Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Journal of the Southern African Institute of Mining and Metallurgy
versión On-line ISSN 2411-9717
versión impresa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.122 no.8 Johannesburg ago. 2022
http://dx.doi.org/10.17159/2411-9717/1154/2022
PROFESSIONAL TECHNICAL AND SCIENTIFIC PAPERS
Production of Al(HI)-K(I)-Ti(IV)-sulphate-containing leach liquor from metakaolinite-containing ash derived from South African coal fines
A.C. CollinsI; C.A. StrydomII; R.H. MatjieIII; J.R. BuntIII; J.C. van DykI, IV
IChemical Resource Beneficiation, North-West University, Potchefstroom, South Africa. https://orcid.org/0000-0002-5134-9638
IICentre of Excellence in Carbon-based Fuels, School of Physical and Chemical Sciences, NorthWest University, Potchefstroom, South Africa. https://orcid.org/0000-0001-5295-2095
IIICentre of Excellence in Carbon-based Fuels, School of Chemical and Mineral Engineering, NorthWest University, Potchefstroom, South Africa. R.H. Matjie: https://orcid.org/0000-0002-2839-3729; J.R. Bunt: https://orcid.org/0000-0003-3051-2528
IVAfrican Carbon Energy, South Africa
SYNOPSIS
South African discard coal fines and K2CO3 blends were heated in a laboratory-scale rotary kiln to produce ashes for H2SO4 leaching tests. The optimized H2SO4 leaching conditions of 6.12 mol.dm3 (M) H2SO4, solid to liquid ratios 1:5 and 1:10, and 8o°C for 8 hours were used. K2CO3 was added to increase the dissolution efficiency of K. The objective was to determine if the Al present in metakaolinite (Al2O3.2SiO2), the Al, K, and Ti in the alumino-silicate glasses, and the Ti in rutile (TiO2) in the ashes could be selectively dissolved in H2SO4. XRF results show that the ashes formed at 700°C dissolved more efficiently (87% Al, 89% K and 23% Ti) compared to the ashes formed at 1050°C. This can be attributed to the presence of Al2O3.2SiO2, K2CO3 melt, K2CO3 remnants, KAl(SO4)2, and K-aluminosilicate glass in these ashes. XRD results indicate that the ashes prepared at 1050°C contained anorthite (CaAl^Og), microcline (KAlSi3O8), pseudomullite (Al2.SiO2), and silicon spinel (2Al2O3.3SiO2), which are either insoluble or only sparingly soluble in H2SO4. These minerals resulted in the lower dissolution efficiencies of Al and K. Based on the high dissolution efficiencies of Al and K for the ashes produced at 700°C, coal fines blended with K2CO3 could possibly be utilized as feedstocks for the production of aluminium(III), potassium(I), and titanium(IV) and a sulphate-containing leach liquor. Furthermore, the environmental issues and costs associated with the handling and disposal of large volumes of coal fines will also be resolved.
Keywords: sulphuric acid leaching, aluminium, titanium, potassium, coal ash, XRD, XRF.
Introduction
Industrial and population growth in developing countries has necessitated the generation of more power to meet increasing energy demands. Coal-based power generation is still one of the most important and effective processes (Izquierdo and Querol, 2012). The increase in power generation results in energy demands being met (Nayak and Panda, 2010), but at the cost of an increase in waste generation (coal fines, coal ash, and gaseous emissions) which give rise to associated environmental and health risks.
South African collieries and gasification and combustion plants dispose of more than 60 Mt of coal fines (>75<1000 µm) per annum that are unavoidable by-products (Reddick, von Blottnitz, and Kothuis, 2007; Matjie et al., 2018). Furthermore, other sources of coal fines (>1 mm < 5 mm coal particles) produced from carbon conversion plants using Highveld coal are pulverized to <75 µm and blown into pulverized fuel boilers to generate electricity and high-pressure steam for the fixed-bed gasification process.
Current disposal methods for coal fine discards are associated with environmental and health problems, i.e. the spontaneous combustion and dust particle emissions to the environment (Moyo et al., 2018; Department of Environmental Affairs, 2012; Muzenda, 2014). Spontaneous combustion is caused by the oxidation of pyrite (FeS2) present in the coal, and can result in fires and pyrite oxidation products being released during handling and storage. In addition, high costs are incurred by the disposal of coal fine wastes into slimes dames or disposal areas.
South African commercial power stations produce more than 50 Mt of coal ash per annum, which comprises both bottom ash and coal fly ash (Department of Environmental Affairs, 2018). These ashes contain significant amounts of aluminium (>7.9 Mt), potassium (>0.2 Mt), silicon (>13 Mt), and titanium (>0.5 Mt) (Badenhorst, 2019). According to Ginster and Matjie (2005) and Reynolds-Clause and Singh, (2019) approximately 7.5 Mt of coal ash are re-used in cement applications as extender, wastes treatment, concrete applications, road construction, brickmaking, rubber, paint, geopolymer, soil amelioration, mine backfilling, landfill, and mine drainage treatment. However, more than 43 Mt of coal ash is still being dumped at the disposal sites of South African power stations every year.
Furthermore, coal ash from overseas power stations is utilized in small amounts in industrial applications, i.e., brick manufacturing, building materials, road construction, and ceramics (Nayak and Panda, 2010). The ash by-products, containing significant amounts of Al and smaller amount of Ti (a higher-value inorganic element), also have potential for the recovery of these elements by leaching (Barry et al., 2018; Matjie, Bunt, and van Heerden, 2005; Wu, Yu, and Zang, 2012).
Studies have shown that approximately 80% of the aluminium which is present in South African coal fly ash produced during the pulverized fuel combustion process at approximately 1600°C is associated with mullite (Al6O13Si2), which is not soluble in the sulphuric acid (Matjie, Bunt, and van Heerden, 2005; Freeman, 1993). The balance of the aluminium (20%) is reported to be present in the amorphous aluminosilicate glasses (Freeman, 1993; Matjie, Bunt, and van Heerden, 2005). The leachability of aluminium from the glassy phase will be influenced by the form in which the amorphous materials are present (Seferinoglu, 2003) and surface associated elements which have been found to be more susceptible to leaching (Izquierdo and Querol, 2012).
All inorganic elements that are associated with inactive or non-reactive minerals formed at elevated temperatures are sparingly soluble or insoluble in all mineral acid solutions apart from hydrofluoric acid solution (Paul et al., 2006). These minerals are formed in coal ashes through partial or complete mineral transformation during the coarse coal gasification (1400°C) process and high-temperature (1600°C) combustion (Matjie, Bunt, and van Heerden, 2005; Torma, 1983; Neupane and Donahoe, 2013). All of South Africa's bottom ash and coal fly ash, as well as gasification coal ash containing mullite and anorthite (CaAl2Si2O8), formed at elevated temperatures during the pulverized fuel combustion and gasification processes respectively, are unsuitable leaching using sulphuric acid solution (Matjie, Bunt, and van Heerden, 2005; Torma, 1983). However, it has been shown that the leaching of a heated blend of South African fly ash and quicklime (CaO) with 6.12 M H2SO4 at 80°C and 1:3% or 1:5 solid to liquid ratios for 4 hours results in high aluminium and titanium extractions of 80-85% and 50% respectively in (Matjie, Bunt, and van Heerden, 2005). Moreover Sangita, Nayak, and Panda, (2017) and Torma (1983) leached a blend of fly ash from an overseas power station and CaO which was heated to between 1000°C and 1200°C with sulphuric acid solution and achieved an aluminium extraction of 80-99%. This implies that CaO reacted with the alumina that is associated with mullite in the coal ash to form calcium aluminate (CaAl2O4), which is soluble in sulphuric acid solution (Torma, 1983; Matjie, Bunt, and van Heerden, 2005).
