Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.122 n.4 Johannesburg Apr. 2022
http://dx.doi.org/10.17159/2411-9717/1968/2022
PROFESSIONAL TECHNICAL AND SCIENTIFIC PAPERS
Improving the lead flotation recovery at Lakan lead-zinc processing plant using high-intensity conditioning
K. Barani; M. Godarzi; F. Moradpouri
Department of Mining Engineering, Faculty of Engineering, Lorestan University, Khorramabad, Iran
SYNOPSIS
A sampling campaign carried out on the lead flotation circuit at Lakan lead-zinc processing plant demonstrated that the Pb recovery was only about 68-69%. Characterization of the Pb flotation tailing showed that 61.4% of the Pb is in the -37 um size fraction, which is difficult to recover by flotation. Batch flotation experiments were carried out on a plant sample of the lead flotation feed and high-intensity conditioning (HIC) was used to improve flotation recovery. The results showed that increasing the collector conditioning time from 1 to 3 minutes significantly increases the Pb recovery from 42.5% to 57.3%. Further increases in conditioning time to 5 to 7 minutes decreased the Pb recovery. With a collector conditioning time of more than 3 minutes, due to the surface cleaning effect, increasing collector and frother dosage improved the flotation selectivity and recovery, even at higher collector dosages. The Pb recovery increased from 69.1% to 79.8% with increasing impeller speed from 600 to 1250 r/min. HIC increases the flotation kinetics and greatly improves the flotation recovery and concentrate grade.
Keywords: flotation, lead-zinc ore, high-intensity conditioning, Lakan plant.
Introduction
Plant experience, as well as experimental work, has shown that fine particles (<io pm) float more slowly and less selectively than particles in the intermediate size range (10-70 pm). A number of hypotheses have been advanced to explain the poor floatability of ultrafine particles. (Engel, Middlebtook, and Jameson, 1997; Aldrich et al., 1997; Bulatovic and Salter, 1989; Wang et al., 2020; Ulusoy and Kursun 2021):
> The mass of fine particles decreases and consequently the probability of collision and adhesion is reduced m
> The increased surface area reduces the suspension stability, collector coating, and froth stabilization
> The decrease in particle size increases the surface energy, and accordingly the adsorption of
collectors on the particles becomes non-specific > The oxidation of fine particle increases > Slime coatings occur on coarse particles.
Several alternative processes such as shear flocculation, carrier flotation, oil agglomeration, selective flocculation, and desliming have been suggested to overcome the problem of recovering fine mineral particles in froth flotation (Valderrama and Rubio, 1998; Chen et al., 1999a). In shear flocculation, the selective aggregation of hydrophobic particles occurs due to the high turbulence (Warren 1975). Many researchers have extended the concept of shear flocculation to the conditioning stage by increasing conditioning time or increasing impeller speed (Rubio, 1978; Bulatovic and Salter, 1989; Valderrama and Rubio, 1998).
High-intensity conditioning (HIC) is effective only if it is done in the presence of collectors and modifiers (Liu, Zhang, and Chen, 2021). After HIC, fine hydrophobic particles (-10 pm) become selectively agglomerated and readily floatable, the hydrophilic species are removed from the intermediate (-10+75 pm) and coarse (+75 pm) fractions (surface cleaning), and the hydrophobicity of particle surfaces is improved. Also due to surface cleaning, collector adsorption onto the particle surfaces is improved so the flotation performance becomes more sensitive to collector and frother addition (Bulatovic and Salter, 1989; Chen et al., 1999a).
HIC has been investigated in laboratory and pilot plant test work to improve the flotation of fine particles from finely disseminated copper-zinc, copper-nickel, copper-lead-zinc-silver, and copper ores (Bulatovic and Salter, 1989). The results showed that HIC without additions of collector and frother has no positive effect on the flotation of ultrafine mineral slimes. By contrast, HIC with the addition of collector, frother, and suitable modifiers can significantly improve both recovery and selectivity of ultrafine sulphide slimes.
