Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.122 n.10 Johannesburg Oct. 2022
http://dx.doi.org/10.17159/2411-9717/2070/2022
PROFESSIONAL TECHNICAL AND SCIENTIFIC PAPERS
Evaluation of pre-treatment methods for gold recovery from refractory calcine tailings
P. Mutimutema; G. Akdogan; M. Tadie
Department of Process Engineering, Stellenbosch University, South Africa. ORCID: M. Tadie: https://orcid.org/0000-0003-3111-5188
SYNOPSIS
The South African gold mining industry has a legacy of abundant tailings dams, which have attracted the attention of investors because of their potential as a cheaper secondary gold resource. In this we investigate study gold recovery from a refractory calcine tailings dam. Bulk mineralogy of the tailings indicated silicates and iron oxides to be the most abundant phases. Scanning Electron Microscopy (SEM) showed gold to exist in submicrometre and micrometre sizes, as free gold, and associated with arsenic, sulphur, and silicates e.g. quartz and talc. Gold recovery by direct cyanidation was low at 17.3%. Mechanical (ultra fine grinding P80 -16µm) and chemical (alkaline, NaOH) pre-treatment and microwave roasting and microwave-assisted cyanide leaching were investigated to increase gold recoveries. Ultrafine grinding was the most effective, producing recoveries of 66.5%. NaOH pre-leaching of ultrafine milled material increased recovery to 71.5%. Alkaline pre-leaching overall increased recoveries for non-pre-treated material, making this process the most preferred because it is less costly than ultrafine grinding. Microwave roasting and microwave-assisted leaching did not achieve higher recoveries than alkaline pre-treatment or fine grinding. The investigation highlights and confirms that chemical treatment with NaOH is a powerful tool for gold extraction from refractory tailings.
Keywords: gold recovery, refractory calcine tailings, ultrafine grinding, alkaline pre-treatment, microwave.
Introduction
South Africa has over 500 gold tailings dumps, several of which have the potential to be profitably reprocessed (Janse van Rensburg, 2016). Gold lost to the dumps is attributed to inherent process inefficiencies. Historically and up to 1993, gold lost annually to tailings in South African mines was considered to equate to R8.5 billion, indicating a significant amount of value present in tailings (Metzner, 1993).
The reprocessing of tailings presents an opportunity, via modern technologies, for low-cost recovery of gold. Such activities can be used to supplement revenue for existing operations. Reprocessing tailings for gold recovery can be complex and is dependent on the mineralogy of the dump, which is in turn related to the original orebody characteristics or prior processing. Prior processing of tailings adds to the complexity of the mineralogy, which is either refractory and non-refractory. Typical refractory ores have a high sulphide content, such as those found in the Barberton greenstone belt. High-sulphide ores have been processed in the past by methods such as roasting and calcination. These processes oxidize sulphur species at high temperatures, liberating gold from cyanide-consuming sulphur matrices. High energy costs and SO2 emissions associated with these processes have led to their discontinuation.
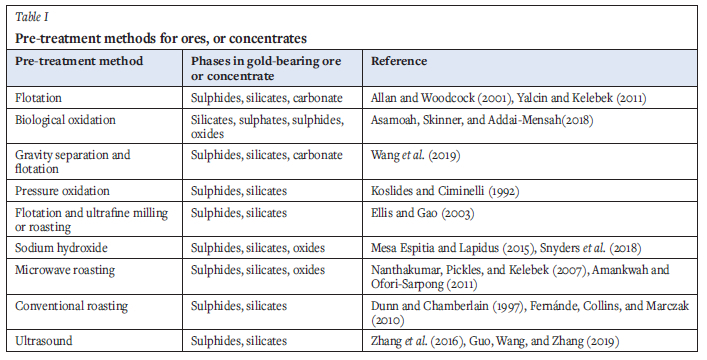
Research is ongoing for pre-treatment methods that can be applied to aid gold recovery by cyanidation. Roasting, pressure oxidation (acidic and alkaline), biological oxidation, chlorination, microwaves, and ultrafine grinding have been applied to process refractory ores (Fraser, Walton, and Wells, 1991). Table I shows several pre-treatment methods for gold ores or concentrates. Flotation, biological oxidation, gravity separation, pressure oxidation, ultrafine milling, and sodium hydroxide pre-leach are applied commercially in metallurgical plants (Anderson and Twidwell, 2008; Anderson and McDonald, 2016; Das and Sarkar, 2018). Microwaves are being investigated at the pilot plant level by the University of Nottingham's microwave process engineering department (Buttress et al., 2017). The use of ultrasound is also being considered (Wang, and Zhang, 2019).
In this research we investigated through gold recovery an analysis of the tailings mineralogy and application of different pre-treatment methods. The aim was to unlock gold from the complex mineralogy and formulate relationships between the prior treatment of the tailings and the gold extraction behavior. Several potentially viable pre-treatment methods were considered as options, i.e. ultrafine milling as a mechanical technique, sodium hydroxide pre-leach as a chemical technique, and microwave technology (González-Anaya, Nava-Alonso, and Pecina-Treviño, 2011; Nanthakumar, Piddes, an Kelebode, 2007; Snyders et al., 2018). These pre-treatment methods were factored in as a mechanism to circumvent any challenges encountered due to the previous roasting and the associated refractory nature of the calcine tailings. Heating using microwave energy was explored to demonstrate the energy requirements to unlock gold by the lixiviant at normal operating conditions.