In addition, Sangita, Nayak, and Panda (2017) used sulphuric acid solution to solubilize aluminium from coal fly ash from Chinese power stations, which contained 62% reactive amorphous aluminosilicate glasses, to form pure aluminium sulphate crystals. Furthermore, van der Merwe et al. (2017) heated a blend of South African ultrafine fly ash containing cenospheres and either ammonium sulphate or ammonium bisulphide to 400°C and 600°C to produce a solid sample. A conventional hydrometallurgical water method was subsequently applied to selectively solubilize aluminium (47% Al solubilized) from the heated cenosphere blends. These cenospheres are ultrafine particles and roughly 8-1000 µm in diameter with a density lower than 1 g/cm3 (Kolay, 2001; Vassilev, 2004). Cenospheres, which comprise approximately 1-2% of the fly ash produced during pulverized fuel combustion, comprise a mixture of aluminosilicate glasses with mullite and quartz (SiO2) (Kolay, 2001; Vassilev, 2004). Sulphuric acid is mostly used for recovering high-value metals due to its effectiveness, availability, and affordability as a leaching solution (Seidel, 1999). However, the high energy input required to break the bonds between alumina and silica in mullite and K-feldspar crystals, the gas and alkali emissions, as well as the cost of the chemicals, make the proposed H2SO4 leaching process economically unviable as well as environmentally.
Since aluminium, titanium, and potassium species in coal ashes produced from the combustion and gasification processes cannot directly react with sulphuric acid, coal fine waste and a blend of coal fine waste and potassium carbonate were heated at either 700°C or 1050°C in air to produce lower temperature combustion coal ash samples. These temperatures are more typical of what can be expected in a fluidized-bed combustion unit. The low-temperature ashes contain metakaolinite, and amorphous glasses of illite (K, H3O)(Al,Mg,Fe)2(Si,Al) and microcline. Soluble potassium melt and K2CO3 remnants were produced at either 700°C or 1050°C via combustion of the coal fines, and blends of coal fines and K2CO3. The Al2O3 from metakaolinite and aluminosilicate glasses, and titanium, and potassium compounds contained in the low-temperature combustion ash samples were dissolved during the sulphuric acid leaching experiments. The possible chemical reactions of Al2O3 from metakaolinite, TiO2, K2O, and H2SO4 to form the aluminium(III), titanium(IV), potassium(I), and sulphate-containing leach liquor are as follows:
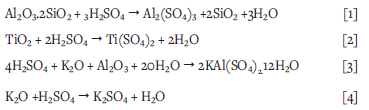
Ash samples derived from kaolinite-containing coal fines, blends of coal fines and K2CO3 have not previously been utilized as an alternative feedstock to heated kaolinite-bearing clays in the production of technical-grade aluminium sulphate salts using sulphuric acid solution (Altiokka et al., 2010; Alquacil, Amer, and Luis, 1987; Numluk and Chaisena, 2012). In addition, Phillips and Wills (1982) used the nitric acid leaching route to dissolve alumina from the micaceous residues from smelter grade China clay.
The commercial leaching methods and conditions used during this investigation are applied in the recovery of aluminium from deactivated clay minerals (non-bauxite ores) with Al dissolution efficiencies reaching up to 90% (Ibrahim, Moumani, and Mohammad, 2018; Mark et al., 2019; Numluk and Chaisena, 2012). High-temperature pulverized fuel combustion conditions (approximately 1600°C) (van Alphen, 2005) are used in the commercial power stations to produce fly ash and bottom ash samples. These conditions were not followed in this study as they are not suitable for the production of low-temperature combustion ashes (containing soluble Al, K, and Ti species) of coal fines and blends of coal fines and K2CO3, which can be leaching using H2SO4 leaching experiments.
Potential uses of the liquid products
The aluminium(III), potassium(I), titanium(IV), and sulphate-containing leach liquor produced during the acid leaching tests of these coal ashes can possibly be utilized in other chemical industries, z'.e. technical aluminium sulphate or potassium aluminium sulphate in the form of either liquid or solid can be used as coagulants in water treatment plants. Furthermore, the aluminium(III), potassium(I), titanium(IV), and sulphate-containing leach liquor could be purified through solvent extraction for the production of pure aluminium chemicals, including iron-free aluminium sulphate or iron-free potassium aluminium sulphate. The potassium sulphate solution could be used in potassium chemicals, or in the fertilizer industry, whereas a pure titanium dioxide could be used in the paint/paper industry. The ash residues obtained after leaching could possibly be used as an aggregate in brick manufacturing and masonry concrete.
X-ray fluorescence (XRF) and X-ray diffraction (XRD) techniques were used to characterize the coal fines and blends of coal fines and K2CO3, the ash samples, and leached ash residues produced from the experiments. The XRF results and the masses of ash samples before and after the H2SO4 leaching tests were utilized in calculating the dissolution efficiencies of inorganic elements after acid leaching of the ash samples prepared at either 700°C or 1050°C.
Materials and methods
Coal sampling, preparation, and composition of coals
The sampling of coals from the collieries as well as the preparation methods are reported in Collins et al. (2018). Due to the similarities in the chemical and mineralogical properties of coal samples SA1 and SA2, sample SA2 was blended with K2CO3. SA1, SA2 blend, and SA3 were subjected to low-temperature combustion tests to produce samples for the leaching experiments, XRD and XRF analyses. In addition, SA2, SA1, SA2 blend, and SA3 were submitted for XRD analyses. The characterization analytical methods including proximate, ultimate, and XRF analyses used to determine the chemical composition of coals and the results obtained are also published in Collins et al. (2018). The reagents (H2SO4 and K2CO3) used in this investigation are also described in Collins et al. (2018).
Ash samples
The coal samples used in this investigation have similar properties to those of discarded coal fines produced during coal mining and beneficiation at the South African coal preparation plants. The prepared coal samples were subjected to oxidation in air, using specific conditions to produce the desired ash samples. These ashes have not been used for the commercial production of aluminium sulphate salts although, as stated in the introduction they are thought to be suitable for utilization in the commercial process to produce aluminium sulphate leach liquor from heated kaolinite-bearing clay.
According to Collins (2019), the ash samples prepared at 700°C contain a higher proportion of metakaolinite (alumina associated with metakaolinite is soluble in either H2SO4, or HCl or HNO3) and a small proportion of amorphous glasses of illite and microcline. These ashes are also characterized by a small proportion or zero per cent of Al2O3.SiO2 pseudomullite, silicon spinel 2Al2O3.3SiO2, and <1% organic carbon, which are only sparingly soluble or insoluble in these mineral acids. A temperature of 700°C was therefore used in this study to produce ashes which can possibly contain a higher proportion of metakaoilinite, which may be associated with a higher dissolution efficiency of alumina during sulphuric acid leaching. The ash samples produced at 1050°C are characterized by higher proportions of pseudomullite and silicon spinel, a small proportion (or zero per cent) of metakaolinite, and zero organic carbon. Higher proportions of pseudomullite and silicon spinel, which are sparingly soluble in H2SO4, could be formed at this temperature. Lower dissolution efficiencies of Al, K, and Ti during sulphuric acid leaching could be expected from these ashes. In addition, Bryers (1986) and Yanti and Pratiwi (2018) found that metakaolinite transformed to silicon spinel at 925°C, which further formed pseudomullite at 1100°C. At 1200-1400°C, pseudomullite transformed to mullite, which is insoluble in mineral acid solutions apart from hydrofluoric acid (Teklay et al., 2014; Bryers, 1986). According to Osawa and Bertan, (2005) pseudomullite and silicon spinel prepared at pH 4 and pH 8 are formed from mixtures of alumina and silica sols at 1050°C and transform to mullite at 1200°C.
The commercial plants that produce aluminium sulphate by H2SO4 leaching of clay-containing kaolinite is employing temperatures of 600-800°C. Heller-Kallai (1978) and Heller-Kallai and Lapides (2003) heated a mixture of clay containing kaolinite and potassium compounds at 600-700°C to produce synthetic potassium feldspars via the solid state reaction of metakaolinite and potassium cations. Therefore, the temperatures of either 700°C or 1050°C were finally selected for this study, based on the contents of metakaolinite, silicon spinel, and pseudomullite in the coal ashes.
Coal samples of approximately 5 kg were placed to clay fired sample trays and loaded into the hot zone of a rotary kiln. The temperature of the kiln was increased to 700°C at a heating rate of 10°C/min. All sub-samples were heated to 700°C for a residence time of 3 hours under air flow of 80 ml/min to facilitate coal combustion and transformation of the mineral matter and the evolution of volatiles. The furnace was then switched off and allowed to cool for 8 hours and the ash samples were removed once the samples reached ambient temperature. Four representative ash samples were blended to form one homogenous sample. The sample preparation methods set out in ISO 18283 and ISO 13909-4) (Rautenbach et al., 2019) were followed to take representative samples from the bulk ashes prepared at 700°C for the H2SO4 leaching tests and for further heating in a muffle furnace. After placing the representative sample of ash produced at 700°C in the muffle furnace, the temperature was increased to 1050°C at a heating rate of 10°C/ min. This experimental procedure to produce low temperature combustion ashes at either 700°C or i050°C was repeated for all the coal samples used in this study.