The hydrodynamic and chemical aspects of HIC prior to nickel rougher flotation were investigated by Engel, Middlebrook, and Jameson (1997). The Rushton mixing cell design was utilized with variations in impeller type and size. The results showed major improvements to the nickel grade-recovery occurred after HIC. It was shown that impeller speed has some influence on the subsequent flotation performance at any fixed value of power and work.
(Aldrich et al. (1997) investigated the effect of HIC on the batch flotation of sulphide ore. The results showed that HIC significantly increased grade-recovery compared with the baseline floats.
HIC and carrier flotation of fine gold particles were studied at the laboratory scale d by Valderrama and Rubio (1998). After pulp HIC a 24% increase in the recovery of gold, 50% increase in concentrate grade, and faster flotation rates (at least 3-4 times faster) were obtained.
Chen et al. (1999a) studied the effect of HIC on the flotation of a nickel ore. HIC increased the flotation rate and recovery of pentlandite in the 8-75 µm particle size range. A large number of particles before and after HIC were examined using scanning electron microscopy (SEM), image analysis, and X-ray diffraction. It was found that hydrophilic gangue slimes were coated on both pentlandite and gangue mineral surfaces prior to HIC. After HIC, coated slimes were removed from the mineral particle surfaces, the amount of slime removal depending on agitation intensity and time.
Wei, Mei-jiao, and Yue-hua (2009) investigated the influence of HIC on fine particle aggregation and flotation behaviour of sphalerite in a slurry saturated with CO2. The results showed that during HIC using air- or CO2-saturated water, xanthate acts as a frother, and HIC plays a synergistic role in promoting fine particle aggregation and hence flotation.
The removal of serpentine slime from pentlandite surfaces was investigated by Feng et al. (2018). The results illustrated that coarser slimes could be removed by HIC, while the fine slimes could be removed by adding hexametaphosphate reagent.
Feng et al. (2012) studied the effect of the chain length of xanthate on the flotation of nickel ore using different conditioning methods. The results showed that HIC could increase pentlandite flotation recovery significantly. Xanthates with different hydrocarbon chain lengths had a different effect on pentlandite flotation when different conditioning methods were used
It can be concluded from the literature that a significant improvement in the floatability and selectivity of slime particles can be obtained by HIC conditioning. In this research work, we investigated the application of HIC for increasing lead recovery in the lead flotation circuit at the Lakan lead and zinc processing plant.
Materials and methods
Surveying and sampling
Lakan processing plant is located in the Markazi province of Iran. The main ore minerals are galena (PbS) and sphalerite (ZnS) along with small amounts of serosite (PbCO3), hemimorphite (ZnCO3), anglesite (PbSO4), and zensite (ZnO). The gangue minerals are pyrite (FeS2), calcite (CaCO3), dolomite (CaMg[CO3]2) and silica (SiO2).
Figure 1 shows the process flow sheet of the Lakan plant. The dry solids feed rate to the plant is about 40 t/h. The run-of-mine ore (ROM) is crushed by jaw and cone crushers to 100% passing 10 mm (P100 10 mm). The crusher product is ground to a D80 of 150 urn by primary (open circuit) and secondary (closed circuit) ball mills. The ground product is fed to the Pb rougher flotation circuit (16 Denver mechanical cells with 1.4 m3 volume). At the Pb rougher flotation stage, the Pb is floated at a pH of 7.7-7.8 and 40% solids with potassium ethyl xanthate (45 g/t) as galena collector, zinc sulphate (550 g/t) as sphalerite depressant, sodium silicate (150 g/t) as the gangue depressant, and MIBC (40 g/t) as frother. The Pb concentrate from the rougher enters the multi-stage cleaner circuit (four Denver mechanical cells with a 1.4 m3 volume) for further upgrading. The tailings of the Pb rougher flotation stage proceeds to the Zn flotation circuit for zinc recovery.
