Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.122 n.8 Johannesburg Aug. 2022
http://dx.doi.org/10.17159/2411-9717/2001/2022
COAL EDITION
Novel ceramic composites produced from coal discards with potential application in the building and construction sectors
O. Eterigho-IkelegbeI; R. TrammellII; S.O. BadaI
IDSI-NRF SARChI Clean Coal Technology Research Group, School of Chemical & Metallurgy, Faculty of Engineering and the Built Environment, University of the Witwatersrand, South Africa. ORCID: S.O. Bada: https://orcid.org/0000-0002-1079-3492; O. Eterigho-Ikelegbe: https://orcid.org/0000-0001-6384-7741
IISemplastics, United States
SYNOPSIS
In response to the enormous amounts of coal discard generated during coal mining and preparation, the development of an efficient and sustainable strategic use of this resource is essential. Furthermore, the rising urban population over the next decades is confronted with the depletion of quality raw materials for building components. To this end, this study reports new information on the morphology, water absorption, and flexural strength properties of ceramic composites produced from three different coal discards and polysiloxane pre-ceramic polymer (PCP) resin. In addition, test results relating to the continuous operating temperature, chemical resistance, and efflorescence potential of the composites are presented. The results show that the water absorption and flexural strength of the coal composites, up to 1.94% and 36.46 MPa respectively, exceed the requirements for ceramic and clay roof tiles. The continuous operating temperature of composites is found to be more thermally stable than conventional roofing tiles (concrete and ceramic) between ambient temperature and 600°C. In addition, the excellent chemical resistance of the composites (94.43%-99.98%) compared to conventional roofing tiles (67.82%-99.97%) eliminates the need for additional external coatings. The interesting results documented so far suggest that this technique could be used to produce low-temperature application building products such as bricks, panels, roofing tiles, etc. This new recycling technique offers an excellent opportunity to eliminate enormous volumes of coal discard and to advance the circular economy in the coal industry.
Keywords: coal discard, waste utilization, composites, chemical resistance, circular economy.
Introduction
Coal is a relatively low-cost source of energy that has contributed enormously to global economic development. The mining and processing of coal in the industrialized and developing countries has led to the production of billions of tons of coal discard. With coal meeting about 77% (http://www.energy.gov.za) of the energy needs in South Africa, coal production will likely remain significant, at least for the foreseeable future. According to the website, about 60 Mt coal waste are produced each year, adding to an already accumulated surplus of 1 Gt. These coal discards heaped on arable land or retention ponds could pose enormous environmental hazards such as spontaneous combustion if not properly managed. Most of these dumps are known to contain low- to high-quality rich inorganic-organic compounds, depending on the source. Although some coal discard dumps can be beneficiated to yield clean coal, the process could be somewhat unattractive when the age of the coal is taken into consideration. Other factors that are considered are the degree of oxidation or weathering of the coal, the potentially high cost of treatment, and the subsequent generation of discard after beneficiation.
A panoply of innovative research on the beneficiation of South Africa's coal discard into alternative products exists in the literature. Tambwe, Kotsiopoulos, and Harrison. (2020) demonstrated the laboratory-scale heap leaching potential of pyrite from sulphidic coal discard to reduce its risk of disposal. Nevertheless, the process still leaves behind desulphurized coal discard as waste. The combustion of SA coal discard in a circulating fluidized bed (CFB) boiler was demonstrated and reported by Belaid et al. (2013). Due to the similar combustion profile of the co-combustion of 70% coal discard and 30% refuse-derived fuel compared to 100% coal, Isaac and Bada (2020) proposed that the blend could be considered an alternative fuel for use in existing pulverized boilers. The use of different South African coal discards to produce activated carbon as porous adsorbents for natural gas storage was reported by Abdulsalam et al. (2019; 2020). Amaral Filho et al. (2020) demonstrated that low-sulphur ultrafine coal discard fabricated topsoil outperformed native soil for rehabilitating coal mine sites. Despite the numerous ongoing investigations on coal discard utilization, it is worth mentioning that these approaches would not fully utilize the over 1Gt of coal discard in South Africa.