Analytical methods
XRF analysis
XRF analysis of coal ash samples (SAi, SA2 blend, and SA3) prepared at either 700°C or i050°C to determine the proportions of inorganic elements in these samples was conducted according to the methods proposed by other researchers (Norrish and Hutton, 1969; Matjie et al., 20i8).
XRD analysis
XRD analysis of coal (SAi, SA2, SA2 blend and SA3) and ash (SAi, SA2 blend and SA3) samples was carried out to qualify and quantify the proportions of crystalline and amorphous phases in these samples using the methods suggested by other researchers (Rietveld, 1969; Speukman, 2012; Matjie et al., 2018). The percentage XRD error is 0.2-0.3 and the detection limit is between 0.5 and 5 weight per cent. This implies that XRD results for the trace minerals with smaller crystallite sizes (<1 nm crystallites) in the solid samples which are above this detection limit may not be accurate (Chinchon et al., 1993).
Experimental procedure
A schematic diagram of the process and analytical methods used in this study for dissolving Al, K, and Ti from coal ash and blend ash samples, is presented in Figure 1.
Acid leaching
Technical grade sulphuric acid (10 M H2SO4 or 98% H2SO4, a stable or less reactive and viscous mineral acid) with small proportions of ions (H3O+, SO42-) was used to prepare a 6.12 M H2SO4 or 60% H2SO4, which is very reactive with significant amounts of ions (H3O+, SO42-), using deionized water, for the leaching experiments. A 500 cm3 round-bottomed container, with sealing lid and cooler, was used as the leaching vessel. An automatic overhead stirrer with base plate (model 720), coupled with a temperature controller with a sensor probe, was used as the heat source. The leaching conditions used in the experiments are stated below.
6.12 M H2SO4 leaching
The influence of solid to liquid ratio on the dissolution of the inorganic elements contained in the ash samples was investigated during the acid leaching experiments. The solid to liquid ratios used were 1:5 and 1:10. A 10 g ash sample was accurately weighed out and added into the leaching vessel. The optimized H2SO4 leaching conditions for sintered pellets of a mixture of South African fly ash, coal, and CaO - a solid to liquid ratio of 1:5, temperature of 80°C, time of 8 hours and (6.12 M) H2SO4 (Matjie, Bunt, and van Heerden, 2005)- which achieved the highest leaching efficiency of Al and Ti, were used in this study. A known 6.12 M H2SO4 was transferred into the vessel containing value of coal ash to maintain the solid to liquid ratio of the specific experiment. The slurry was stirred at 200 r/min with an automatic overhead stirrer at 80°C for 8 hours. The hot slurry sample was filtered to produce the leached wet ash residue and leach liquor (filtrate). The ash residue was washed with deionized water to ensure that all dissolved inorganic species were recovered. The leached ash residue was placed in a vacuum furnace and dried at 60°C for 24 hours. The wash solution was combined with the original leach liquor to form the final leach liquor sample. In this investigation, only coal ash and leached ash residue samples were submitted for XRD and XRF analyses. Due to the cost constraints associated with the inductively coupled plasma atomic emission spectrometry (ICP-AES) analysis of the liquid samples, all the leach liquor samples were not submitted for this analysis but were kept for the other purification steps.
Calculation of dissolution efficiencies
Equation [5] was used to calculate the percentage dissolution efficiency for Al, K, and Ti using the XRF results for the ash samples and the leached ash residues as well as the masses of ashes. In this study the aluminium in the form of the alumina is expected to be selectively dissolved from metakaolinite during sulphuric acid leaching of the coal ash. Al, Ti, and K contained in the aluminosilicate glasses of illite and microcline and Ti in either rutile or anatase, as well as the K in the potassium melt and K2CO3 remnants, are also expected to be solubilized. The dissolution efficiency was calculated using Equation [5]:

where
N (MO) is the dissolution efficiency of the specific inorganic element (Al, K, or Ti)
mCA is the mass of the specific inorganic element in the coal ash and leached ash residues samples
mRES is the mass of ash residue (after leaching).
The mass of the specific inorganic element in the coal ash and leached ash residue samples can be calculated by using Equation [6].

where
mCA is the mass (g) of inorganic element (Al, K, or Ti) in the coal ash or leached ash residues
%E is the percentage of the inorganic element in the coal ash or leached ash residue, as calculated by Equation [7]

msampk is the mass of the ash sample or leached ash residue sample.
The percentage (%E) of inorganic element present in each of the ash and leached ash residue samples was calculated using Equation [7].
where
%E is the percentage of the element in the coal ash or ash residue sample
%EO is the percentage elemental oxide in the sample (provided through XRF analysis)
Melement is the molecular weight of the element in the oxide
MEO is the molecular weight of the elemental oxide.
Results and discussion
Coal mineralogy
The XRD results of the coal samples are presented in Table I. The results indicate that all the coal samples consist mainly of amorphous (non-crystalline) organic carbon. The SA1 sample contains a higher proportion of kaolinite than the other coals tested, while the SA2 coal and SA2 blend samples contain less quartz than SA1 and SA3. Minor proportions of other minerals (microcline, illite, calcite (CaCO3), pyrite (FeS2), and anatase (TiO2)) and a high proportion of dolomite (CaMgCO3), were found in all the coal samples evaluated in this study. Other minerals are present in trace quantities. As expected, the SA2 blend also contained a low proportion of K2CO3 compared to the percentage of potash added to this coal). This can be attributed to the hydration of K2CO3 by the 4% moisture contained in this coal. Also, these XRD results, excluding the SA2 blend, match previous XRD data for other South African coals (van Dyk et al., 2009; van Dyk, Waanders, and Hack, and 2008).
The occurance of extraneous calcite and extraneous dolomite discrete particles indicates that these minerals could transform at temperatures of 700°C and 1000°C to CaO (quicklime) and a mixture of MgO (periclase) and CaO respectively (Matjie et al., 2018; van Dyk, Waanders, and Hack, 2008). The inherent calcite or dolomite associated with clays (kaolinite, illite, muscovite) and microcline and carbon matrix in the coals can transform at 1200-1400°C to form a molten solution or partial melt (Rautenbach et al., 2021). Furthermore, either the extraneous calcite and dolomite react with each other at 1200-1400°C to form melts during heat treatment. The high-temperature minerals (mullite, anorthite, K-feldspar) formed, which are insoluble in sulphuric acid solution, crystallized at low temperatures from the molten solutions. These minerals can also be formed at elevated temperatures via solidstate reactions.
The high-temperature products (CaO and MgO) derived from transformation of either extraneous calcite or dolomite have been found to be responsible for the in-situ capturing of sulphur dioxide to form calcium sulphate (Rautenbach et al., 2021). These oxides account for the reduction of gas emissions during coal combustion. In addition, the carbonation reaction will form either calcite or dolomite in the ashes, resulting in the minimization of CO2 emissions.
XRF analysis
The XRF results of the ash samples prepared from coals at elevated temperatures are presented in Table II. The dominant constituents in the SA1, SA2 blend, and SA3 ashes prepared at either 700°C or 1050°C under air are SiO2, and Al2O3, with lesser but still significant proportions of CaO, Fe2O3, MgO, TiOa MgO, and in some cases K2O (>18%) and SO3 (>4%) (Table II). Other oxides, including Cr2O3, Mn3O4, P2O5, BaO, Na2O, and SrO, each make up less than 1% of the ashes. These XRF results, with the exception of the ash of the coal sample spiked with K2CO3, are in good agreement with those of the ash samples prepared from other South African coals using thermochemical processes (Hattingh et al., 2011; van Alphen 2005; van Dyk et al., 2009).