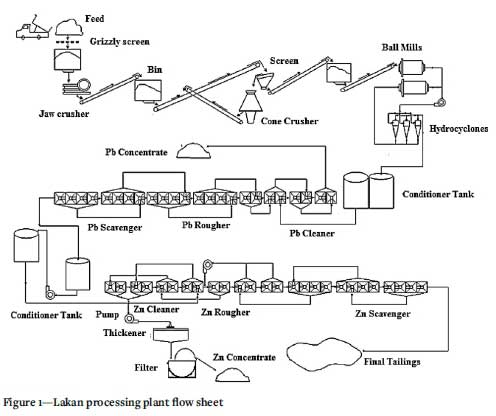
A sampling campaign was carried out on the Pb flotation circuit for a period of one month. The Pb flotation feed, the Pb final concentrate (the cleaner concentrate), and the Pb final tailings (Zn flotation circuit feed) were sampled and the particle size distributiosn and Pb grades determined. The average metallurgical performance of the Pb flotation circuit for a period of one month is shown in Table I. It will be observed that the Pb recovery was about 68.6%, with the remaining 31.4% passing through to the Zn flotation circuit.
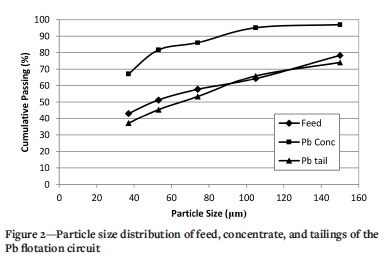
Figure 2 shows the particle size distribution of the feed, concentrate, and tailings of the Pb flotation circuit. It can be seen that the Pb concentrate has a much finer size distribution than the feed and tailings, with 67.2% of the particles smaller than 37 um compared with 43.1% and 37.1% for the Pb feed and tailings, respectively. This indicates that the Pb-bearing particles have undergone selective grinding in the comminution process.
Characterization of Pb flotation tailings
To identify the problems in the Pb flotation, it is important to determine the Pb and Zn distributions in the various size fractions of the Pb flotation tailings. A sample of the tailings was divided into six size fractions of +150, -150+105, -105+74, -74+53, -53 +37, and -37 um and the mass and the Pb and Zn grades determined in each fraction. Polished sections were prepared from each fraction and examined using optical microscopy and backscattered electron imaging by energy-dispersive X-ray spectroscopy (EDS) using scanning electron microscopy (SEM).
Table II shows the Pb and Zn distributions in the Pb flotation tailings. As can be seen, 61.4% of the Pb is in the -37 um fraction.
The optical microscopy studies showed that the main minerals in the Pb flotation tailings are sphalerite (sp), galena (gn), and pyrite (py). In the +150 and -105 +150 um fractions, metallic minerals, which include sphalerite (5.5-6.0%), pyrite (0.5-1.0%), and galena (approx. 0.5%), make up about 6-7% of the totals.
Sphalerite is found as large free grains locked with galena, pyrite, and nonmetallic minerals. Small galena inclusions less than 40 um can also be seen in sphalerite. In some cases, sphalerite less than in size 40 µm is observed in the large grains of galena.
As the grain size becomes smaller in the -105+74, -74+53, -57+37, and -37 µm fractions, the volume percentage of sphalerite and pyrite increases and the liberation of these minerals increases to more than 95%. However, in the case of galena, in general the volume percentage decreases and no free galena was observed in the -57+37, and -37 um fractions. In these fractions, galena mostly occurs as very fine micrometre-size inclusions in sphalerite and nonmetallic minerals.
Galena particles constitute less than 0.1% of the -37 um fraction, in contrast with the results in Table II, which show that 61.34% of the Pb in the tailings is in this fraction. Therefore, due to the possibility of the galena particles being below the detection limit of the polarizing microscope, the polished sections were also studied using SEM, which has a much higher resolution. Figure 3 shows backscattered images from the polished sections. The minerals were identified in the surfaces of the sections by EDS analysis and the results compared to the standard mineral samples.
