., El Amari, K., and Yaacoubi, A. 2020. Optimization of chlorite and talc flotation using experimental design methodology: Case study of the MCG plant, Morocco. Journal of the Southern African Institute of Mining and Metallurgy, vol. 120, no. 12. pp. 693-700. [ Links ]
Chadwick, J. 2007. Water management: Minimizing water use and its pollution, International Mining. pp. 38-47. [ Links ]
Chandra, A.P. and Gerson, A.R. 2009. A review of the fundamental studies of the copper activation mechanisms for selective flotation of the sulfide minerals, sphalerite and pyrite. Advances in Colloid and Interface Science, vol. 145. pp. 97-110. [ Links ]
Deng, M., Liu, Q., and Xu, Z. 2013. Impact of gypsum supersaturated water on the uptake of copper and xanthate on sphalerite. Minerals Engineering, vol. 49. pp. 165-171. [ Links ]
Deng, J., Wen, S., Liu, J., Wu, D., and Feng, Q. 2014. Adsorption and activation of copper ions on chalcopyrite surfaces: A new viewpoint of self-activation. Transactions of Nonferrous Metals Society of China, vol. 24. pp. 3955-3963. [ Links ]
Dávila-Pulido, G.I., Uribe-Salas, A., Álvarez-Silva, M., and López-Saucedo, F. 2015. The role of calcium in xanthate adsorption onto sphalerite. Minerals Engineering, vol. 71. pp. 113-119. [ Links ]
Hochstrat, R., Wintgens, T., and Melin, T. 2008. Development of integrated water reuse Strategies. Desalination, vol. 218. pp. 208-217. [ Links ]
Ikumapayi, F., Makitalo, M., Johansson, B., and Rao, K.H. 2012. Recycling of process water in sulphide flotation: Effect of calcium and sulphate ions on flotation of galena. Minerals Engineering, vol. 9. pp. 77-88. [ Links ]
Jing, X., Runqing, L., Wei, S., Yuehua, H., and Jingping, D. 2012. Effect of mineral processing wastewater on electrochemistry of galena. Journal of Environmental Science and Engineering, vol. A1. pp. 279-285. [ Links ]
Liu, W., Moran, C.J., and Vink, S. 2013. A review of the effect of water quality on flotation. Minerals Engineering, vol. 53. pp. 91-100. [ Links ]
Levay, G., Smart, R.S.C., and Skinner, W.M. 2001. The impact of water quality on flotation performance. Journal of the Southern African Institute of Mining and Metallurgy, vol. 101. pp. 69-76. [ Links ]
McIntyre, J.P. 2006. Industrial water reuse and wastewater minimization, GE Water and process technologies technical report, General Electric Company, pp. 1-7. [ Links ]
Mudd, G.M. 2008. Sustainability reporting and water resources: A preliminary assessment of embodied water and sustainable mining. Mine water and environment, vol. 27. pp. 136-144. [ Links ]
Muzenda, E. 2010. An investigation into the effect of water quality on flotation performance. World Academy of Science, Engineering and Technology, vol. 69. pp. 237-241. [ Links ]
Molina, G.C., Cayo, C.H., Radrigues, M.A.S., and Bernades, A.M. 2013. Sodium isopropylxanthate degradation by advanced oxidation processes. Minerals Engineering, vol. 45. pp.88-93. [ Links ]
Managem Group, 2014. Annual Report. Casablanca, Morocco. [ Links ]
N'gandu, D.E. 2001. The effect of underground mine water on performance of the Mufulira flotation process. Journal of the Southern African Institute of Mining and Metallurgy, vol. 101. pp. 367-380. [ Links ]
Nedved, M. and Jansz, J. 2006,. Waste water pollution control in the Australian mining Industry. Journal of Cleaner Production, vol. 14. pp. 1118-1120. [ Links ]
Orona, G., Gillermana, L., Bickd, A., Mnaore, Y., Buriakovskya, N., and Haginc, J. 2007. Advanced low quality waters treatment for unrestricted use purposes: Imminent challenges. Desalination, vol. 213. pp.189-198. [ Links ]
Rao, S.R. and Finch, J.A. 1989. A review of water re-use in flotation. Minerals Engineering, vol. 2. pp. 65-85. [ Links ]
Seke, M.D. and Pistorius, P.C. 2006. Effect of cuprous cyanide. dry and wet milling on the selective flotation of galena and sphalerite. Minerals Engineering, vol. 19. pp. 1-11. [ Links ]
Slatter, K.A., Plint, N.D., Cole, M., Dilsook, v., de Vaux, D., Palm, N., and Oostendorp, B. 2009. Water management in Anglo Platinum process operations: Effects of water quality on process operations, [Abstract] Proceedings of the International Mine Water Conference, Pretoria, South Africa, 19-23 October. International Mine Water Association. pp. 46-55. [ Links ]
Van der Bruggen, B. 2010. The global water recycling situation. Sustainability Science and Engineering, vol. 2. pp. 41-61. [ Links ]
Wang, B. and Peng, Y. 2014. The effect of saline water on mineral flotation - A critical review. Minerals Engineering, vol. 66-68. pp. 13-24. [ Links ]
Wang, L., Peng, Y., Runge, K., and Bradshaw, D. 2015. A review of entrainment: Mechanisms. contributing factors and modelling in flotation, Minerals Engineering, vol. 70. pp. 77-91. [ Links ]
Zeman, C., Rich, M., and Rose, J. 2006. World water resources: Trends challenges and Solutions. Reviews in Environmental Science and Bio/Technology, vol. 5. pp.333-346. [ Links ]
 Correspondence:
Correspondence:
K. Boujounoui
Email: boujounoui.kh@gmail.com
Received: 28 Nov. 2019
Revised: 2 Dec. 2019
Accepted: 1 July 2022
Published: November 2022