Coal ash mineralogy
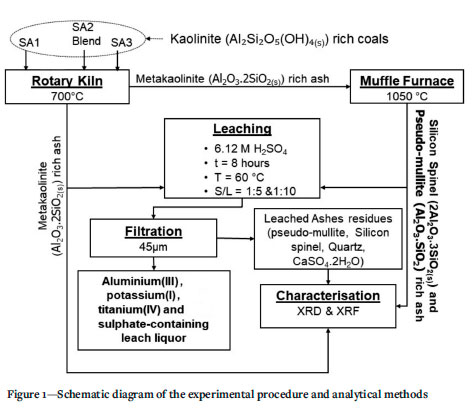
XRD results of the selected ashes of SAi, SA2 blend, and SA3 samples prepared at 700°C and 1050°C are presented in Figures 2-4 and Tables III and IV. These results indicate that the SA2 blend and SA3 ash samples prepared at 700°C, consisted of higher proportions of total amorphous phases (metakaolinite and amorphous glasses of illite and microcline) than the SA1 ash. Higher proportions of total amorphous materials formed at elevated temperatures in the ashes are associated with the mode of occurrence of the fluxing minerals (calcite, dolomite, and pyrite), clays (kaolinite, illite etc.) - and microcline in the coals (Rautenbach et al. 2021). The fluxing minerals lower the ash fusion temperature (AFT) values of clays, promoting the formation of melts during heat treatment of coals. The SA1 ash contains a higher concentration of quartz than the other ash samples. Based on the kaolinite and illite/microcline contents in the coals (Table I), metakaolinite could contribute to 95% of the total amorphous content in the coal ashes produced at 700°C.
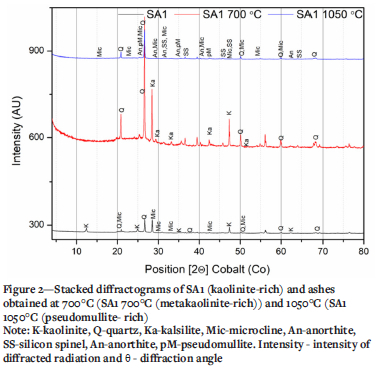
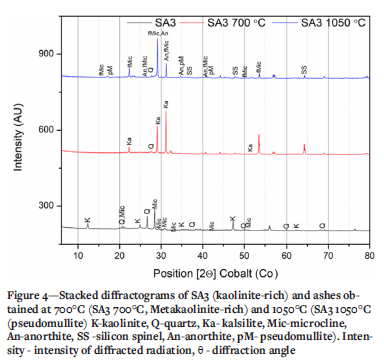
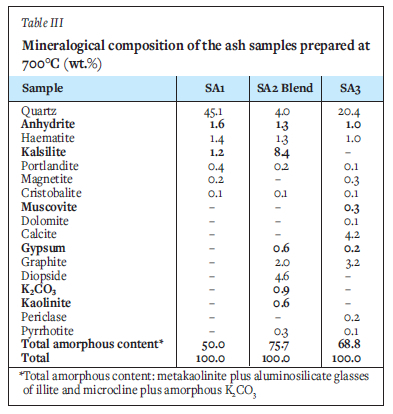
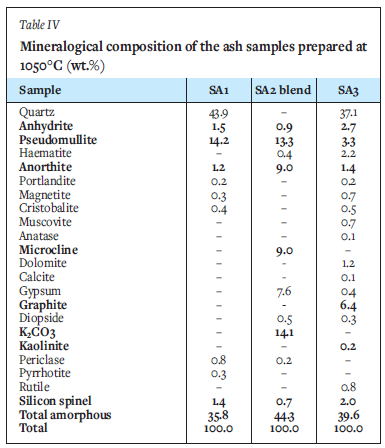
The XRD results show a lower proportion of total amorphous phases in the ashes after heating either SAi coal, SA2 blend, or SA3 coal at 1050°C compared the ashes prepared at 700°C (Tables III and IV). The XRD results of the ash samples reveal that kaolinite in the selected coals transformed at 700°C to form metakaolinite in the ashes (Tables III and IV). The total amorphous material contents of these ashes decrease with increasing temperature. Pseudomullite (transformation product of metakaolinite and silicon spinel at elevated temperatures) increase with increasing temperature during heat treatment (Tables III and IV). The presence of metakaolinite in the ashes of South African coals can be attributed to the transformation of kaolinite at elevated temperatures (600 - 850°C) (Matjie, van Alphen, and Pistorius, 2006; Matjie et al., 2018, Uwaoma et al., 2020).
The SA1 ash sample prepared at i050°C contains a lower or zero percent of metakaolinite, and a higher percentage of silicon spinel and pseudomullite (sparingly soluble in mineral acid solution, apart from HF) compared to the SAi ash samples prepared at 700°C (Tables III and IV). Rautenbach et al. (202i) found that mullite crystallizes from the molten aluminosilicate solution formed at 1200-1400°C during the combustion of coals in a laboratory and gasification study of other South African coals. Furthermore, mullite, which is associated with the transformation of the extraneous kaolinite, can also be formed at elevated temperatures of 1200-1400°C via the solid-state reaction (Rautenbach et al., 2021).
Khan et al. (2017) and Heller-Kallai (1978) studied the transformation of kaolinite in pure kaolinite-containing clay to form metakaolinite at elevated temperatures using XRD analysis, thermogravimetric analysis (TGA), and Fourier transform infrared (FTIR) techniques under oxidizing and inert conditions. They found that almost all of the kaolinite particles in the kaolinite-containing clay samples transformed to metakaolinite at 600-850°C.
The presence of trace periclase in some of the SAi, and SA2 blend ash samples (Table IV) is due to the transformation of the extraneous dolomite at 700°C to form a mixture of CaO and MgO. Hlatshwayo et al. (2009) and Matjie, van Alphen, and Pistorius, (2006) used XRD analysis to detect periclase in ash samples from South African carbon conversion plants that utilize thermochemical processes. The surface temperature and the peak temperature in the combustion zone and ash bed of South African carbon conversion gasifiers are 1200°C and 1400°C respectively (Glover et al., 1995; Bunt, 2006). CaO (the transformation product of either calcite or dolomite associated with kaolinite) can react with metakaolinite at 1400°C to form anorthite either via a solid-state reaction or crystallization from the molten aluminosilicate phase containing Ca, Mg, K, Fe, Ti, and Na impurities (Hlatshwayo et al., 2009). Anorthite is insoluble in sulphuric acid solution.
The low content of haematite (Fe2O3) (Tables III and IV) in the ashes of SA1, SA2 blend, and SA3 prepared at either 700°C or 1050°C was formed by the transformation of pyrite. Anhydrite (CaSO4) (Tables III and IV) was formed via the interaction of organic sulphur and organic calcium. Also, free CaO from the transformation of extraneous calcite at elevated temperatures can capture sulphur dioxide from the reaction of pyrite in the presence of oxygen or air. Furthermore, CaO in these ashes reacts with water to form portlandite (Ca(OH)2). A lower cristobalite (SiO2) content (product of a reaction between silica and alumina) (Tables III and IV) in SA1, SA2 blend, and SA3 ashes prepared at either 700°C or 1050°C, was formed during the transformation of the extraneous kaolinite and illite to metakaolinite, reactive silica, and amorphous glasses of illite. Diopside (CaMgSi2O6) in these ashes (Tables III and IV) was formed by silica reacting with CaO and MgO derived from the transformation of dolomite at elevated temperatures. These XRD results are consistent with those obtained during characterization studies of South African coal ashes produced from thermochemical processes (Hlatshwayo et al., 2009; Rautenbach et al., 2021; Uwaoma et al., 2020).
As expected, the total amorphous content of the ash samples decreases with increasing ashing temperature due to the transformation of metakaolinite in the amorphous phases to silicon spinel during combustion. Silicon spinel subsequently transforms at 1100°C to pseudomullite (Bryers, 1986; Yanti and Pratiwi, 2018). This is supported by the observed decrease in the total amorphous content, which is accompanied by an increase in the content of gypsum (CaSO4.2H2O), K2CO3, microcline originally detected in the coals, and synthetic microcline and pseudomullite in the ashes (Tables III and IV). An increase in the proportion of insoluble microcline in the SA2 blend ash samples prepared at 1050°C can be attributed to the reaction between potassium ions from the K2CO3 melt or K2O reacting with metakaolinite, at elevated temperatures (Tables III and IV) (Rautenbach et al., 2021, Matjie et al., 2018).
