Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Journal of the Southern African Institute of Mining and Metallurgy
versão On-line ISSN 2411-9717
versão impressa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.122 no.1 Johannesburg Jan. 2022
http://dx.doi.org/10.17159/2411-9717/1638/2022
PROFESSIONAL TECHNICAL AND SCIENTIFIC PAPERS
A new preconcentration technique for the determination of PGMs and gold by fire assay and ICP-OES
A. MasasireI, III; F. RwereII; P. DzombaIII; M. MupaIII
ISiyancla Union Mine,, Limpopo, RSA. https://orcid.org/0000-0002-7403-4962
IIChinhoyi University of Technology, Department of Chemistry, Chinhoyi, Zimbabwe. https://orcid.org/0000-0002-7037-6850
IIIBindura University of Science Education, Department of Chemistry, Bindura, Zimbabwe. P. Dzomba: https://orcid.org/0000-001-6821 2606; M. Mupa: https://orcid.org/0000-0003-2185-9386
SYNOPSIS
Accurate determination of platinum group metals (PGMs) and gold (Au) has always proven to be a difficult task, due to their low concentrations in platiniferous ores. The most common preconcentration technique used in analysis of these metals is fire assay with a flux containing nickel or lead. This technique can be improved by using co-collectors. Here we hypothesise that Fe, Co, and Cu can be used as co-collectors to enhance separation and preconcentration of PMGs and gold by fire assay. To test this hypothesis, geological exploration samples from Siyanda Union Mine (Northam, South Africa) were analysed by an inductively coupled plasma-optical emission spectrometer (ICP-OES) for PGMs (Pt, Pd, and Rh) and Au. A control sample (certified reference material AMIS 0426) was also analysed by the same technique. The PGM and Au recoveries from the control sample ranged from 83% to 105% for all three co-collectors, with relative standard deviations less than 10% for the control sample and 7% for the geological samples. The PGM and Au recoveries by Fe and Co co-collectors were modestly higher than that of the Cu-collector. These results indicate that Fe and Co are better co-collectors than Cu, presumably due to the loss of analyte when using Cu. Taken together, Fe and Co co-collectors can be viable alternatives for analysis of PGMs and gold using the fire assay method.
Keywords: Platinum group metals, ICP-OES, co-collection, fire assay.
Introduction
South Africa is a prominent global supplier of platinum group metals (PGMs). The main reserves of PGMs are the Bushveld Complex (Tanner et al., 2019), where platiniferous ores are obtained from the Merensky Reef (Creech et al., 2014). The PGMs are found in low concentrations, typically less than 10 g/t (Serbin, Bazel, and Ru, 2017). The low PGM grade of these ores contributes to their high market value.
The analysis of the platiniferous ores and flotation concentrate samples is challenging because of the low PGM concentrations and their heterogeneous distribution in the matrix. Therefore, their determination is usually preceded by isolation from the gangue material and preconcentration (Berezhnaya and Dubinin, 2016; Bayrak et al., 2017). In South African mining and metallurgical testing laboratories this is accomplished mainly by fire assay using nickel sulphide or lead collection followed by spectrometric determination (Vanhaecke et al., 2010).
The growing demand for PGMs and Au has led to concerns about their future supply. This has resulted in a renewed interest in the recycling of end-of-life materials. The supply of PGMs from recycling has doubled over the past decade. Makua et al. (2019) recovered PGMs from a pregnant leach solution by using solvent extraction and cloud-point extraction. They concluded that the efficiency of the cloud-point extraction method depends on the pH of the solution, the surfactant and the complexing agent, the hydrochloric acid concentration, and the presence of a reducing agent. Although the cloud-point extraction method is more environmentally friendly than fire assay, it is time-consuming.
Carelse et al. (2020) assessed the distribution of gold and silver in alloys produced from the smelting of printed circuit boards, using SEM-EDS, EPMA, and LA-ICP-MS analyses. Gold and silver were found to be most enriched in the lead phase of the tap, which indicates that lead is a good collector of gold.
Different analytical techniques have been used to determine PGMs and Au in geological work because of their economic importance (Hughes, McDonald, and Kerr, 2015; Jansen et al., 2016). Liipo et al. (2019) characterized the South Georgian complex copper-gold ores by fire assay and ICP-OES. Other authors have attempted to determine PGMs and Au in ores using X-ray fluorescence (XRF), but have encountered sensitivity problems (Hinds and Burgess, 2014; Díaz, Hahn, and Molina, 2017). In addition, a variety of techniques have been employed for sample decomposition and preconcentration of PGMs and gold prior to ICP-OES measurement. These studies have concentrated on fire assay with nickel sulphide (NiS) collection to characterize the metals in geological samples. However, low recoveries of the PGMs using NiS collection are reported in the literature (Morcelli et al., 2004). In other studies, co-precipitation with Te after HCl digestion of the NiS button has been shown to improve PGM recoveries (Morcelli et al., 2004). However, the results for Pd and Pt are reportedly lower than the certified values for reference materials (Morcelli et al., 2004).