Analysis of the SEM images showed that galena particles exist in all fractions. The free galena particles occur more in the coarse fractions, and with decreasing particle size, fine galena and sphalerite particles can be seen as inclusions in each other, which indicates the phenomenon of 'galena disease'. The important point to note in the SEM images is the large amount of very fine, scattered galena particles (light grey phase) in the -37 µm fraction. Most of these particles are in the size range 5-10 µm. This is consistent with the results in Table II, which indicate about 61.3% of the Pb is in the -37 µm fraction. This is below the resolution of the optical microscope.
It can be concluded from the plant sampling campaign and characterization of the Pb flotation tailing that a large amount of lead is lost as slime and is difficult to recover. Therefore, any changes in the flotation process should be done with the aim of improving the slimes flotation.
Flotation experiments
The feed sample for the flotation experiments was collected during the plant sampling campaign. Table III shows the elemental analysis of the sample. Flotation experiments were conducted with 2800 g dry solids of the flotation feed in a 2.5-litre conventional laboratory cell (Denver model) in tap water, at 36% solids by weight. The pulp was added to the flotation cell and conditioned for 3 minutes. The pH was adjusted to 7.7-7.8 by adding NaOH or H2SO4. Sodium silicate (0.15 g) as silicates depressant, zinc sulphate (0.55 g) as sphalerite depressant, and sodium sulphide (0.2 g) as pyrite depressant were added to the cell and the suspension was conditioned for 5 minutes. Then, ethyl xanthate potassium (various concentrations) was added as collector. The collector conditioning time and the impeller speed during the collector conditioning time were varied. Finally, methyl isobutyl carbinol (MIBC, various concentrations) was added as the frother. After 1 minute the impeller speed was set to 600 r/ min, the air was delivered to the cell, and froth products were collected for 6 minutes. The froth and tailings were collected, filtered, dried, analysed for Pb and Zn content, and the Pb and Zn recoveries calculated.
First, two flotation experiments were carried out according to the plant conditions (baseline experiments) with a collector dosage of 45 g/t, frother dosage of 20 g/t, and conditioning time 1 minute. The flotation experiments with HIC were then performed and the effect of collector conditioning time, collector dosage, impeller speed,, and frother dosage were investigated. Finally, flotation kinetic experiments were conducted using the baseline experimental conditions and the optimum HIC conditions.
Each flotation experiment at each set of conditions was repeated three times and the average of the results reported.
Results and discussion
Baseline experiments
Table III shows the results for the baseline flotation experiments. This test was repeated two times. The average Pb and Zn recoveries are 42.5% and 9.7%, respectively, compared with 68.6% in the industrial circuit 68.6% (Table I). It should be noted that the industrial flotation process includes rougher, scavenger, and cleaner stages, and the flotation time is much longer than for the the batch flotation experiment (which is only a rougher flotation).
High-intensity conditioning (HIC)
Effect of conditioning time
Figure 4 shows the effect of collector conditioning time on the Pb and Zn recoveries. The other flotation parameters were as for the baseline experiments. As can be seen, increasing the collector conditioning time from 1 to 3 minutes significantly increases the Pb recovery from 42.5% to 57.3% (about 15%). Further increases in the conditioning time from 3 to 5 and 7 minutes decrease the Pb recovery. Also, the Zn recovery to the lead concentrate increases from 9.7% to 16.3%. Excessively strong conditioning intensity could cause interparticle collisions or attrition, thereby destroying sthe reagent film and decreasing the concentrate recovery (Li et al., 2020; Quast, 2015). Longer conditioning times may cause the collector to detach from the galena particle surfaces, thereby reducing the Pb recovery.