Matjie et al. (2021) studied the reaction between metakaolinite and potassium cations to form synthetic K-feldspars by heating a blend of 10% K2CO3 and coal at 700°C and 1050°C in air. In addition, Collins et al. (2018) used FACTSAGE™ modelling to predict microcline and kalsilite crystallization at temperatures between 1050°C and 1250°C from the melt of the SA2 blend. Furthermore, potassium cations from K2CO3 react with metakaolinite derived from kaolinite-containing clay at 600-700°C to form synthetic potassium feldspars (kalsilite (KAlSiO4), kaliophilite (KAlSiO4), nepheline ((Na, K)AlSiO4), and microcline via solid-state reactions (Heller-Kallai and Lapides, 2003; Heller-Kallai, 1978).
It can be concluded that all selected ash samples from SA1, SA2 blend, and SA3 coal samples produced at elevated temperatures of 1050°C contain a lower proportion of total amorphous material and higher proportions of silicon spinel, pseudomullite, and anorthite than those produced at 700°C. The mineralogical composition of the coal ash samples thus changed as the ashing temperature increased.
XRF analysis of the leached ash samples
The XRF results for the ash samples prepared at 700°C and 1050°C which were leached with the 6.12 M H2SO4 at 80°C and solid to liquid ratios of 1:5 and 1:10 for 8 hours are presented in Tables V and VI. The leached SA1 and SA3 ash samples prepared at 1050°C contain a lower proportion of SiO2 and a higher proportion of Al2O3 (Table VI) than the leached SA1 and SA3 ash samples prepared at 700°C (Table V). On the other hand, the leached SA2 blend ash contain a lower proportion of Al2O3 and a higher proportion of SiO2 than the other ash samples (Tables V and VI). Furthermore, the leached SA2 blend ash is characterized by a higher content of CaO in relation to the other leached ash samples prepared at either 700°C or 1050°C. The presence of SiO2, Al2O3, and CaO in the leached ash samples prepared at both 700°C and 1050°C is consistent with those of the original SA1, SA2 blend, and SA3 ash samples (Table II) before H2SO4 leaching at 80°C for 8 hours.
The SA2 blend and SA3 ash samples leached with 6.12 M H2SO4 contained less Al2O3, K2O, Fe2O3, and TiO2 compared to the original ashes prepared at either 700°C or 1050°C (Tables V and VI). The proportions of these inorganic elements decreased significantly after H2SO4 leaching. This can be attributed to the reaction between alumina in the metakaolinite and sulphuric acid (Altiokka et al., 2010; Kyriakogona, Giannopoulou, and Panias, 2017; Equations [1] and [3]). This sulphuric acid solution selectively dissolved alumina from metakaolinite to form aluminium sulphate in the leach liquor.
Silica associated with metakaolinite is insoluble in the sulphuric acid solution and remained in the leached ash residues (Matjie, Bunt, and van Heerden, 2005; Altiokka et al., 2010).
Soluble potassium, iron, and titanium species from the ash reacted with sulphuric acid to form potassium sulphate, iron sulphate, and titanium sulphate respectively. In addition, the dissolution of Al2O3 and K2O from the ash samples contributed to the SiO2 content in the leached ash samples (Equations [1], [3], and [4]).
The proportion of Al2O3 in the leached SA1 ash produced at i050°C and leached SA3 ash samples prepared at i050°C is consistent with that of SA1 and SA3 ash samples prepared at 1050°C (Table VI). This can be ascribed to the significant amounts of the sparingly soluble silicon spinel, and pseudomullite that were formed in these materials. The XRF results of the coal ash samples and leached ash samples are consistent with those for other coal ash samples and their corresponding leached ash samples (Torma, 1983; Nayak and Panda, 2010; Matjie, Bunt, and van Heerden, 2005; Sangita, Nayak, and Panda, 2017; van der Merwe et al., 2017; Burnet, Murtha, and Dunker, 1984).
The leached SAi ash prepared at i050°C contains a lower proportion of CaO (Table VI) than that of the original SA1 ash produced at 1050°C. However, the proportion of CaO (Table V) increased in the leached SAi ash produced at 700°C, leached SA2 blend ash, and leached SA3 ash samples prepared at 1050°C. This can be attributed to the insolubility of CaO in the sulphuric acid solution. However, other researchers found that Ca2+ reacts with SO42- to form gelatinous calcium sulphate, which precipitates during the leaching of the coal ash samples (Nayak and Panda, 20i0; Matjie, Bunt, and van Heerden, 2005; Sangita, Nayak, and Panda, 2017; Freeman, i993; Torma, 1983 (Equation [5]). The chemical reaction between CaO and H2SO4 to form gelatinous calcium sulphate (gypsum) which occurred during the sulphuric acid leaching of coal ash samples is as follows:
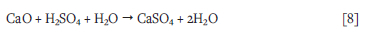
The decrease in the proportions of aluminium, potassium, titanium, and iron in the original ash samples can be attributed to the selective dissolution of alumina from metakaolinite and aluminosilicate glasses, and K from either amorphous K2CO3, K2CO3 remnants, or potassium aluminosilicate glass (Tables V and VI) in the sulphuric acid solution. Fe can be dissolved from haematite, magnetite, and aluminosilicate glasses , while Ti as TiO2 can be dissolved from rutile/anatase (Equation [2]) and aluminosilicate glasses.
Finally, the XRF results of the leached ash samples are in good agreement with the XRD results (Tables III, IV, V and VI). The chemical properties of the ash samples and their corresponding leached ash samples varied substantially after H2SO4 leaching.
Mineralogy leached ash samples mineralogy
XRD results of the ash samples leached with 6.12 M H2SO4 using 1:5 and 1:10 S/L ratios are shown in Table VII. These samples, apart from the leached SA2 blend ash prepared at 1050°C, contain major quartz, anhydrite, and amorphous materials which are insoluble in sulphuric acid. Minor minerals (dolomite, calcite, gypsum, portlandite, and pseudomullite) along with trace minerals (kaolinite, graphite, illite, muscovite, cristobalite) are insoluble in sulphuric acid. In addition, other minor minerals (pyrrhotite, haematite, K2CO3, rutile, periclase, and anatase) are soluble in the sulphuric acid solution. Furthermore, microcline and muscovite are only sparingly soluble or insoluble soluble in H2SO4 (Table VII).
The presence of dolomite, calcite, kaolinite, and graphite in the other leached ash samples could be associated with the small proportion of unburnt coal particles. In addition, the presence of coal char could be due to the encapsulation of coal particles by the molten ash formed during combustion of the coals. Dolomite and calcite in the other leached ash samples are associated with the reactions of CaO/MgO and CO2 during sulphuric acid leaching.
The leached SA2 blend ash produced at 1050°C using 1:10 S/L ratio is characterized by high concentrations of the microcline and anhydrite, and low proportions of quartz and amorphous materials (Table VIII) compared to other samples. Anhydrite crystallizes out from the gelatinous calcium sulphate precipitate and gypsum during drying of leached ashes at 80°C (Freeman, 1993; Matjie, Bunt, and van Heerden, 2005). Furthermore, the XRD results of the leached ash samples (SA1 ash, SA3 ash, and SA2 blend ash) show that the proportion of quartz (Table VIII) in the SAi ash sample produced at 700°C decreased after leaching, due to the formation of the gelatinous calcium sulphate precipitate.
The microcline in the unburnt coal char is insoluble in sulphuric acid. The synthetic potassium feldspar minerals, including microcline, form when potassium species react with metakaolinite at 600-700°C (Heller-Kallai, 1978; Heller-Kallai and Lapides, 2003, Matjie, et al., 202i). Other potassium feldspars present in the coals begin to transform at temperatures of >1050°C (Matjie et al., 2021).
In addition, quartz and metakaolinite could dissolve in the potassium carbonate melt during heating of the SA2 blend at 1050°C, and microcline/anorthite could crystallize during the cooling of the ash melt (Table VIII). As expected, the mineralogical properties of the leached ash samples differ significantly from those of the original ash samples (Tables III, IV, VII, and VIII). This can be ascribed to the dissolution of soluble minerals in the H2SO4 solution and alumina associated with metakaolinite and Al, Ti, K, Mg and Fe from other aluminosilicate glasses of illite, muscovite and microcline. Furthermore, the significant difference in the mineralogical properties could be linked to the carbonation of calcium and magnesium oxides, as well as the in-situ capturing of sulphur dioxide to form calcium sulphates during leaching.