Experimental methods
Material
100 kg of calcine tailings was obtained from a tailings dump in South Africa. The tailings were blended and divided into representative aliquots through cone and quartering, followed by riffle splitting, and finally rotary splitting to obtain 500 g samples.
Sample characterization
The tailings were characterized using multiple techniques to determine bulk mineralogy gold content and associations, as well as trace element chemistry. Figure 1 summarizes the different techniques applied to sample characterization and the nature of the information obtained.
Grade and particle size
Nine 100 g samples were analysed by fire assay to determine the head grade. Fire assay results showed an average head grade of 2.96 ± 0.26 g/t, a high grade relative to some run-of-mine operations. The tailings had a P80 of 56 µm, determined using a Micromeritics Saturn Digisizer 5200 laser diffraction particle size analyser.
Chemical analysis
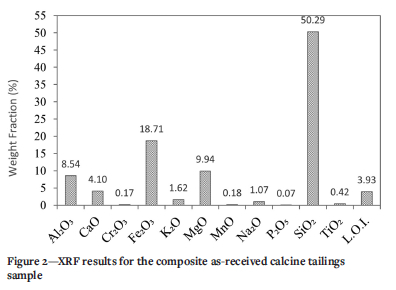
X-ray fluorescence spectroscopy (XRF) was conducted using a PANalytical Axios wavelength-dispersive spectrometer for chemical analysis. Silicon and iron were the major elements in the sample (Figure 2). Significant amounts of sodium, magnesium, potassium, and aluminium were detected. Loss on ignition (LOF; represents the elements (sulphur, arsenic, etc.) that were volatized during analysis.
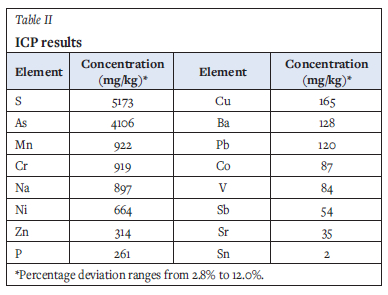
Trace element analysis was conducted on three composite samples. Samples were digested using six parts nitric acid to one part hydrogen peroxide in a MARS microwave digester. The solutions were analysed using an Agilent 7900 inductively coupled plasma mass spectrometry (ICP-MS) instrument and a Thermo ICap 6200 inductively coupled plasma optical emission spectrometry (ICP-OES) instrument. Results shown in Table II indicate arsenic and sulphur in appreciable quantities, 4106 ± 246 mg/kg and 5 173 ± 620 mg/kg respectively.
Bulk mineralogy
Quantitative X-ray diffraction (QXRD) was performed to determine bulk mineralogy. The sample was analysed with a PANalytical Aeris diffractometer with a pixcel detector and fixed slits with Fe-filtered Co-Ka radiation. Phases were identified using X'Pert Highscore plus software.
Gold particle size and associations were investigated using scanning electron microscopy (SEM)using a Zeiss MERLIN field emission scanning electron microscope. A Zeiss 5-diode back-scattered electron (BSE) detector (Zeiss NTS BSD) and Zeiss SmartSEM software generated BSE images. An Oxford Instruments X-Max 20 mm2 detector attached to the SEM and Oxford Aztec software were used to chemically quantify the samples by semi-quantitative energy dispersive X-ray spectrometry (EDS) and generation of corresponding EDS maps,
Finally, quantitative scanning electron microscopy (QEMSCAN) analysis was performed on the calcine sample. 500 g of the sample was screened into four particle size ranges; +75 µm, -75+38 µm, -38+25 µm, and -25 pm. Two polished sections were prepared for each size fraction. The sections were analysed by a QEMSCAN 650F with two Bruker EDS detectors. Bulk mineral analysis and trace minerals were measured in each section.
Leaching tests
Leaching tests were conducted using a 24-hour bottle roll with sodium cyanide as lixiviant. Unless otherwise stated, the cyanide addition was 2 kg/t. A solids density of 30% solids was used for the leaching tests. Lime was added to adjust the pH 10.5. Liquid samples were collected at 1-hour, 2-hour, and 24-hour intervals and analysed for gold using ICP-OES. The bottle roll tails were water-washed and filtered before oven drying at 50°C. Dry tailings samples were sent for fire assay.
Pre-treatment methods
Ultrafine milling
Ultrafme milling was performed using a pulverizer. A 100 g sample was pulverized for 120 seconds to achieve a P8o of 16 µm.
Sodium hydroxide pre-leach
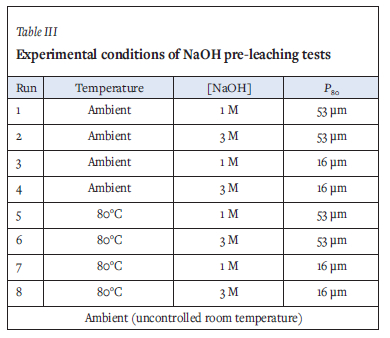
Analytical-grade sodium hydroxide was used for chemical pre-treatment. The pre-leach was conducted at 25% solids concentration (Gonzalez-Anaya, Nava-Alonso, and Pecina-Treviho, 2011) for 4 hours. Pre-leaching was conducted in a top-driven stirred glass vessel with temperature-controlled heater at the base of the vessel. Solution samples were collected after 4 hours and analysed for Au, As, Al, Fe, S, Si, and Zn using IGP-MS. Solid tails were washed with deionized water, filtered, and dried in an oven at 50°G before preparation for cyanidation tests. Cyanidation tests were conducted using the methodology described later. A full factorial design was conducted at two levels to investigate the effects of temperature, [NaOH], and particle size. Table III shows the levels and conditions investigated.