An article by Haywood et al. (2019) calls for the introduction and use of mineral waste into the South African economy. The author reported that the secondary utilization of mining waste is a preferred choice over waste disposal. In a sense, it opens the door to value creation, sustainable development, and reducing the responsibilities associated with the waste stream. Globally, the repurposing of coal discard aligns with the concepts of waste valorization, the circular economy, and sustainability. In this context, it could be considered a practical solution to pollution problems caused by coal discard dumps. To this end, among the potential re-use strategies of South African coal discard, for the first time, Eterigho-Ikelegbe, Trammell, and Bada. (2021) reported on the density, porosity, compression strength, pyrolysis shrinkage, etc. of coal composites produced from South African coal discard and a polysiloxane preceramic polymer (PCP) resin. PCPs are known to convert into thermoset polymers and Si-based ceramics upon crosslinking and pyrolysis, respectively. The polysiloxanes (R1R2SiO)n, contains -Si-O- as the basic backbone or inorganic network with various side reactive and/or inert groups on the Si atom site. These polymers are ideal precursors for producing SiCO bulk ceramic, coatings, foams, energy storage materials, ceramic fibres, and ceramic matrix composites (Bernardo et al., 2014; Wen et al., 2022; Wen, Yu, and Riedel 2020). By blending a suitable coal discard dump of about lMt with a known quantity of PCP resin, following heat treatment, structural coal composites could be produced without generating secondary waste. Whereas with the washing of this discard dump and co-firing with biomass, additional wastes like flyash and discard are generated.
In this article, we report new scientific information on the morphology, water absorption, and flexural strength of the produced coal composites. Furthermore, the continuous operating temperature, chemical resistance, and efflorescence potential of the coal discard composites are presented. Some of the results obtained are then compared with those of standard building materials (concrete and ceramic roof tiles). The performance of the coal composites produced in this study in terms of their temperature resistance up to 600°C and chemical resistance equivalent to 'thermal stability and durability', respectively, would be of great interest to the building/construction and coal industries. These results suggest the prospect of repurposing South African coal discard to produce ceramic composites for building/construction purposes.
Experimental
Materials and method
Three coal discards were selected which were sourced from the Witbank coalfield and the KwaZulu-Natal coal area. The coal discards are denoted GTS (Greenside tailing slurry), PCD (Proteas coal discard), and LCS (Likeflow coal slurry). A specially formulated PCP resin by XMAT®, the Advanced Materials Division of Semplastics, Florida, USA was used as a binder to develop the coal composites. The as-received coal discard lumps were crushed and pulverized to about 80% passing 45 μm. The coal particles were thoroughly mixed with the PCP resin, after which the mixture was pressed to 6.89 MPa in low-cost rectangular moulds. The moulds are machined steel with a smooth matte finish, and a PTFE release film used to line the mould was in direct contact with the material being pressed. The processing of the coal composites occurred in two stages - curing and pyrolysis. The moulded bodies were cured to about 120°C in an oven to harden and fuse the resin and the coal particles. During cross-linking, the functional groups in the PCP and coal particles presumably undergo a series of reactions - condensation and combination reactions. After curing, the cured bodies were pyrolysed in a furnace under a nitrogen flow at 236 mL/min up to 1000°C for 10 hours. The pyrolysis step facilitates the molecular and chemical interactions between the PCP and the coal constituents. In parallel, the elimination of some volatile and hydrogen species occurs during pyrolysis, thereby converting the cured bodies to ceramic-like composites. A schematic of the production process of the coal composites is depicted in Figure 1.
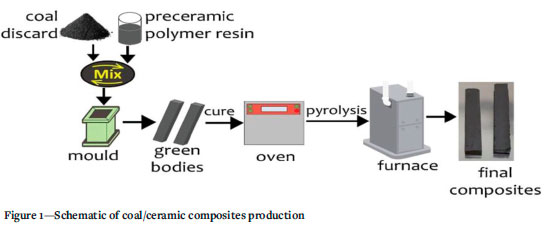
Characterization of coal discard and coal/ceramic composite
The following techniques were used to characterize the coal discard and the produced coal composites.
> Proximate analysis was conducted using approximately 1 g of each sample in a thermogravimetric analyser (TGA701, LECO Corp., USA) according to ASTM D5142.
> A bench-top energy-dispersive X-ray fluorescence (EDXRF) elemental analyser (NEX QC+ QuantEZ) was used to semi-quantitatively determine the major and minor elements of the coal discard.
> Carbon and sulphur contents were obtained using a LECO SC-132 analyser (LECO Corp., USA) following the ASTM D4239 standard.
> The morphology of the composites was observed under a Zeiss Sigma 300 VP scanning electron microscope (Carl-Zeiss, Germany).
> Measurement of water absorption and flexural strength of the composites is described in Eterigho-Ikelegbe, Trammell, and Bada (2021).
> The X-ray diffraction patterns of the powdered coal composites were recorded using a Bruker D2 phaser diffractometer (Bruker Corp., Germany). X-ray source Cu Ka radiation (X =1.54184 A) operating at 30 kV and 10 mA was used to scan the sample through a range of 20 angles from 10° to 70°.
> The thermogravimetric analyser was used to estimate the continuous operating temperature of the composites. For the analysis, 1 g of chunk composites were heated in an air atmosphere from room temperature to 950°C at a ramp rate of 10°C/min.