Dissolution efficiencies of Al, K, and Ti as calculated from XRF results
The dissolution efficiencies for Al, K, and Ti as calculated from the XRF results after H2SO4 leaching of the coal ash samples are presented in Figure 5. Sulphuric acid leaching of the SA1 ash, produced at 700°C with the 6.12 M H2SO4 using a 1:5 S/L ratio, achieved higher dissolution efficiencies of Al, K, and Ti compared to the SA1 ash prepared at 1050°C (Figure 5). The 1:10 S/L ratio attained higher dissolution efficiencies of Al, K, and Ti compared to the SA1 ashes prepared at 1050°C (Figure 5). Also, as the volume/concentration of sulphuric acid solution is increased, precipitation of gelatinous calcium sulphate increases. This can subsequently result in lower dissolution efficiencies of Al and K species during H2SO4 leaching of the ash samples with higher proportions of CaO. This gelatinous calcium sulphate lost water when heated at 80°C to form anhydrite (Tables VII and VIII).
The low dissolution efficiencies of Al and K for the SA1 ash samples produced at 1050°C are due to the presence of springly soluble and insoluble minerals (pseudomullite, silicon spinel, and Ca-anorthite/K-microcline in the ash samples. The interactions of association minerals (calcite/dolomite/K2CO3 associated with kaolinite) at temperatures of >1000°C resulted in the formation of aluminosilicate melts with K, Fe, and Ca impurities (Klopper, Strydom, and Bunt, 2012). Pseudomullite, anorthite, and synthetic potassium feldspars can subsequently crystallize from this molten solution or partially melted particles. These minerals, which are sparely soluble or insoluble in the sulphuric acid solution, can also be formed at 600-700°C via solid-state reactions (Heller-Kallai, 1978; Heller-Kallai and Lapides, 2003, Matjie et al., 2021).
The low Ti dissolution efficiencies could be due to Ti being captured in the insoluble crystalline phases in ashes formed during combustion. Also, insufficient free sulphuric acid in the leach liquor could contribute to the lower dissolution efficiency of Ti from rutile and anatase.
The SA2 blend ash samples prepared at 700°C achieved the highest dissolution efficiency of Al, and K (Figure 5) when leached with the 6.12 M H2SO4 using S/L ratios of either 1:5 or 1:10 compared to those of SA2 blend ashes produced at 1050°C. A similar dissolution efficiency of Ti was attained after leaching SA2 blend ashes produced at either 700°C or 1050°C with 6.12 M H2SO4 using S/L ratios of 1:5 and 1:10. The leaching of SA1 ash prepared at 1050°C with a S/L ratio of 1:10 achieved higher dissolution efficiencies of Al and K than those of SA3 ashes samples prepared at 1050°C. The high dissolution efficiencies for Al and K (Figure 5) from the SA2 blend ash samples prepared at 700°C are due to the dissolution of Al and K from metakaolinite, the aluminosilicates of illite and microcline, K2CO3 remnants, and soluble K melt present in these ash samples. These soluble potassium-containing species in the ash could react with sulphuric acid to form potassium sulphate, which in turn could prevent the formation of gelatinous calcium sulphate precipitate (Klopper, Strydom, and Bunt, 2012).
Leaching experiments of the SA3 ash sample prepared at 700°C, with 6.12 M H2SO4 using a 1:5 S/L ratio, yielded higher dissolution efficiencies of Al and K (Figure 5) compared to those of the SA3 ash produced at 700°C using a solid to liquid ratio of 1:10. Lower or zero dissolution efficiency of Ti was achieved by H2SO4 leaching of SA3 ashes using solid to liquid ratios of either 1:5 or 1.10. The lower dissolution efficiency of Ti from the SA3 ash, could be due to either a lower Ti content in the original coal sample or the association of Ti with minerals and glassy phases in these ashes which are insoluble in sulphuric acid. The SA3 ash sample prepared at 1050°C achieved the lowest dissolution efficiency of Al, K, and Ti (Figure 5) when leached with the 6.12 M H2SO4 at either 1:5 S/L or 1:10 S/L.
Low dissolution efficiencies of these elements are attributed to the formation of significant amounts of gelatinous calcium sulphate precipitate and the presence of pseudomullite, silicon spinel, microcline, and anorthite in the ash samples. An Al dissolution efficiency of 85% was obtained by Matjie, Bunt, and van Heerden (2005) when sintered pellets of another South African fly ash, and quicklime (CaO) were leached with 6.12 M H2SO4 at a temperature of 80°C for 8 hours. Torma (1983) obtained a maximum Al dissolution efficiency of 99% after the H2SO4 leaching of calcined pellets, which comprise fly ash from an overseas power station and calcium carbonate. Van der Merwe et al. (2017) showed a low dissolution efficiency of 47% for Al using conventional hydrometallurgical methods to selectively solubilize Al from an ultrafine South African coal fly ash using ammonium sulphate/sulphite solution. All ash samples containing metakaolinite (alumina soluble in H2SO4 and silica insoluble in H2SO4), amorphous glasses of illite and microcline, and potassium carbonate remnants prepared at 700°C achieved the highest dissolution efficiencies of aluminium and potassium compared to the 1050°C ash samples containing pseudomullite, silicon spinel, microcline, and anorthite. Microcline and potassium feldspar, which are insoluble in H2SO4, are associated with the calculated negative dissolution efficiencies of Al and K and resulted in the extrapolation errors. Furthermore, the calculated negative dissolution efficiency of titanium could be attributed to the polymerization or hydrolysis of titanium species to produce agglomerates that are insoluble in sulphuric acid. According to Han, Rubcumintara, and Fuerstenau (1987) and Lasheen (2009) titanium polymerizes to produce hydrolysed titanium agglomerates which are insoluble in sulphuric acid.
Conclusions
In this study, the Al as alumina from metakaolinite, K from potassium carbonate melt and potassium carbonate/sulphate, and Ti from rutile/anatase contained in the coal ash samples were selectively dissolved in concentrated sulphuric acid solution.
Based on the results the following conclusions can be drawn:
Ashes of kaolinite-rich coals (SA1 and SA3) have comparable chemical and mineralogical properties. The corresponding metakaolinite-rich ash samples from kaolinite-rich coals that were produced at either 700°C or 1050°C have similar chemical properties but different mineralogical properties, apart from the ash of coal SA2 blend with 10% K2CO3 additive.
All ash samples produced at 1050°C contain lower proportions of amorphous phase (metakaolinite and aluminosilicate glasses of illite, muscovite, and microcline), higher proportions of pseudomullite, silicon spinel, K-feldspar, and anorthite than all ash samples prepared at 700°C, except for the SA2 blend ash. The metakaolinite in coal ash samples transformed at 1050°C to silicon spinel and pseudomullite as the ashing temperatures increased during combustion of the coals.
As expected, the mineralogical and chemical properties of the ashes leached with sulphuric acid are significantly different to those of the original ash samples (Tables II, III and IV). This can be attributed to the dissolution of K2CO3 remnants and K ions contained in the potassium melt, Al associated with metakaolinite, and soluble Al, K, and Ti species contained in the aluminosilicate glasses in sulphuric acid. Low carbonation of calcium oxide and magnesium oxide to form calcite and dolomite (estimated to be <20% CO2 in-situ captured) respectively, as well as the in-situ capturing of sulphur dioxide with free CaO to form calcium sulphates (estimated to be >90% SO2 in-situ captured) in the leaching tests and are associated with the significant differences in mineralogical and chemical properties of the leached ashes. Also, the gelatinous calcium sulphate precipitation that forms during the sulphuric acid leaching of coal ashes can contribute to different mineralogical properties of the leached ashes.
All ash samples containing H2SO4 -soluble species of Al associated with metakaolinite, soluble K associated with the potassium melt, and the added K2CO3 remnants formed at 700°C achieved higher dissolution efficiencies of aluminium and potassium compared to the ash samples containing pseudomullite, silicon spinel, microcline and anorthite prepared at i050°C. The SA2 blend ash prepared at 700°C achieve the highest Al dissolution efficiencies of 87% and 82%, and highest K dissolution efficiencies of 89% and 91% during leaching with 6.12 M H2SO4 using 1:5 ratios of and 1:10, respectively. The higher dissolution efficiencies can be attributed to the presence of metakaolinite containing acid-soluble Al2O3 and aluminosilicate glasses with soluble species of Al, K, Ti and potassium carbonate remnants in these ash samples, which are soluble in the sulphuric acid solution.