Microwave treatment
Microwave energy was used to facilitate the breakdown of refractory mineral matrices to liberate gold before and during leaching experiments. Microwaves were used as an energy source due to their ability to apply a high energy density over a short period of time. The experiments are a demonstration of the energy required, or thermodynamic thresholds to be broken, in order to circumvent the chemical and physical barriers created by prior processing of these tailings. The power densities applied during the experiments are not reflective of economic conditions for the application of industrial microwave technology to these ores, and the results are not interpreted as such.
Microwave roasting
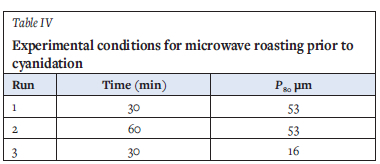
A 1000 W, 2.45 GHz Samsung domestic microwave oven was used for the experiments. A standalone beaker containing 300 ml of water was included in the apparatus to prevent excessive reflection of microwaves, thereby protecting the magnetron. The energy received by the sample was therefore not 100% of the energy dissipated by the microwave device. 60 g of the feed was used for the microwave roasting experiments. Roasting experiments were conducted under two conditions, with moisture in sample and without moisture, for a total roasting time of 20 minutes.
Variables considered in these tests are summarized in Table IV Leaching of the roasted material followed the same procedure and conditions as described previously.
Microwave-assisted leaching
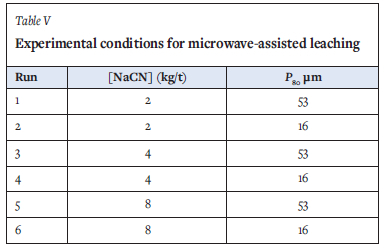
A 1000 W, 2450 MHz domestic microwave oven was modified to accommodate an overhead stirrer and 1 L reactor vessel. The microwave unit was insulated and operated in an insulated room for safety. A liquid-to-solids ratio of 6:1 was used for the leaching tests. The pH was set at 10.5, and the pulp was allowed to condition in the reactor for 3 minutes prior to addition of NaCN. Leaching was conducted for 50 minutes. Liquid samples were extracted at intervals of 10,30 and 50 minutes via a sampling port. At the end of each experiment, the microwave unit was switched off and the pulp was allowed to cool before washing, filtering, and oven-drying. Variables tested under microwave-assisted leaching included the cyanide concentration and the particle size distribution, as provided in Table V.
Results and discussion
Gold deportment and bulk mineralogy
XRD, QEMSCAN, and SEM with EDS were used to develop an understanding of the occurrence of gold in the sample and relevant mineral associations that might constitute a barrier to extraction. The bulk mineralogy, therefore, played a critical role in identifying and quantifying cyanides as well as understanding the alterations in the sample due to calcination.
X-ray diffraction analysis
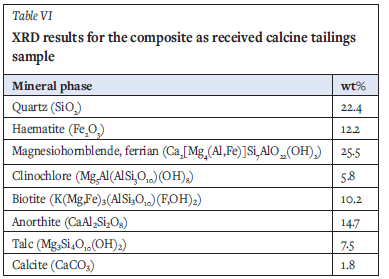
XRD analysis was performed to determine the crystalline phase constituents. Results (Table VI) indicate that the major nonmetallic and metallic oxides in the sample are silica and haematite, together making up 37% of the sample. It is proposed that haematite in the calcine tailings is a roasting product of minerals such as pyrite and arsenopyrite in the original ore. Magnesiohornblende-ferrian, biotite, clinochlore, anorthite, and talc are characteristic of minerals originating from amphibolite-facies minerals occurring in the host orogenic gold deposit. These minerals, together with quartz, constitute 8696 of the ore. Base metal sulphides were below detection limit, probably due to alteration during calcination. Table II, however, shows sulphur and arsenic content to be as much as 5.2 g/kg and 4.1 g/kg respectively, indicating the presence of some of these base metals.
QEMSCAN analysis
QEMSCAN results are is shown in Figures 3 and 4 for the bulk mineralogy and the base metal sulphide analysis. The predominant phases are confirmed to be silicates (quartz, feldspar, amphibole and mica) and iron oxides that are a product of the roasting process and from the original orebody. Gold particle size was below the detection limit for the QEMSCAN, indicating the fineness of the gold that is typical in tailings.
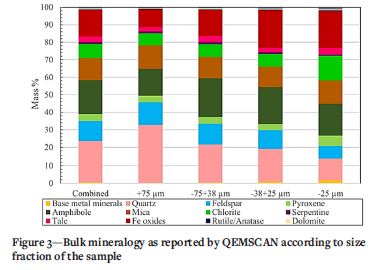
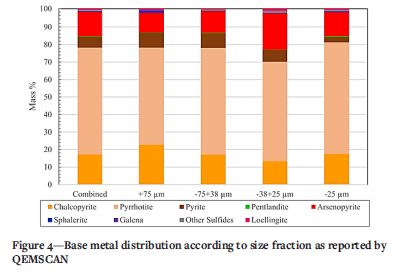
Base metal sulphide phases (Figure 4) were identified as predominantly pyrrhotite, arsenopyrite, chalcopyrite, and pyrite. Although Fe oxides (Figure 3) which constitute up to 12 96 of the tailings, include sulphides altered from roasting and from prolonged exposure to weathering on the dump, the bulk of the Fe oxides is likely to have originated from the host orebody, since they are present in appreciable amounts.