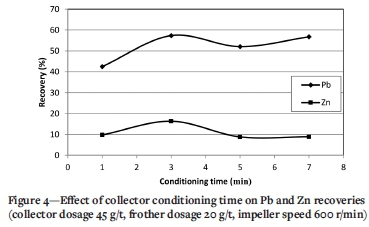
At this stage, considering the fact that our goal is to achieve the highest Pb recovery, 3 minutes was considered the best conditioning time and was applied in the next steps.
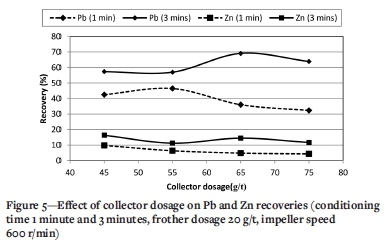
Effect of collector dosage
Figure 5 shows the effect of collector dosage for collector conditioning times of 1 and 3 minutes. At 1 minute conditioning time the Pb recovery increases from 42.5% to 46.5% with increasing collector dosage from 45 to 55 g/t. The Pb recovery decreases to 36.0 and 32.3% with further increase in collector dosage from 55 to 65 and 75 g/t. This could be explained by the interactions between the bubbles and solids. When the collector dosage increases, the solid surfaces become completely covered with an immobilized collector monolayer, and then the frother molecules cannot penetrate to the mineral surface. Consequently, bubble adhesion is suppressed, which weakens the attachment of particles to the air bubbles (Sis and Chander, 2003; Azizi, 2014). Figure 5 also shows that at a collector conditioning time of 3 minutes, increasing the collector dosage from 45 to 65 and 75 g/t increases the Pb recovery from 57.3% to 69.1 and 63.9%, respectively. The Zn recovery at both conditioning times shows a decreasing trend with increasing collector dosage. It can be concluded that at long collector conditioning times, due to the surface cleaning effect, collector adsorption onto the particle surfaces is improved, which leads to improved flotation and recovery even at higher collector dosages.
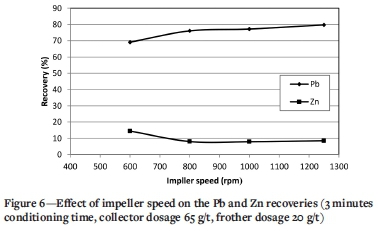
Effect of impeller speed
HIC can be carried out by increasing either the collector conditioning time or the impeller speed. Figure 6 shows the effect of impeller speed on the Pb and Zn recoveries at a collector conditioning time of 3 minutes. It should be noted that the impeller speed was reduced to 600 r/min for all experiments before air was released to the flotation cell. As can be seen, increasing the impeller speed from 600 to 1250 r/min resulted in a Pb recovery increase from 69.1% to 79.8% and a decrease in Zn recovery to the Pb concentrate from 14.4 to 8.5%. Increasing the impeller speed causes better collector diffusion and increases collector adsorption on the particles. As the impeller speed increases, the amount of air that enters the cell and disperses in pulp increases. The air is released in the form of fine bubbles, resulting in an increase in the flotation rate for all particles.
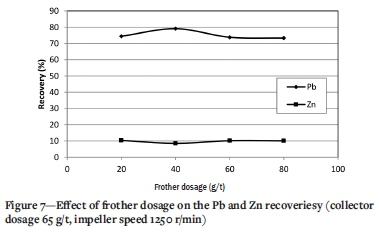
Effect of frother dosage
Figure 7 shows the effect of frother dosage at a collector conditioning time of 3 minutes, collector dosage of 65 g/t, and impeller speed of 1250 r/min. The Pb recovery increases from 74.4 to 79.8% with increasing frother dosage from 20 to 40 g/t. Further increases in frother dosage have a negative effect on the Pb recovery. Also, the Zn recovery increases from 8.5 to 10.2% with increasing frother dosage, which is not desirable. Excessive frother dosage causes deformation of the bubbles, which has an adverse effect on flotation performance.