> The resistance of the coal composites to different analytical reagent grade chemicals was evaluated according to ISO 10545-13 for ceramic tiles. The dried composites were immersed in glass bottles containing the chemical for 17 days at ambient temperature and kept covered to minimize evaporation. The chemicals used were
• Ammonium chloride solution (100 g/L)
• Hydrochloric acid (18 % V/V)
• Lactic acid solution (5 % V/V)
• Potassium hydroxide solution (100 g/L and 30 g/L)
• Citric acid solution (100 g/L)
After immersion, the composites were washed with running water and dried in an oven at 105°C to a constant weight until the difference between two successive weighings was less than 0.1 g. The chemical resistance of all the composites was measured in terms of weight loss (WL) using Equation [1].
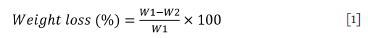
where W1 and W2 are the weights of the composites before immersion and after drying, respectively.
>The efflorescence potential of the coal composites was evaluated according to the ASTM C67 standard test method for structural clay tiles.
Results and discussion
Properties of the coal discard samples
The coal discards differ widely and exhibit different chemical compositions due to the diversity of the coal sources (Table I).
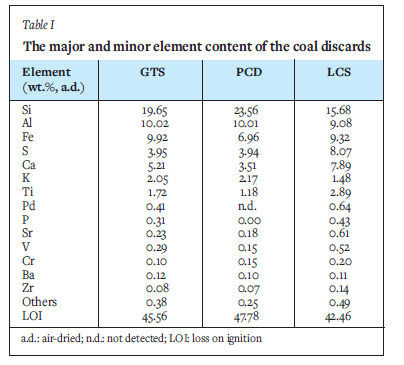
The data presented in Table I shows in quantitative terms that the coal discards contain predominantly Si, Al, and Fe followed by S and Ca in varying quantities. Although the coal discards have similar contents of Al, they differ widely in Si content. This variance is the first evidence of potential differences in the curing and pyrolysis activity of the three coal/PCP blends. Of the coal discards, PCD possesses the highest amount of Si and Al combined (33.57%), followed by GTS (29.67%) and LCS (24.76%). The abundance of Si and Al, especially for PCD2, depends largely on the silica minerals (mostly quartz) and aluminosilicates (especially kaolinite) associated with coal (Huggins, 2002; Mishra et al., 2016). Due to the refractory nature of quartz PCD, with the highest content of Si (23.56%) will degrade at relatively high temperatures compared with GTS and LCS, which contain less Si. The alkali and alkaline earth (K + Ca) metal content in the coal discard average 7.27%, 5.68%, and 9.37% for GTS, PCD, and LCS respectively, and are related to the feldspar species in the discard. Feldspar-containing coal discard could promote the formation of the glassy or vitreous phase (Nicolas et al., 2020) with the PCP resin during pyrolysis of the mixture. This reaction is important for the densification of coal composites during pyrolysis.
Table II presents a summary of the physicochemical properties of the coal discard and coal composites. The ash content of PCD (39.76%) compared with GTS (30.69%) and LCS (15.57%) infers that PCD contains a significant amount of refractory and incombustible minerals. It is anticipated that this property might cause PCD to be weakly reactive and thus lead to a weak bonding interaction between PCD and the PCP resin during pyrolysis. The volatile matter of PCD (10.67%) and LCS (23.72%) infers that PCD and LCS might originate from lean anthracite coal and medium-volatile bituminous coal, respectively (Eterigho-Ikelegbe, Trammell, and Bada 2021). Compared to PCD, higher volatile matter coal discards (GTS and LCS) could have a positive influence on the reactivity of the mixture during pyrolysis and ultimately on the final properties of the composites. From an environmental, scalability, and cost perspective, this straightforward technique is attractive because the coal discard does not require beneficiation before utilization to produce the composites. In addition, the carbon within the coal discard is retained in the composites, as seen in Table II.
Morphological characterization of the coal composites
Visualization of the surface morphology of the composites (Figure 2) shows that there is a relationship between the physiochemical properties of the coal discards presented in Table II and the surface smoothness of the coal composites.
From Figure 2 it appears that the surface of GTS and LCS composites is smoother than that of PCD composites. The surface unevenness and lack of fusibility of PCD composite might be related to the physicochemical properties of the coal discard, in particular the oxygen content. As stated in (Table II), GTS and LCS discards recorded higher oxygen contents compared to PCD. Based on this, it is likely that GTS and LCS are weathered coals and contain a significant amount of oxygen functional groups (Gao et al., 2017; Jian et al., 2019; Nicolas et al., 2020). It could also suggest that GTS and LCS discards likely contain more vitrinite (reactive macerals) whose chemical structure is generally characterized by a relatively high oxygen content compared to other maceral groups (Whitehurst, 1978; ICCP, 1998; van Niekerk, 2008; Roberts, 2015). Based on this, it is possible that coal discard with significant vitrinite maceral and oxygen functional groups, especially if the groups are polar such as -OH and -COOH, could have enhanced reactivity (Jian et al., 2019; Ahamed et al., 2019) with the PCP during the curing and pyrolysis stages of the blend. This phenomenon might be responsible for the good fusibility and smooth surfaces of these composites (GTS and LCS). In summary, since the major raw material of the blend is coal discard, to produce composites of satisfactory fusibility and low surface roughness it is deemed necessary to select coal discard with a significant oxygen content for this process.