Scanning electron microscopy
To determine the gold associations in the sample, SEM was used to detect gold particles, and EDS analysis around the targeted gold particles was used to determine their elemental associations. Based on the known bulk mineralogy, conclusions were drawn to identify the gold associations within these samples. Figure 5 shows SEM images of different gold particles from the sample along with chemical overlay maps from EDS analysis.
Figure 5a shows gold occluded in a silicate matrix, probably quartz, which is abundant in the sample. In Figure 5b a gold particle is associated with sulphur and arsenic in a silicate matrix. The absence of iron in the sulphur and arsenic association with gold is likely due to incomplete arsenopyrite oxidation during roasting of the host ore. The presence of Mg and Si in the silicate matrix also suggests that the mineral is an amphibole like talc. In Figure 5c a free gold particle is identified. It is therefore evident that the maximum recovery from the sample depends on effective pre-treatment strategies to release trapped gold.
Mineralogical implications for processing
Ultrafine milling does not effectively unlock gold-bearing particles smaller than 1 µm and therefore additional pre-treatment would be necessary to either complement or replace ultrafine grinding in liberating gold (Corrans and Angove, 1991; Harbort et al, 1996). An economic energy input for such an operation will be a strong determinant for proceeding with this strategy.
Gold occluded within quartz is a cause of refractoriness since quartz consitutes a barrier for lixiviant contact. Occlusion of gold in quartz is also known to be typical of calcine (Yannopoulus, 1991), resulting from mobilization of gold into molten quartz during roasting. Liberation of such gold is achieved by both mechanical (fine grinding) and chemical strategies (alkaline pre-treatment). NaOH leaches the quartz matrix and renders the gold accessible to the lixiviant (Crundwell, 2014; Snyders et al, 2018).
Gold associated with talc has similar effects to quartz, sulphur, and arsenic. Sulphur is a known oxygen and cyanide consumer, and the primary motivation for calcination. Alkaline pre-treatment may reduce the amount of arsenic, sulphur and talc present in the sample (Darban et al, 2011; Crundwell, 2014; Mesa Espitia and Lapidus, 2015; Snyders et al, 2018).
When the mineralogical barrier constitutes a thermodynamic threshold that cannot be readily crossed by conventional processing methods, alternative techniques with higher energy intensity are necessary. The presence of sulphide phases in this sample is a potential advantage for the application of microwaves as these minerals are known irradiation absorbers and far more effective than quartz and talc (Haque, 1999; Amankwah and Ofori-Sarpong, 2011). Thermal stresses and differential heating responses are a therefore a potential advantage of microwaves and effect the physical unlocking of encapsulated gold. Microwave-assisted leaching, on the other hand, may facilitate specific heating of minerals associated with gold and thus increase the kinetics of dissolution.
Direct cyanidation
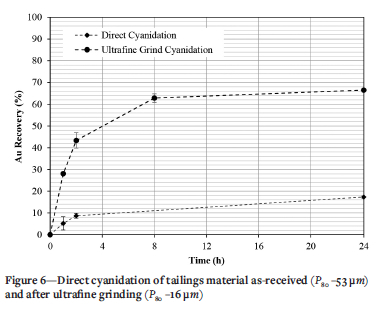
Figure 6 shows the results for direct cyanidation of the tailings after ultrafine grinding that without pre-treatment. The refractory nature of the tailings is indicated by a gold recovery after of 17.3 ± 0.54% 24 hours. The low recovery classifies the calcine tailings in the category of highly refractory material. This low recovery confirms of the need for pre-treatment to liberate occluded gold prior to cyanidation. Ultrafine grinding increases recoveries by up to three times (66.5 ± 0.87%) compared with direct cyanidation of the as-received material (Yannopoulos, 1991). It is clear that the gold occurs in this sample as fine gold which is liberated at particle sizes corresponding to those of the material after further grinding.
Alkaline (NaOH) pre-treatment
NaOH pre-leaching has been demonstrated to be a useful hydrometallurgical technology for the release of encapsulated precious metals (Snyders et al, 2018). Results after 4 hours of pre-leaching and subsequent cyanidation for 24 hours are presented in Figure 7.
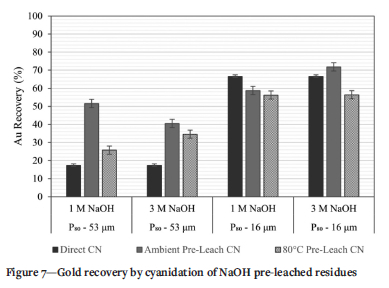
The as-received tailings material (p80-53 µm) responded differently to NaOH pre-leaching, with a low concentration of NaOH (lM) producing higher recovery at ambient conditions, 51.6%, compared to a higher concentration (3 M NaOH) which resulted in a Au recovery of 40.5%. Both conditions improved recovery from direct cyanidation without any pre-treatment (17.3%), indicating the effectiveness of chemical pre-treatment in releasing gold from silicate matrices and arsenic- and sulphur-bearing material.
At ambient conditions, the recovery of gold from finely ground tailings material was lower than that without pre-treatment, especially at NaOH concentrations of 1 M and 3 M. Pre-leaching at 3 M NaOH under ambient conditions, however, produced a response that was marginally higher than that of the ultrafine ground material without pre-treatment and was the only condition to exceed that recovery. The chemical response of the tailings to pre-treatment indicates that the species released from mineral matrices during NaOH oxidation were detrimental to gold leaching and might have been cyanicides.