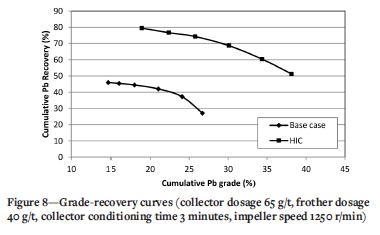
Flotation kinetics
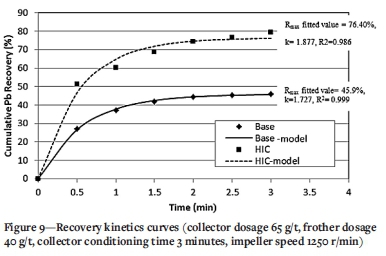
A collector dosage of 65 g/t, frother dosage of 40 g/t, collector conditioning time 3 minutes, and impeller speed 1250 r/min were selected as the optimum conditions for HIC. The flotation kinetics were evaluated from recovery times under the baseline experimental conditions and the optimum HIC conditions. Six concentrate samples were collected after 0.5, 1.0, 1.5, 2.0, 2.5, and 3.0 minutes, and the cumulative Pb recoveries calculated. Figure 8 presents the cumulative Pb grade-recovery curves for the baseline experiments and optimum HIC conditions.
The flotation kinetics were modelled after Agar (1985) using Equation [1]:

where R is the cumulative recovery (%) at a given time t (min), Rmax is the maximum flotation recovery, and k is the first-order rate constant (min-1). Rmax and k are determined by fitting Equation [1] to the experimental data. The model parameters are estimated by least-squares regression. The kinetics curves and the fitted kinetics model parameters are presented in Figure 9. The results show significant improvement in the rate constant and the maximum recovery after HIC - so much so that with HIC the Pb recovery after 0.5 minutes (51.2%) is greater than the maximum recovery of baseline experiments that is achieved after 3 minutes (46%).
Conclusions
In the lead flotation circuit at the Lakan minerals processing plant, 31.4% of the Pb is not recovered and reports to the Pb tailings. Also, 61.4% of the Pb in the tailing is in the -37 pm fraction. This indicates that a large amount of lead is lost as slime and is difficult to recover. The current study proved that high-intensity conditioning (HIC) by increasing either the collector conditioning time or the impeller speed improves the Pb flotation recovery. Also, with HIC, increasing the collector dosage and frother dosage has a greater impact on the Pb flotation recovery due to the surface cleaning effect. A collector dosage of 65 g/t, frother dosage of 40 g/t, collector conditioning time 3 minutes, and impeller speed of 1250 r/min are the optimum conditions for HIC.
The flotation kinetics show that HIC causes a considerable improvement in the rate constant and a concentrate with a higher grade and recovery can be obtained.
However, these results were obtained on a laboratory scale; pilot or industrial scale tests will be required prior to the possible industrial implementation of HIC.
There are other ways to improve the flotation process, including reducing the production of fine particles by modifying the grinding and classification circuit or improving the hydrodynamic performance of the flotation cells. It is suggested that the impact of these factors be investigated in future research.