Flexural strength and water absorption properties
High-quality coal composites proposed for use as building materials must be able to satisfy specifications for flexural strength and water absorption. Flexural strength is a parameter used to determine the strength requirement for building materials. For example, ISO 15045-4 specifies that the minimum average flexural strength for ceramic tiles should be 35 MPa. Three flexural tests were performed on each composite and the mean values are presented in Figure 3. It can be seen from the figure that PCD composites failed to meet the ISO requirement. However, the flexural strength of GTS and LCS composites, 36.46 MPa and 35.23 MPa respectively, exceeding the 35 MPA requirement for ceramic tiles.
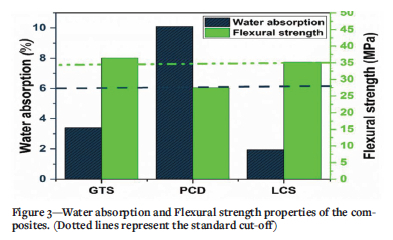
A water absorption test provided valuable information on the degree of compactness of the composites. Based on the standard for clay roof tile, the water absorption must not exceed 6% (ASTM C1167; ASTM C373; Taha et al., 2017). Figure 3 shows that the GTS (3.4%) and LCS (1.94%) composites recorded lower water absorption compared to PCD composites (10.1%). In general terms, the GTS and LCS composites with water absorption that meets the ASTM C1167 standard displayed higher strength. This trend is similar to observations by other researchers (Phonphuak and Thiansem, 2011; Rahman et al., 2015; Yague et al., 2018).
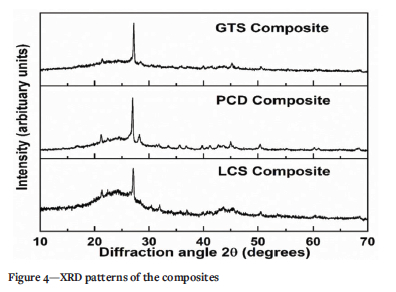
Composites GTS and LCS, which meet the required ASTM C1167 standard for water absorption, may have more compact structures. This could be used as a good indicator for predicting the durability of the composites in terms of efflorescence and frost resistance (Eliche-Quesada et al., 2011; Makarov, Suvorova, and Masloboev, 2019). The superior water absorption and flexural strength properties of GTS and LCS composites over PCD composites can be explained based on the studies by Hu et al. (2017) and Hill and Easter (2019). These authors reported that the molecules of low-ranked or weathered coals are rich in hydrogen bonds that could easily be broken to form hydrogen bonds with other materials. As stated previously, the hydrogen content of the PCD (1.64%) reflects that this coal discard might contain a low amount of hydrogen-bonded structures required to form adequate interactions with the PCP resin during pyrolysis. Furthermore, it has also been reported that hydrogen bonding probably accounts for the glassy properties of coal (Painter, Sobkowiak, and Youtcheff, 1987, Painter 2010). Therefore, the ability to generate a glassy phase during pyrolysis would enhance the fusibility and compaction of the mixture. Hence, LCS composites conforming well with the ASTM C1167 standard specification might be a result of the glassy phase formed during the pyrolysis of LCS coal discard and the PCP resin. This is evidenced in the broad hump around 20= 24° of the XRD patterns of the composites (Figure 4). Taha et al. (2017) reported that the formation of the glassy phase had a positive effect on the mechanical strength of ceramic composites produced from coal.
Continuous operating temperature
The extent of the degradation of 1 g chunk coal composites heated in a thermogravimetric analyser under an air atmosphere from 25°C to 900°C until constant weight loss is depicted in Figures 5a and 5b. As with sintered ceramic, polymer-derived ceramic or ceramic composites produced from PCP resins are notable for displaying high heat resistance (Ionescu et al., 2010; Ding et al., 2012; Fonblanc et al., 2018; Shen, Barrios, and Zhai, 2018). Therefore, it was anticipated that the composites produced in this study would display appreciable heat resistance.