No direct correlations between low- and high- temperature effects and low and high NaOH concentration effects were observed. These variations indicate that the response of the tailings to gold leaching is likely to be due to a combination of the oxidized species (cyanicides) reducing leaching efficiency and the occlusion of gold (reactivity with CN-) in the minerals reducing the lixiviants access.
The highest recovery (71.8%) was achieved after ultrafine grinding and 3 M NaOH pre-treatment at ambient conditions. The comparison of gold recovery at this condition with that of direct cyanidation suggests that gold liberation is almost at its maximum. The unrecovered gold is likely to be very finely disseminated and inaccessible without significant energy input.
Increasing the temperature during NaOH pre-leaching is expected to increase the rate of reaction according to an Arrhenius relationship and expose more gold (Mesa Espitia and Lapidus, 2015; Snyders et al, 2018). From Figure 7 it is observed that increasing the temperature during pre-leaching resulted in increased recovery of gold for the sample as-received compared to direct cyanidation. However, at ultrafine grind size (P80 -16pm) the recovery of gold after pre-leaching at 8o°C does not exceed the 66.5% obtained without pre-leaching. Au recoveries at 1 M and 3 M NaOH do not show any significant differences. Overall, the low Au recoveries after pre-leaching at high temperature indicate a possible interaction between the pre-leaching products and the tailings material that prevents maximum recovery of gold. This would be in the form of species produced at elevated temperature. The chemical system and aqueous chemistry during pre-leaching are therefore a critical factor in determining the efficiency of NaOH pre-leaching. To investigate the possible chemical reactions occurring in the pre-leach solutions an elemental analysis of the pre-leach solution is an important first step.
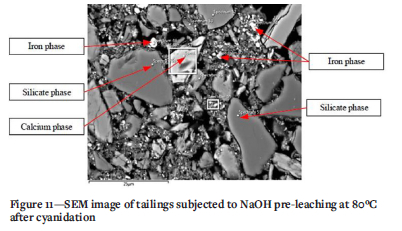
The XRD results (Table VI) on the tailings indicated a predominance of amphibolies as gangue components and a large proportion of quartz. Analyses of the extraction of Al and Si are presented in Figure 8 as a proxy for the dissolution of these minerals. SEM analysis (Figure 11) indicates an association of gold particles with arsenic, which may have originated from arsenopyrite. Figure 9 shows the extraction of As and Fe as a proxy for Fe- and As- bearing phases hosting gold.
From Figure 8, at ambient conditions Si and Al notably show lower extraction than at 8o°C, which is consistent with an Arrhenius relationship on the kinetics of dissolution. Increasing the concentration of NaOH for all conditions consistently results in increased elemental recovery for quartz and amphibole minerals. These high recoveries of elements from the gangue phases would explain why the Au recoveries under all conditions, but specifically at ambient conditions, were higher than those for direct cyanidation with no pre-treatment.
Au recovery from the ultrafine ground material, however, is not consistent with the Al and Si recoveries as this was shown to be lower than that of direct cyanidation. It is possible, however, that the lack of significant improvement in gold recovery after pre-leaching is due to fine grinding exposing available gold to a high degree.
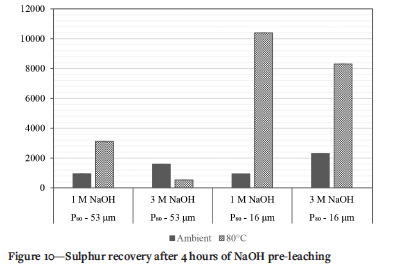
In Figure 9, while Fe extraction is relatively low, As extraction increases with increasing NaOH concentration and temperature. Finally, Figure 10 shows the extraction of sulphur during NaOH pre-treatment analysed as a proxy for sulphide minerals that were determined by QEMSCAN (Figure 5) to account for up to 0.3% of the tailings. S recovery at ambient conditions increases with increasing NaOH concentration. The release of As and S due to alkaline dissolution also liberates any gold encapsulated in mineral matrices containing these elements and would also account for increased gold recoveries compared to direct cyanidation (Darban et al. 2011, Mesa Espitia and Lapidus 2015).
A comparison of gold recovery after pre-leaching and the extraction of significant elements from the tailings indicates that the pre-leaching mechanism for these tailings is far more complex than the release of gold from encapsulated mineral matrices. In general, high extraction of these elements was accompanied by low gold recoveries. It can be hypothesised that the elements of greater concern are As and S and their associated mineral phases, as these are known to be cyanide consumers.
Decomposition of arsenopyrite and pyrite species in alkaline systems is proposed to produce Fe oxide species and soluble As species, (Bhakt, Langhans, and Lei, 1989; Meng, 2005) Equations [1]-[4].
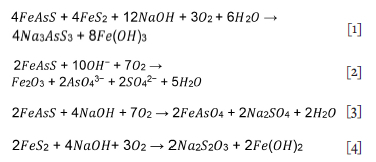
While gold can be mobilized through the oxidation of sulphur and arsenic, the remaining Fe oxide species can act as cyanide consumers and likewise, sulphur species (S2-) and arsenic species reacted with lime to form Ca2AsS (Yannopoulos, 1991).
Figure 11 shows a SEM image of the tailings after pre-leaching. Fe phases are identified along with Ca phases and silicate phases which were not completely broken down in the process. Assuming a chemical system involving oxidation of Fe-As-S phases such as that described above, increased sulphur extraction maybe accompanied by increased liberation of cyanide-consuming species that can be activated in the leaching stage, resulting in reduced Au recovery. This implies that either longer residence times are required for complete oxidation of cyanide consumers, or stronger lixiviant conditions are required to ensure that the material is completely leached. For the specific samples tested, alkaline pre-treatment, while beneficial due to the release of gold from silicate and amphibolite matrices, is counteracted by the generation of competing cyanide consumers that limit the extent of gold extraction.