The results indicate that the leaching efficiency of certain metallic and non metallic species from metakaolinite-rich coal ash containing soluble K, Al, and Ti species in the aluminosilicate glasses produced at lower' temperatures can be improved by the addition of K2CO3. This is particularly applicable to fluidized-bed combustion ashes.
Recommendations
The recovery of soluble aluminium and potassium species from coal ash and ash with a potassium additive prepared under conditions used in this study may be possible with 6.12 M H2SO4 leaching step. The aluminium(III), potassium(I), titanium(IV) and sulphate-containing leach liquor could be utilized as coagulant in water treatment. The leach liquor could also be subjected to solvent extraction produce pure iron-free aluminium sulphate solution for utilization in paper mills and dye manufacturing. Titanium sulphate solution is used as a mordant in dyeing, and TiO2 in paint, coatings, paper, pigments, and chemical industries and water treatment; and potassium sulphate liquid as fertilizer. In addition, the leached residues, neutralized with quicklime, could be utilized as aggregate in the road and building industries, brick manufacturing, and masonry concrete applications. Furthermore, the ashes derived from the existing low-temperature combustion of coal via fluidized-bed combustion or gasification technologies could be an excellent feedstock for mineral recovery.
The proposed process for recovery of Al, Ti, and K from metakaolinite-rich coal ashes and ashes of coal fines blended with i0% K2CO3 additive using the H2SO4 leaching should be subjected to an economic evaluation.
Acknowledgements
The information presented in this paper is based on research financially supported by the South African Research Chairs Initiative (SARChI) of the Department of Science and Technology and National Research Foundation of South Africa (Coal Research Chair Grant No. 86880). Any opinion, finding, or conclusion or recommendation expressed is that of the authors and the NRF does not accept any liability in this regard. The authors would also like to acknowledge Mrs B. Venter for the XRF and XRD analyses. We also would like to acknowledge Mintek researchers for providing the laboratory rotary kiln and muffle furnace for the preparation of coal ashes at lower temperatures for the H2SO4 leaching experiments. Mr Katlego Mphahlele is thanked for preparing the figures and tables, as well as the preparation of coal and K2CO3 blends for XRD and XRF analysis.
References
Alguacil, F.J., Amer, S., and Luis, A. 1987. The application of Primene 81R for the purification of concentrated aluminium sulphate solutions from leaching of clay minerals. Hydrometallurgy, vol. 18. pp. 75-92. [ Links ]
Altiokka, M.R., Akalin, H., Melek, N., and Akyalcin, S. 2010. Investigation of the dissolution kinetics of meta-kaolin in H2SO4 solution. Industrial and Engineering Chemistry Research. vol. 49. pp. 12379-12382. [ Links ]
Badenhorst, C. 2019. Char extracted from coal ash as a replacement for natural graphite - 'Charphite'. PhD thesis University of Johannesburg, South Africa. pp. 21-31 [ Links ]
Barry, T.S., Uysal, T., Birinci, M., and Erdemoglu, M. 2018. Thermal and mechanical activation in acid leaching processes of non-bauxite ores available for alumina production-A review. The Society for Mining, Metallurgy and Exploration. [ Links ]
Bryers, R.W. 1986. Influence of segregated mineral matter in coal. Mineral Matter and Ash in Coal. Vorres, K., (ed). American Chemical Society. pp. 351-374 [ Links ]
Bunt, J.R. 2006. A new dissection methodology and investigation into coal property transformational behaviour impacting on a commercial-scale Sasol-Lurgi MK IV fixed-bed gasifier. PhD thesis, North-West University: Potchefstroom, South Africa. [ Links ]
Burnet, G., Murtha, M.J., and Dunker, J.W. 1984. Recovery of metals from coal ash. DOE Iowas State University Ames: Ames Laboratory. [ Links ]
Chinchón, J.S.E., Va'zquez, A., Alastuey, A., and López-Soler, A. 1993. X-ray diffraction analysis of oxidisable sulphides in aggregates used in concrete. Materials and Structures, vol. 26. pp. 24-29. [ Links ]
Collins, A. C. 2019. Extraction of K, AI and Ti containing compounds from ash produced by low temperature combustion. PhD thesis, North-West University: Potchefstroom, South Africa. [ Links ]
Collins, A.C., Strydom, C.A., van Dyk, J.C., and Bunt, J.R. 2018. FACTSAGE™ thermo-equilibrium simulations of mineral transformations in coal combustion ash. Journal of Southern African Institute of Mining and Metallurgy, vol. 218. pp. 1059-1066. [ Links ]
Department of Environmental Affairs. 2012. National waste information baseline. Pretoria. [ Links ]
Department of Environmental Affairs. 2018. South Africa - State of waste report. 2nd draft report. Government Printer, Pretoria. [ Links ]
Freeman, M.J. 1993. The manufacture of alumina in South Africa Mintek, Randburg, South Africa. [ Links ]
Ginster, M. and Matjie, R.H. 2005. Beneficial utilisation of Sasol coal gasification ash. Proceedings of World of Coal Ash (WOCA) Conference. Center for Applied Energy Research, Lexington, University of Kentucky, KY. http://www.flyash.info/ [ Links ]
Glover, G., van Der Walt, T.J., Glasser, D., Prinsloo, N.M., and Hildebrandt, D. 1995. DRIFT spectroscopy and optimal reflectance of heat-treated coal from a quenched gasifier. Fuel, vol. 74. pp. 1216-1219. [ Links ]
Han, K.N., Rubcumintara, T., and Fuerstenau, M.C. 1987. Leaching behavior of ilmenite with sulphuric acid. Metallurgical Transaction B, vol. h18. pp. 325-330. [ Links ]
Hattingh, B.B., Everson, R.C., Neomagus, H.W.J.P., and Bunt, J.R. 2011. Assessing the catalytic effect of coal ash constituents on the CO2 gasification rate of high ash, South African coal. Fuel Processing Technology, vol. 92. pp. 2048-2054. [ Links ]
Hlatshwayo, T.B., Matjie, R.H., Li, Z., and Ward, C.R. 2009. Mineralogical characterisation of Sasol feed coals and corresponding gasification ash constituents. Energy & Fuels, vol. 23. pp. 2867-2873. [ Links ]
Heller-Kalla, L. 1978. Reactions of salts with kaolinite at elevated temperatures. Clay Miner, vol. 13. pp. 221. [ Links ]
Heller-Kallai, L. and Lapides, I. 2003. Thermal reactions of kaolinite with potassium carbonate. Journal of Thermal Analysis and Calorimetry, vol. 71. pp.689-698. [ Links ]
Ibrahim, K.M., Moumani, M.K., and Mohammad, S.K. 2018. Extraction of y-alumina from low-cost kaolin. Resources, vol. 7, no. 63. pp. 2-12. [ Links ]
Izquierdo, M. and Querol, X. 2012. Leaching behaviour of elements from coal combustion fly ash: An overview. International Journal of Coal Geology, vol. 94. pp. 54-66. [ Links ]
Khan, M. I., Ullah, K.H., Khairun Azizi, A., Sufian, S., Man, Z., Siyal, A., Muhammad, N., and Rehman, M. 2017. The pyrolysis kinetics of the conversion of Malaysian kaolin to metakaolin. Applied Clay Science, vol. 146. pp. 152-161. [ Links ]
Klopper, L., Strydom, C.A., and Bunt, J.R. 2012. Influence of added potassium and sodium carbonates on CO2 reactivity of the char from a demineralised inertinite rich bituminous coal. Journal of Analytical and Applied Pyrolysis, vol. 96. pp. 188-195. [ Links ]
Kolay, P.K. 2001. Physical, chemical, mineralogical, and thermal properties of cenospheres from an ash lagoon. Cement and Concrete Research, vol. 31. pp. 539-542. [ Links ]