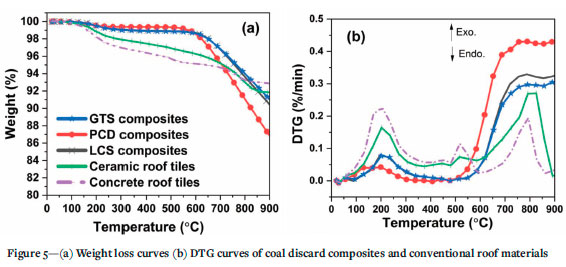
Weight loss in all the samples occurred in two separate stages. No significant change in weight was noted between 25 °C and 125°C, whereas above 125°C a pronounced degradation in the molecular structure of the conventional roof tiles is observed compared to the coal composites. The peaks at 200°C (Figure 5b) indicate that the coal composites contain fewer volatile compounds then the conventional materials. This observation agrees with the data presented in Table II. Of the three composites, PCD composites possessed the lowest volatile matter after devolatilization. The coal composites are also noted to be more stable up to 600°C, with about 99% of the original mass of the composites remaining (Figure 5a). It can be deduced that the compact structure of GTS and LCS composites, as confirmed by their low water absorption values, might be responsible. In addition, the ceramic phases formed during pyrolysis could also have contributed to the heat-resistant nature of the composites up to 600°C. The dramatic weight loss experienced by the PCD composites above 600°C (Figure 5a) could be a result of a loosely compacted structure/bonding as explained under the morphological characterization section.
The weight loss data for the composites at 900°C and the temperature at 5% weight loss (T5%) extracted from the thermograms are presented in Table III. The T5% values of the coal/ceramic composites are slightly higher than for the conventional roofing tiles, confirming their superiority, and that thermally stable structures were indeed formed. Furthermore, the percentage weight loss of the composites is comparable with that for the conventional materials after being subjected to thermal degradation up to 900°C, with PCD displaying the highest degradation of 13%.
Overall, the results demonstrate that the coal composites are superior to conventional roof tiles at temperatures up to 600 °C. The thermal degradation in this range indicates the suitability of the composite as building materials such as roof tiles, bricks, or panels. In addition, the impressive resistance of the composites to degredation at temperatures up to 600°C could be considered a desirable property for fire-resistant construction materials.
Chemical resistance
The ability of the coal composites to resist degradation or physical corrosion caused by chemical media attacks is of paramount importance. To confirm this, the composites were exposed to different chemical reagents for 17 days to evaluate their chemical resistance (Figure 6).
Table IV provides valuable information on the compatibility of the coal composites in different chemical environments. The results show that the composites were moderately affected by base solutions and least affected by acid solutions. This indicates that the bonds of the coal composite structure are more resistant to attack or diffusion of Cl- and H+ ions than OH- ions. In contrast, conventional roofing materials displayed limited resistance to acidic solutions, as indicated by high level of deterioration in terms of weight loss.
Interestingly, the chemical resistance of the coal composites can be correlated with the amorphous nature of the composites (XRD spectra in Figure 4). This amorphous peak, which was attributed to the presence of glassy-like structures (Eterigho-Ikelegbe, Trammell, and Bada, 2021), is more noticeable in LCS composites. The amorphous nature of LCS composites could have been responsible for the unprecedented chemical stability (99.21% -99.98%) compared to GTS and PCD composites. Furthermore, we recorded the porosity of GTS, PCD, and LCS composites to be 7.22%,i7.o8%, and 3.44%, respectively (Eterigho-Ikelegbe, Trammell, and Bada, 2021). Therefore, composites with porosities that meet the ASTM C1167 standard may have restricted chemical absorption. Thus, in addition to the porosities of GTS and LCS composites that meet the ASTM C1167 standard, the stabilized bond network of these composites (as mentioned earlier) possibly serves as a protective mechanism against weight loss caused by chemical attack. The results mean that little to no chemical activity took place at the material-solution interface during immersion in the solutions, making the composites ideal for long-lasting performance.
Equally important, the physical appearance and colour changes of the reagents at the end of the test validated the superiority of the coal discard composites to chemical attack. It was observed that the colourless chemical solutions remained unchanged after testing. In terms of the physical appearance of the composites, we observed no surface damage or visual corrosion effects. In contrast, the conventional roof materials formed solid sediments, and the appearance of the clear, colourless chemical solution altered, especially the acid solutions. Admittedly, the composites must be checked to see whether they retained their mechanical strength, water resistance absorption, and thermal resistance properties. In conclusion, the results so far present the first evidence that coal discard composites would perform well in aggressive environments. They may not require additional surface protective coating, additives, or inhibitors to prevent degradation.
Efflorescence
Construction products are constantly exposed to water for long periods of time, and this can result in functional or aesthetic deterioration. Therefore, the efflorescence potential of as-produced coal composites was analysed according to the method of ASTM C67. This test was performed to assess the potential for migration of water-soluble salts to the surfaces of composites. To simulate realistic conditions, the composites were partially immersed in distilled water for 7 days at ambient temperature, and then dried in an oven for 24 hours. Based on visual inspection of the faces of the tested composites compared to freshly produced composites, there was no deposition of white deposits (crystal line salts) on the surface. The absence of crystalline salts suggests that the composites contain no soluble salts. This indicates that calcium compounds such as Ca(OH)2 and/or CaO that cause efflorescence are not present in the composites. According to Ibraeva, Tarasevskii, and Zhuravlev, (2017), the migration of soluble salts could weaken the binding network of structural bodies and deteriorate the technical and aesthetic performance of building materials. Furthermore, it should be noted that there was no significant change in the weight and dimension of the coal composites after the efflorescence experiment. This indicates that they are of good quality and can be classified as 'not efflorescent'.