Microwave roasting pre-treatment
Heating in the form of roasting by microwaves was applied to the tailings sample to determine whether the increased energy input could be exploited to further alter the minerals encapsulating gold, and determine whether differential heating of microwave absorbers and non-absorbers could induce lixiviant pathways through generation of cracks for improved gold extraction (Amankwah and Ofori-Sarpong, 2011). In Figure 12, Au recovery from microwave roasting of the sample as received at 30 minutes and 60 minutes is shown compared to an ultra-fine ground sample.
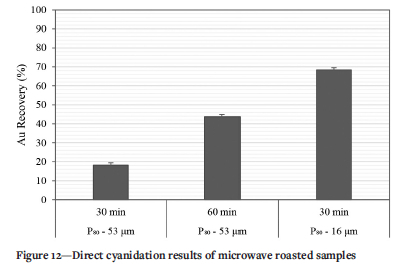
Although it is not expected that all the energy supplied by the microwaves in the set-up is absorbed by the sample, it is apparent that prolonged energy input is required to induce the release of gold from the gangue matrices. After 60 minutes of microwave roasting 43.7% Au recovery is achieved through cyanidation compared to 18.2% after 30 minutes of roasting. For finer material gold recovery increases to (68.4%) after 30 minutes of roasting. This value is marginally higher than that obtained after direct cyanidation of ultrafine ground material with no pre-treatment (66.5%). The observations suggest that ultrafine grinding in this case resulted in maximum exposure of gold for subsequent leaching and defines the maximum recovery barrier.
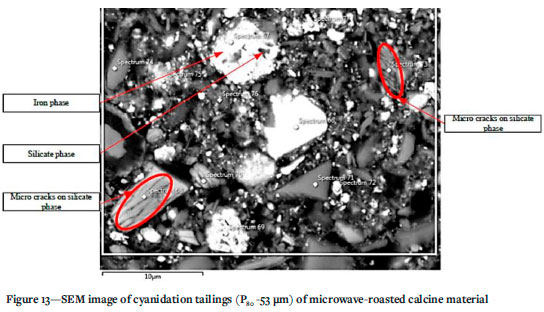
SEM analysis (Figure 13) of the material after microwave roasting shows fracture within the silicate matrices which might have been induced by differential heating of adjacent sulphide phases. Figure 13 shows a silicate particle adjacent to an Fe phase that might be an alteration product of an Fe-S phase. As with NaOH pre-leaching, while the oxidation of sulphur phases is beneficial towards liberation of locked or encapsulated gold, the remaining Fe species are still cyanide consumers and therefore the refractory nature of the tailings material is only partially reduced.
Microwave-assisted leaching
Microwaves were further used to aid direct cyanide leaching and investigate improvement in recoveries from the as-received (P80-53 µm) and ultrafine (P80-16 µm) samples (Figure 14). Leaching at 2 kg/t NaCN for both the as-received sample and ultrafine sample resulted in low gold recoveries of 3.2% and 15.0% respectively. Increasing the NaCN dosage to 4 kg/t and 8 kg/t, also yielded low gold recoveries at 20.3% and 29.0% respectively from the as-received sample. Although the recovery increases with NaCN dosage, the low values confirm the chemical refractoriness of the as-received sample due to the high concentration of cyanicides as discussed for these tailings (Pooley, 1987; Yannopoulos, 1991).
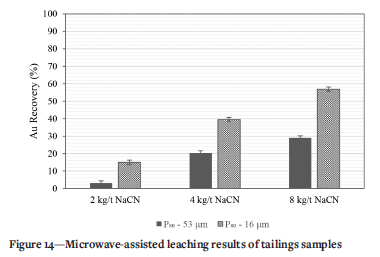
Increasing the NaCN dosage to 4 kg/t for the ultrafine milled sample had a significant effect on the gold recovery. The attained recovery was 39.5%. Furthermore, doubling the NaCN dosage to 8 kg/t resulted in a 17.4% increase in gold recovery to 56.9%. The significant improvement in gold recovery from the ultrafine milled sample brought about by 8 kg/t NaCN, highlights the benefits of the rapid kinetics associated with microwave-assisted leaching. According to Huang and Rowson (2002) and Krishnan, Mohanty, and Sharma, (2007), thermal convection currents are generated by differential heating responses of the various ore constituents. These convection currents facilitate the rapid removal of reaction products from the solid-liquid interface, allowing rapid exposure of unleached surfaces to continuous lixiviant action, The vigorous nature of the leaching was found to be effective in washing away any passivated gold surfaces, thus exposing them to cyanide action. In an earlier work, Mukendi, Handfield-Jones, and Akdogan, (2000) carried out microwave-assisted cyanide leaching tests on a different refractory materials containing 35% pyrite, 13% arsenopyrite, and 5% pyrrhotite which resulted in gold recoveries of 59%. However, the Au recove^ from these tailings was not comparable to that of direct cyanidation after ultrafine grinding, and with such high cyanide consumption, assisted leaching would be uneconomical.