References
Aldrich, C. et al., 1997. Relationship between surface froth features and process conditions in the batch flotation of a sulphide ore. Minerals Engineering, vol. 10, no. 11. pp. 1207-1218, [ Links ]
Azizi, A. 2014. Influence of collector dosage and pulp chemistry on copper flotation. Geosystem Engineering, vol. 17, no. 6. pp. 311-316. [ Links ]
Bulatovic, S.M. and Salter, R.S. 1989. High intensity conditioning - a new approach to improving flotation of mineral slimes. In G. S. Dobby & S. R. Rao, eds. Processing of Complex Ores. Proceedings of Metallurgical Society of Canadian Institute of Mining and Metallurgy. Amsterdam: Pergamon. pp. 169-181. http://dx.doi.org/10.1016/B978-0-08-037283-9.50020-2. [ Links ]
Chen, G., Grano, S., Sobieraj, S., and Ralston, J. 1999a. The effect of high intensity conditioning on the flotation of a nickel ore, Part 2: Mechanisms. Minerals Engineering, vol. 12, no. 11. pp. 1359-1373. [ Links ]
Chen, G. et al., 1999b. The effect of high intensity conditioning on the flotation of a nickel ore. Part 1: Size-by-size analysis. Minerals Engineering, vol. 12, no. 10. pp. 1185-1200. [ Links ]
Engel, M.D., Middlebrook, P.D., and Jameson, G.J. 1997. Advances in the study of high intensity conditioning as-ameans of improving mineral flotation performance. Minerals Engineering, vol. 10, no. 1. pp. 55-68. [ Links ]
Feng, B. et al., 2018. Removal behavior of slime from pentlandite surfaces and its effect on flotation. Minerals Engineering, vol. 125 (January). pp. 150-154. https://doi.org/10.1016/j.mineng.2018.06.011. [ Links ]
Feng, B., Feng, Q., Lu, Y., and Lv, P. 2012. The effect of conditioning methods and chain length of xanthate on the flotation of a nickel ore. Minerals Engineering, 39. pp. 48-50. Available at: http://dx.doi.org/l0.l0l6/j.mineng.2012.05.022, [ Links ]
Li, D., Zhang, C., Li, X., Yang, L., Yan, X., Wang, K., Liu, Q., and Zhang, H. 2020. Experimental study on the preconditioning of fine coal particles surface modification using a new type flow mixer. Fuel, 268. p.117361. [ Links ]
Liu, D., Zhang, G., and Chen, Y. 2021. Studies on the selective flotation of pyrite from fine serpentine by using citric acid as depressant. Minerals Engineering, vol. 165. p.106742. [ Links ]
Quast, K. 2015. Use of conditioning time to investigate the mechanisms of interactions of selected fatty acids on hematite. Part 1: Literature survey. Minerals Engineering, vol. 79. pp.295-300. [ Links ]
Rubio, J. 1978. Conditioning Effects of Flotation of Finely Divided Non-Sulphide Copper Ore. Transactions of the Institution of Mining and Metallurgy C, vol. 87. pp.284-287. [ Links ]
Sis, H.I. and Chander, S. 2003. Improving froth characteristics and flotation recovery of phosphate ores with nonionic surfactants. Minerals engineering, vol. 16, no. 7. pp. 587-595. [ Links ]
Ulusoy, U. and Kursun, H. 2021. Comparison of ultrasonically aided zinc beneficiation by mechanical flotation and column flotation cell. EUREKA: Physics and Engineering, vol. 1. pp. 3-13. [ Links ]
Valderrama, L. and Rubio, J. 1998. High intensity conditioning and the carrier flotation of gold fine particles. International Journal of Mineral Processing, vol. 52, vol. 4. pp. 273-285. [ Links ]
Wang, H., Yang, W., Li, Q., Zhang, C., Yan, X., Wang, L., and Zhang, H. 2020. Enhancement of coal flotation using impact flow conditioning pulp. Journal of Cleaner Production, vol. 267. p.122124. https://doi.org/10.1016/j.jclepro.2020.122124. [ Links ]
Warren, L.J. 1975. Shear- flocculation of ultrafine scheelite in sodium oleate solutions. Journal of Colloid and Interface Science, vol. 50, no. 2. pp. 307-318. [ Links ]
Wei, S.U.N., Mei-jiao, D., and Yue-hua, H.U. 2009. Fine particle aggregating and flotation behavior induced by high intensity conditioning of a CO2 saturation slurry. Mining Science and Technology (China), vol. 19, no. 4. pp. 483-488. http://dx.doi.org/10.1016/S1674-5264(09)60090-9 [ Links ]
 Correspondence:
Correspondence:
K. Barani
Email: barani.k@lu.ac.ir
Received: 28 Dec. 2021
Revised: 22 Feb. 2022
Accepted: 22 Feb. 2022
Published: April 2022