Conlusions
This study provides additional information on the properties of coal ceramic composites produced using South African coal discard and a polysiloxane PCP resin. The composites displayed excellent resistance to different chemical solutions, are stable at operating temperatures up to 600°C and contained no soluble salts. As regards suitability for blending with polysiloxane PCP resin, high-ash and quartz-rich coal discard (PCD) was deduced to be only weakly reactive until a relatively high temperature is achieved. This resulted in composites with weak molecular bonding. On the other hand, GTS and LCS coal discard formed composites of good quality that meet building material standards. Coal/ceramic composites that meet or/and exceed building material requirements may also be obtained by investigating other coal discard stockpiles in South Africa and PCP resins. The interesting properties of the coal composites reported in this study indicate that this innovative approach could bode well for avoiding the environmental impact of coal discard and for producing environmentally friendly composite materials for construction applications.
Acknowledgments
The authors wish to thank the Advanced Materials Division of Semplastics, X-MAT®, for developing the coal composite specimens. This research was funded by the Department of Science and Innovation National Research Foundation (DSI-NRF) South African Research Chairs Initiative (SARChI) Clean Coal Technology Grant (Grant Number: 86421).
References
Abdulsalam, J., Mulopo, J., Bada, S.O., and Oboirien, B., 2020. Equilibria and isosteric heat of adsorption of methane on activated carbons derived from South African coal discards. ACS Omega, vol. 5, no. 50. pp.32530-32539. https://doi.org/10.1021/acsomega.0c04744 [ Links ]
Abdulsalam, J., Mulopo, J., Oboirien, B., Bada, S. and Falcon, R. 2019. Experimental evaluation of activated carbon derived from South Africa discard coal for natural gas storage. International Journal of Coal Science & Technology, vol. 6, no. 3. pp. 459-477. https://doi.org/10.1007/s40789-019-0262-5 [ Links ]
Ahamed, M.A.A., Perera, M.S.A., Matthai, S.K., Ranjith, P.G., and Dong-yin, L. 2019. Coal composition and structural variation with rank and its influence on the coal-moisture interactions under coal seam temperature conditions - A review article. Journal of Petroleum Science and Engineering, vol. 180. pp.901-917. https://doi.org/10.1016/j.petrol.2019.06.007 [ Links ]
Amaral Filho, J.R., Firpo, B.A., Broadhurst, J.L., and Harrison, S.T.L., 2020. On the feasibility of South African coal waste for production of 'FabSoil', a Technosol. Minerals Engineering, vol. 146. p. 106059. https://doi.org/10.1016/j.mineng.2019.106059 [ Links ]
ASTM C373-18. 2018. Standard test methods for determination of water absorption and associated properties by vacuum method for pressed ceramic tiles and glass tiles and boil method for extruded ceramic tiles and non-tile fired ceramic whiteware products. https://www.astm.org/c0373-18.html [accessed 24 January 2022]. [ Links ]
ASTM C1167-11. 2017. Standard specification for clay roof tiles. https://www.astm.org/c1167-11r17.html [accessed 24 January 2022]. [ Links ]
ASTM C67. Standard test methods for sampling and testing brick and structural clay tile. https://www.astm.org/c0067-07.html [accessed 24 January 2022]. [ Links ]
Belaid, M., Falcon, R., Vainikka, P., and Patsa, K.V. 2013. Potential and technical basis for utilising coal beneficiation discards in power generation by applying circulating fluidised bed boilers. Proceedings of the 2nd International Conference on Chemical, Ecology and Environmental Sciences. pp.17-18. [ Links ]
Bernardo, E., Fiocco, L., Parcianello, G., Storti, E., and Colombo, P. 2014. Advanced ceramics from preceramic polymers modified at the nano-scale: A review. Materials, vol. 7. pp.1927-1956. https://doi.org/10.3390/ma7031927 [ Links ]