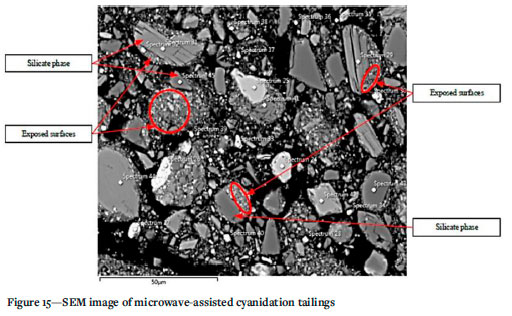
Figure 15 shows a SEM image of the tailings after microwave-assisted leaching. It can clearly be observed that there is exposure of some specifically silicate matrices within the or. However, the liberation of gold to a significant extent remains unattainable.
Conclusions
Calcination is an oxidative roasting process historically used in gold mining to remove cyanide-consuming species such as Fe-As-S minerals. The tailings from such a process therefore comprise heat-altered minerals with residual cyanide-consuming species and often with entrapped valuable gold. This research investigated gold recovery from calcine tailings and various pre-treatment processes (mechanisms) most suitable for enhancing gold recovery, such as fine grinding, NaOH leaching, microwave roasting, and microwave-assisted leaching. The refractory nature of the tailings was evident from direct cyanidation of the material (P80 -53 µm) reaching a gold recovery of 17.3% after 24 hours. Fine grinding (P80 -16 µm) resulted in improved liberation of the gold in the sample, increasing the gold recoveries to 66.5%.
Alkaline pre-treatment (NaOH) resulted in the highest gold recovery in the study, 71.6% at a P80 of 16 pm, ambient temperature pre-leaching, and 3 M NaOH. The 5.6% difference with alkaline pre-treatment of finely ground material reflects the percentage of gold that was not mechanically liberated. A recovery of 51.6% was achieved for the tailings sample without fine grinding (P80 -53 pm) at ambient temperature pre-leaching conditions and of 1 M NaOH. High-temperature pre-leaching consistently produced low gold recoveries compared to ambient temperature pre-leaching, High recoveries of detrimental elements such as Fe, As, and S into leaching solutions under these conditions might possibly lead to precipitation of species that increase cyanide consumption during leaching. Although the recovery after alkaline pre-treatment is 14.9% lower than that after two pre-treatment steps including further grinding, the two pre-treatment steps correspond to significant energy consumption and therefore, increased costs.
Microwave roasting and assisted leaching improved recovery by 17.3% but were not comparable to alkaline pre-treatment. NaOH pre-leaching is therefore concluded to be the most feasible process option based on low energy consumption and moderate recovery of gold.
Acknowledgments
The authors acknowledge the funding and support of the Royal Society and African Academy of Sciences through the Future Leaders African Independent Researchers (FLAIR) grant FLR\ R1VL91541. The authors would also like to acknowledge Pan-African Mining resources, Barberton Mines.
References
Allan, G.C. and Woodcock, J.T. 2001. A review of the flotation of native gold and electrum. Minerals Engineering, vol. 14, no.9. pp. 931-962. [ Links ]
Amankwah, R.K. and Ofori-Sarpong, G. 2011. Microwave heating of gold ores for enhanced grindability and cyanide amenability. Minerals Engineering, vol. 24, no. 6. pp. 541-544. [ Links ]
Anderson, G.S. and McDonald, N.W. 2016. IsaMills at Kalgoorlic Consolidated Gold Mines - from the M3000 to the M10000 and Replacement of the Roasters at Gidji Processing Plant. Proceedings of the 13th AUSIMM Mill Operators' Conference, Penh. Australasian Institute of Mining and Metallurgy, Melbourne, pp. 29-38. [ Links ]
Anderson, C.G. and Twidwell, L.G. 2008. The alkaline sulphide hydrometallurgical separation, recovery and fixation of tin, arsenic, antimony, mercury and gold. Proceedings of the 2008 Global Symposium on Recycling, Waste Treatment and Clean Technology, REWAS 2008, vol. 3. pp. 159-168. [ Links ]
Asamoah, R.K., Skinner, W., and Addai-Mensah, J. 2018. Leaching behaviour of mechano-chemically activated bio-oxidised refractory notation gold concentrates. Powder Technology, vol. 331. pp. 258-269. [ Links ]
Bhakta, P., Langhans, J.W., and Lei, K.P., 1989. Alkaline oxidative leaching of gold-bearing arsenopyrite ores. US Department of the Interior, Bureau of Mines. [ Links ]
Buttress, A.J., Katrib, J., Jones, D.A., Batchelor, A.R., Craig, D.A., Royal, T.A.. Dodds, G, and Kingman, S.W. 2017. Towards large scale microwave treatment of ores: Part 1 - Basis of design, construction and commissioning. Minerals Engineering,vol. 109. pp. 169-183. [ Links ]
Crundwell, F.K. 2014. The mechanism of dissolution of minerals in acidic and alkaline solutions: Part II Application of a new theory to silicates, aluminosilicates and quartz. Hydrometallurgy, vol. 149. pp. 265-275. [ Links ]
Darban, A.K., Aazami, M., Melendez, A.M., Abdollahv, M., and Gonzalez, I. 2011. Electrochemical study of orpiment (As2S2) dissolution in a NaOH solution. HydrometaUurgy, vol. 105, no. 3-4. pp. 296-303. [ Links ]