Kyriakogona, K., Giannopoulou, I., and Panias, D. 2017. Extraction of aluminium from kaolin: A comparative study of hydrometallurgical processes. Proceedings of the 3rd World Congress on Mechanical, Chemical, and Material Engineering (MCM'17), Paper no. MMME 133. https://doi:10.11159/mmme17.133 [ Links ]
Lasheen, T.A. 2009. Sulphate digestion process for high purity TiO2 from titania slag. Front. Chem. Eng. China, vol. 3. pp. 155-160. [ Links ]
Mark, U., Anyakwao, C.N., Onyemaobi, O., and Nwobodo, C.S. 2019. The thermal activation of Nsu clay for enhanced alumina leaching response. International Journal of Engineering and Technologies, vol. 16. pp. 34-46. [ Links ]
Matjie, R., Bunt, J., Stokes, W., Bijzet, H., Mphahlele, K., Uwaoma, R., and Strydom, C. 2021. Interactions between kaolinite, organic matter, and potassium compounds at elevated temperatures during pyrolysis of caking coal and its density-separated fractions. 2021. Energy Fuels, vol. 35. pp. 13268-13280. [ Links ]
Matjie, R.H., Bunt, J.R., and van Heerden, J.H.P. 2005. Extraction of alumina from coal fly ash generated from a selected low rank bituminous South African coal. Minerals Engineering, vol. 18. pp. 299-310. [ Links ]
Matjie, R.H., van Alphen, C., and Pistorius, P.C. 2006. Mineralogical characterisation of Secunda gasifier feedstock and coarse ash. Minerals Engineering, vol. 19. pp. 256-261. [ Links ]
Matjie, R.H., Lesufi, J. M., Bunt, J.R., Strydom, C.A., Schobert, H.H., and Uwaoma, R. 2018. In situ capturing and absorption of sulfur gases formed during thermal treatment of South African coals. ACS Omega, vol. 3. pp. 14201-14212. [ Links ]
Moyo, A., Filho, J.A., Harrison, S., and Broadhurst, J. 2018. Characterising the environmental risks of coal preparation wastes: A study of fine coal slurry waste and discards from South African collieries. Proceedings of the11th ICARD | IMWA | MWD Conference - 'Risk to Opportunity'. pp. 417-423. [ Links ]
Muzenda, E. 2014. Potential uses of South African coal fines: A review. Proceeidngs of the 3rd International Conference on Mechanical, Electronics and Mechatronics Engineering. pp. 37-39. [ Links ]
Neupane, G. and Donahoe, R.J. 2013. Leachability of elements in alkaline and acidic coal fly ash samples during batch and column leaching tests. Fuel, vol. 104. pp. 758-770. [ Links ]
Norrish, K. and Hutton, J.T. 1969. An accurate X-ray spectrographic method for the analysis of a wide range of geological samples. Geochimica et Cosmochimica Acta, vol. 33. pp. 431-453. [ Links ]
Numluk, P. and Chaisena, A. 2012. Sulfuric acid and ammonium sulphate leaching of alumina from Lampang clay. E-Journal of Chemistry, vol. 9. pp. 1364-1372, [ Links ]
Osawa, C.C. and Bertran, C.A. 2005. Mullite formation from mixtures of alumina and silica sols: Mechanism and pH Effect. Journal of the Brazilian Chemical Society, vol. 16. pp. 251-258. [ Links ]
Paul, M., Seferinoglu, M., Aycik, G.A., Sandstrom, A., Smith, M.L., and Paul, J. 2006. Acid leaching of ash and coal: Time dependence and trace element occurrences. International Journal of Mineral Processing, vol. 79. pp. 27-41. [ Links ]
Phillips, C.V. and Wills, K.J. 1982. A laboratory study of the extraction of alumina of smelter grade from China clay micaceous residues by nitric acid route. Hydrometallurgy, vol. 9. pp. 15-28. [ Links ]
Rautenbach, R., Matjie, R., Strydom, C., and Bunt, J. 2021. Transformation of inherent and extraneous minerals in feed coals of commercial power stations and their density-separated fractions. Energy Geoscience, vol. 2. pp. 136-147. [ Links ]
Rautenbach, R., Strydom, C. A., Bunt, J. R., Matjie, R. H., Campell, Q. P., and van Alphen, C. 2019. Mineralogical, chemical, and petrographic properties of selected South African power stations' feed coals and their corresponding density separated fractions using float-sink and reflux classification methods. International Journal of Coal Preparation and Utilization, vol. 39. pp. 421-446. [ Links ]
Reddick, J.F., von Blottnitz, H., and Kothuis, B. 2007. A cleaner production assessment of the ultra-fine coal waste generated in South Africa. Journal of the Southern African Institute of Mining and Metallurgy, vol. 107. pp. 55-60. [ Links ]
Reynolds-Clause, K. and Singh, N. 2019. South Africa's power producer's revised coal ash strategy and implementation progress. Coal Combustion and Gasification Products, vol. 11. pp. 10-17. http://www.flyash.info/ [ Links ]
Rietveld, H.M. 1969. A profile refinement method for nuclear and magnetic structures. Journal of Applied Crystallography, vol. 2. pp. 65-71. [ Links ]
Sangita, S., Nayak, N., and Panda, C.R. 2017. Extraction of aluminium as aluminium sulphate from thermal power plant ashes. Transaction of the Nonferrous Metals Society of China, vol. 27. pp. 2082-2089. [ Links ]
Seferinoglu, M. 2003. Acid leaching of coal and coal-ashes. Fuel, vol. 82. pp. 1721-1734. [ Links ]
Seidel, A. 1999. Self-inhibition of aluminium leaching from coal fly ash by sulfuric acid. Chemical Engineering Journal, vol. 72. pp. 195-207. [ Links ]
Speukman, S.A. 2012. Basics of X-ray powder diffraction. http://prism.mit.edu/xray [accessed 14 February 2019]. [ Links ]
Teklay, A., Yin, C., Rosendahl, L., and B0jer, M. 2014. Calcination of kaolinite clay particles for cement production: A modelling study. Cement and Concrete. Research, vol. 61-62. pp. 11-19. [ Links ]
Torma, A.E. 1983. Extraction of Aluminium from fly ash. Metals, vol. 37. pp. 589-592. [ Links ]
Van Alphen, C. 2005. Factors influencing fly ash formation and slag deposit formation (slagging) on combusting a South African pulverised fuel in a 200MWe boiler. PhD thesis, University of the Witwatersrand. [ Links ]
Van der Merwe, E.M., Gray, C.L., Castleman, B.A., Mohamed, S., Kruger, R.A., and Doucet, F.J. 2017. Ammonium sulphate and/or ammonium bisulphate as extracting agents for the recovery of aluminium from ultrafine coal fly ash. Hydrometallurgy, vol. 171. pp. 185-190. [ Links ]
Van Dyk, J.R., Benson, S.A., Laumb, M.L., and Waanders, B. 2009. Coal and coal ash characteristics to understand mineral transformations and slag formation. Fuel, vol. 88. pp. 1057-1063. [ Links ]
Van Dyk, J.C., Waanders, F.B., and Hack, K. 2008. Behaviour of calcium-containing minerals in the mechanism towards in situ CO2 capture during gasification. Fuel, vol. 87. pp. 2388-2393. [ Links ]
Vassilev, S.T. 2004. Phase-mineral and chemical composition of coal fly ashes as a basis for their multicomponent utilisation. 2. Characterisation of ceramic cenosphere and salt concentrates. Fuel, vol. 83. pp. 585-603. [ Links ]
Uwaoma, R.C., Strydom, C.A., Matjie, R.H., Bunt, J.R., and Van Dyk, J.C. 2020. The influence of the roof and floor geological structures on the ash composition produced from coal at UCG temperatures. International Journal of Coal Preparation and Utilisation, vol. 40. pp. 247-265. [ Links ]
Wu, C., Yu, H., and Zang, H. 2012. Extraction of aluminium by pressure acid-leaching method from coal fly ash. Transaction of the Nonferrous Metals Society of China, vol. 22. pp. 2282-2288. [ Links ]
Yanti, E.D., and Pratiwi, I. 2018. Correlation between thermal behaviour of clays and their chemical and mineralogical composition: a review. Earth and Environmental Science, vol. 118. pp. 1-4. [ Links ]
 Correspondence:
Correspondence:
R.H. Matjie
Email: matjie4@gmail.com
Received: 10 Mar. 2020
Revised: 13 Jun. 2022
Accepted: 3 Aug. 2022
Published: August 2022