Ding, D., Zhou, W., Zhou, X., Luo, F., and Zhu, D. 2012. Influence of pyrolysis temperature on structure and dielectric properties of polycarbosilane derived silicon carbide ceramic. Transactions of Nonferrous Metals Society of China, vol. 22, no. 11. pp.2726-2729. https://doi.org/10.1016/S1003-6326(11)61524-0 [ Links ]
Eliche-Quesada, D., Martínez-García, C., Martínez-Cartas, M.L., Cotes-Palomino, M.T., Pérez-Villarejo, L., Cruz-Pérez, N., and Corpas-Iglesias, F.A. 2011. The use of different forms of waste in the manufacture of ceramic bricks. Applied Clay Science, vol. 52, no. 3. pp.270-276. https://doi.org/10.1016/j.clay.2011.03.003 [ Links ]
Eterigho-Ikelegbe, O., Trammell, R., and Bada, S. 2021. Preparation and characterization of ceramic composites from South Africa coal discard. Construction and Building Materials, vol. 302. p. 124164. https://doi.org/10.1016/j.conbuildmat.2021.124164 [ Links ]
Fonblanc, D., Lopez-Ferber, D., Wynn, M., Lale, A., Soleilhavoup, A., Leriche, A., Iwamoto, Y., Rossignol, F., Gervais, C., and Bernard, S. 2018. Crosslinking chemistry of poly (vinylmethyl- co -methyl) silazanes toward low-temperature formable preceramic polymers as precursors of functional aluminium-modified Si-C-N ceramics. Dalton Transactions, vol. 47, no. 41. pp. 14580-14593. https://doi.org/10.1039/C8DT03076F [ Links ]
Gao, Z., Ding, Y., Yang, W., and Han, W. 2017. DFT study of water adsorption on lignite molecule surface. Journal of Molecular Modeling, vol. 23, no. 1. p. 27. https://doi.org/10.1007/s00894-016-3194-7 [ Links ]
Haywood, L.K., de Wet, B., de Lange, W., and Oelofse, S., 2019. Legislative challenges hindering mine waste being reused and repurposed in South Africa. The Extractive Industries and Society, vol. 6, no. 4. pp. 1079-1085. https://doi.org/10.1016/j.exis.2019.10.008 [ Links ]
Hill, A. and Easter, W. 2019. Carbon ceramic composites and methods. US Patents. 20190292441A1. http://www.energy.gov.za/files/coal_frame.html [accessed 4 May 2022], [ Links ]
Hu, G., Bian, Z., Xue, R., Huang, W., and Komarneni, S. 2017. Polymer-coal composite as a novel plastic material. Materials Letters, vol. 197,. pp. 31-34. https://doi.org/10.1016/j.matlet.2017.03.148 [ Links ]
Huggins, F.E.,2002. Overview of analytical methods for inorganic constituents in coal. International Journal of Coal Geology, vol. 50. pp. 169-214. https://doi.org/10.1016/S0166-5162(02)00118-0 [ Links ]
Ibraeva, Y., Tarasevskii, P., and Zhuravlev, A. 2017. Salt corrosion of brick walls. MATEC Web of Conferences, vol. 106. p. 03003. https://doi.org/10.1051/matecconf/201710603003 [ Links ]
International Committee for Coal and Organic Petrology (ICCP). 1998. The new vitrinite classification (ICCP System 1994). Fuel, vol. 77, no. 5. pp. 349-358. https://doi.org/10.1016/S0016-2361(98)80024-0 [ Links ]
Ionescu, E., Papendorf, B., Kleebe, H.-J., Poli, F., Müller, K., and Riedel, R. 2010. Polymer-derived silicon oxycarbide/hafnia ceramic nanocomposites. Part I: Phase and microstructure evolution during the ceramization process. Journal of the American Ceramic Society, vol. 93, no. 6. pp. 1774-1782. https://doi.org/10.1111/j.1551-2916.2010.03765.x [ Links ]
Isaac, K. and Bada, S.O. 2020. The co-combustion performance and reaction kinetics of refuse derived fuels with South African high ash coal. Heliyon, vol. 6, no. 1. p. e03309. https://doi.org/10.1016/j.heliyon.2020.e03309 [ Links ]
ISO 10545-4. 2019. Ceramic tiles - Part 4: Determination of modulus of rupture and breaking strength. https://www.iso.org/obp/ui/#iso:std:iso:10545:-4:ed-4:v1:en [accessed 24 January 2022]. [ Links ]
ISO 10545-13. 2016. Ceramic tiles - Part 13: Determination of chemical resistance. https://www.iso.org/standard/60975.html [accessed 24 January 2022]. [ Links ]
Jian, K., Chen, G., Guo, C., Ma, G., and Ru, Z. 2019. Biogenic gas simulation of low-rank coal and its structure evolution. Journal of Petroleum Science and Engineering, vol. 173. pp. 1284-1288. https://doi.org/10.1016/j.petrol.2018.11.005 [ Links ]