Das, A. and Sarkar, B. 2018. Advanced Gravity Concentration of Fine Particles: A Review. Mineral Processing and Extractive Metallurgy Review, vol. 39, no. 6. PP- 359-394- [ Links ]
Dunn, J.G. and Chamberlain, A.C. 1997. The recovery of gold from refractory arsenopyrite concentrates by pyrolysis-oxidation. Minerals Engineering, vol. 10, no. 9. pp. 919-928. [ Links ]
Fraser, K.S., Walton, R.H., and Wells, J. A. 1991. Processing of refractory gold ores. Minerals Engineering, vol. 4, no. 7-11. pp. 1029-1041. [ Links ]
Fernandez, R.R., Collins, A., and Marczak, E. 2010. Gold recovery from high-arsenic-containing ores at Newmont's roasters. Minerals and Metallurgical Processing, vol. 27, no. 2. pp. 60-64 [ Links ]
Gonzalez-Anaya, J.A., Nava-Alonso, F., and Pecina-TreviSo, E.T. 2011. Gold recovery optimization of a refractory concentrate by ultrafine grinding - A laboratory study. Minerals and Metallurgical Processing, vol. 28, no. 2. pp. 95-101. [ Links ]
Guo, P., Wang, S., and Zhang, L. 2019. Selective removal of antimony from refractory gold ores by ultrasound. HydrometaUurgy. vol. 190. pp. 105161. [ Links ]
Haque, K.E. 1999. Microwave energy for mineral treatment processes - A brief review. InternationalJournal of Mineral Processing, vol. 57, no. 1. pp. 1-24 [ Links ]
Huang, J.H. and Rowson, N.A. 2002. Hydrometallurgical decomposition of pyrite and marcasite in a microwave field. HydrometaUurgy, vol. 64, no. 3. pp. 169-179. [ Links ]
Iglesias, N. and Carranza, F. 1994. Refractory gold-bearing ores: a review of treatment methods and recent advances in biotcchnological techniques. HydrometaUurgy, vol. 34, no. 3. pp. 383-395. [ Links ]
Janse van Rensburg, S. 2016. Guidelines for retreatment of SA gold tailings: MINTEK's learnings. Proceedings of the 23rd WasteCon Conference, pp. 367-376.[Online], https://iwmsa.co.za/sitcs/dcfault/f1lcs/downloads/56.JansevanRcnsburg%2CS.pdf [ Links ]
Komnitsas, C. and Pooley, F.D. 1989. Mineralogical characteristics and treatment of refractory gold ores. Minerals Engineering, vol. 2, no. 4. pp. 449-457 [ Links ]
Koslides, T. and Ciminelli, V.S.T. 1992. Pressure oxidation of arsenopyrite and pyrite in alkaline solutions. HydrometaUurgy, vol. 30, no. 1-3. pp. 87-106. [ Links ]
Krishnan, K.H., Mohanty, D.B., and Sharma, K.D. 2007. The effect of microwave irradiations on the leaching of zinc from bulk sulphide concentrates produced from Rampura-Agucha tailings. HydrometaUurgy, vol. 89, no. 3-4. pp. 332-336. [ Links ]
Meng, Y.Q. 2005. New extraction process of carbonaceous refractory gold concentrate. Transactions of Nonferrous Metals Society of China (English Edition), vol. 15, no. 5. pp. 1178-1184 [ Links ]
Mesa Espitia, S.L. and Lapidus, G.T. 2015. Pre-treatment of a refractory arsenopyritic gold ore using hydroxyl ion. Hydrometallurgy, vol. 153. pp. 106-113. [ Links ]
Metzner, G. 1993. Multivariable and Optimising Mill Control-The South African experience. Proceedings of the XVIII International Mineral Processing Congress HMPQ, Sydney, pp. 203-299. [ Links ]
Mukendi, N.D., Handfield-Jones, R.V.R., and Akdogan, G. 2000. Microwave assisted leaching of refractory gold concentrates. Proceedings of the International Congress on Mineral Processing and Extractive Metallurgy, Melbourne, Australia. Australasian Institute of Mining and Metallurgy, Melbourne, pp. 197-199. [ Links ]
Nanthakumar, B., Pickles, C.A., and Kelebek, S. 2007. Microwave pre-treatment of a double refractor)' gold ore. Minerals Engineering, vol. 20, no. 11. pp. 1109-1119. [ Links ]
Pooley, F.D. 1987. Use of bacteria to enhance recovery of gold from refractory ores. Minprep 87, Proceedings of the International Symposoum on Innovative Plant and Processes for Mineral Engineering, 31 March-2 April 1987. pp. 1-14. [ Links ]
Snyders, C.A., Akdogan, G., Bradshaw, S.M., van Vreden, J.H., and Smith, R. 2018. The development of a caustic pre-leaching step for the recovery of Au from a refractory ore tailings heap. Minerals Engineering, vol. 121, July 2018. pp. 23-30. [ Links ]
Yalcin, E. and Kelebek, S. 2011. Flotation kinetics of a pyritic gold ore. International Journal of Mineral Processing, vol. 98, no. 1-2. pp. 48-54. [ Links ]
Yannopoulos, J.C. 1991. The Extractive Metallurgy of Gold. Van Nostrand Rcinhold, New York. [ Links ]
Zhang, G., Wang, S., Zhang, L., and Peng, J. 2016. Ultrasound-intensified leaching of gold from a refractor)' ore. ISIJ International, vol. 56, no. 4. pp. 714-718. [ Links ]
 Correspondence:
Correspondence:
M. Tadie
Email: mtaclie@sun.ac.za
Received: 30 Mar. 2022
Revised: 27 Jun. 2022
Accepted: 5 Jun. 2022
Published: October 2022