Makarov, D.V., Suvorova, O.V., and Masloboev, V.A. 2019. Prospects of processing the mining and mineral processing waste in Murmansk Region into ceramic building materials. https://inep.ksc.ru/documents/27_prosp_19%20(1).pdf [accessed 24 January 2022]. [ Links ]
Mishra, V., Bhowmick, T., Chakravarty, S., Varma, A.K., and Sharma, M. 2016. Influence of coal quality on combustion behaviour and mineral phases transformations. Fuel, vol. 186. pp. 443-455. https://doi.org/10.1016/j.fuel.2016.08.092 [ Links ]
Nicolas, M.F., Vlasova, M., Aguilar, P.A.M., Kakazey, M., Cano, M.M.C., Matus, R.A., and Puig, T.P. 2020. Development of an energy-saving technology for sintering of bricks from high-siliceous clay by the plastic molding method. Construction and Building Materials, vol. 242. p. 118142. https://doi.org/10.1016/j.conbuildmat.2020.118142 [ Links ]
Painter, P., Pülati, N., Cetiner, R., Sobkowiak, M., Mitchell, G., and Mathews, J., 2010. Dissolution and dispersion of coal in ionic liquids. Energy & Fuels, vol. 24, no. 3. pp. 1848-1853. https://doi.org/10.1021/ef9013955 [ Links ]
Painter, P.C., Sobkowiak, M., and Youtcheff, J. 1987. FT-i.r. study of hydrogen bonding in coal. Fuel, vol. 66, no. 7. pp. 973-978. https://doi.org/10.1016/0016-2361(87)90338-3 [ Links ]
Phonphuak, N. and Thiansem, S. 2011. Effects of charcoal on physical and mechanical properties of fired test briquettes. ScienceAsia, vol. 37, no. 2. p. 120. https://doi.org/10.2306/scienceasia1513-1874.2011.37.120 [ Links ]
Rahman, M.H., Islam, M.T., Minhaj, T.I., Azad, M.A.K., Hasan, M.M., and Haque, A.M.R. 2015. Study of thermal conductivity and mechanical property of insulating firebrick produced by local clay and petroleum coal dust as raw materials. Procedía Engineering, vol. 105. pp. 121-128. https://doi.org/10.1016/j,proeng.2015.05.019 [ Links ]
Roberts, M.J. 2015. The molecular structure of selected South African coal-chars to elucidate fundamental principles of coal gasification. PhD thesis, North-West University, South Africa. https://repository.nwu.ac.za/handle/10394/16014 [accessed 24 January 2022], [ Links ]
Shen, C., Barrios, E., and Zhai, L. 2018. Bulk polymer-derived ceramic composites of graphene oxide. ACS Omega, vol. 3, no. 4. pp. 4006-4016. https://doi.org/10.1021/acsomega.8b00492 [ Links ]
Taha, y., Benzaazoua, M., Hakkou, R., and Mansori, M. 2017. Coal mine wastes recycling for coal recovery and eco-friendly bricks production. Minerals Engineering, vol. 107. pp. 123-138. https://doi.org/10.1016/j.mineng.2016.09.001 [ Links ]
Tambwe, O., Kotsiopoulos, A., and Harrison, S.T.L. 2020. Desulphurising high sulphur coal discards using an accelerated heap leach approach. Hydrometallurgy, vol. 197. p. 105472. https://doi.org/10.1016/j.hydromet.2020.105472 [ Links ]
Van Niekerk, D. 2008. Structural elucidation, molecular representation and solvent interactions of vitrinite-rich and inertinite-rich South African coals. PhD thesis, Pennsylvania State University. [ Links ]
Wen, Q., Qu, F., Yu, Z., Graczyk-Zajac, M., Xiong, X., and Riedel, R. 2022. Si-based polymer-derived ceramics for energy conversion and storage. Journal of Advanced Ceramics, vol. 11. pp. 197-246. https://doi.org/10.1007/s40145-021-0562-2 [ Links ]
Wen, Q., Yu, Z. and Riedel, R. 2020. The fate and role of in situ formed carbon in polymer-derived ceramics. Progress in Materials Science, vol. 109. pp. 100623. https://doi.org/10.1016/j.pmatsci.2019.100623 [ Links ]
Whitehurst, D.D. 1978. A primer on the chemistry and constitution of coal. Organic Chemistry of Coal, ACS Symposium Series. American Chemical Society. pp. 1-35. https://doi.org/10.1021/bk-1978-0071.ch001 [ Links ]
Yague, S., Sánchez, I., Vigil de la Villa, R., García-Giménez, R., Zapardiel, a., and Frías, M. 2018. Coal-mining tailings as a pozzolanic material in cements industry. Minerals, vol. 8, no. 2. p. 46. https://doi.org/10.3390/min8020046 [ Links ]
 Correspondence:
Correspondence:
O. Eterigho-Ikelegbe
Email: eterighoo@gmail.com
Received: 25 Jan. 2022
Revised: 19 May 2022
Accepted: 22 Jun. 2022
Published: August 2022