It has also been shown in some studies that co-collectors can improve the recovery of PGMs, and allow accurate determination in a possibly cost-effective manner. Modification of the Pb fire assay procedure using Ag or Au as collector for the PGMs from rocks, minerals, and ores has been extensively reviewed (Balcerzak, 2002). Co-collectors normally used in the determination of PGMs include silver, platinum, palladium, and gold (Ndovorwi, 2014). Suominen, Kontas, and Niskavaara (2004) used Au and Ag as co-collectors in the fire assay analysis of Pd, Pt, and Rh in geological reference materials and showed variable recoveries with Ag and better recoveries with Au. Studies on iridium (Ir) and ruthenium (Ru) as co-collectors for PGMs and gold in ores and concentrates showed that Ir was a useful co-collector for concentrate material only at very low concentrations, and Ru was not useful at either low or high concentrations (Ndovorwi, 2014). However, limited research has been done to demonstrate the effectiveness of Fe, Cu, and Co as co-collectors for accurate analysis of PGMs and gold. Using Fe, Cu, and Co can be cost-effective compared to precious metals like silver, platinum, palladium, and gold. Accurate determination of PGMs and Au in geological samples provides important information for mineral exploration (Volzhenin et al., 2018). Therefore, the goal of this study was to develop a lead-based fire assay method for the preconcentration of PGMs and Au using iron, cobalt, and copper as co-collectors and quantitative determination using ICP-OES.
Experimental procedure
Instrumentation
Basic mining laboratory equipment was used for crushing, pulverizing, milling, and splitting of the samples. Industrial Analytical, 2018 model muffle furnaces (0-1300°C) were used for fusion and cupellation. The ThermoFisher ICAP 7400 radial model ICP-OES instrument (Germany) was used to determine the PGMs and Au.
Reagents
Analytical grade nitric acid (55%) and hydrochloric acid (32%) were obtained from Merck (Germany). The lead-based flux was obtained from Terranova (South Africa) and ICP standard reference solutions were obtained from De Bruyne (South Africa). AMIS 0426, a UG2 ore-based certified reference material, was obtained from African Mineral Standards (South Africa).
Sampling and pre-processing
Borehole core sampling was conducted at the Siyanda Union Mine Northam, South Africa as per their mine sampling standards. A total of 30 samples were collected for analysis. The samples were crushed, pulverized, and milled to minimum 95% passing the 75 μπι sieve. The milled samples were homogenized in a blender for 48 hours. A map of the sampling points is shown in Figure 1.
Sample fusion
The fluxing method was adapted from Rodriguez-Rodriguez and Miguel, (2018) with slight modifications. Briefly, 100 g of each sample was mixed with 300 g of the lead-based flux comprising Na2CO3 (39.1%), borax (22.0%), SiO2 (9.23%), starch from corn meal (3.0%), PbO (26.4%), CaF2 (2.3%), and paraffin (900 mL). Then, 20 mL of 20 mg/L solution of Cu, Fe, and Co were added to 10 geological samples and ten certified reference material samples (AMIS 0426). The mixture was introduced into a clay crucible, the surface was covered, and the crucible was placed in a pre-heated muffle furnace. Three fusion conditions were employed, viz. 900 ± 50°C for 60 minutes, 1100 ± 50°C for 60 minutes, and 1200 ± 50°C for 60 minutes. Once the fusion was completed (no effervescence was observed in the melted sample), the crucible was removed from the furnace and its contents poured into an iron mould. After solidification, the crystals that were formed by the slag were crushed with a hammer to release the lead button.
Cupellation
The cupellation method was adapted from Rodríguez-Rodríguez and Miguel, (2018) with slight modifications. The cupellation temperature was increased from 900°C to 1000 ± 50°C. The lead button was put in a magnesite cupel (previously dried at 1000 ± 50°C for 60 minutes) and ignited in a furnace at 1000 ± 50°C until the lead melted. The furnace door was left slightly open so that the lead could be oxidized and most of the lead could be absorbed by the cupel. The temperature was kept constant until all the lead was removed. At the end of this process, a button of PGMs and Au (a prill) was obtained. Table I shows the fusion and cupellation conditions. Figure 2 illustrates the analytical steps involved in the of determination of the PGMs and Au by ICP-OES.
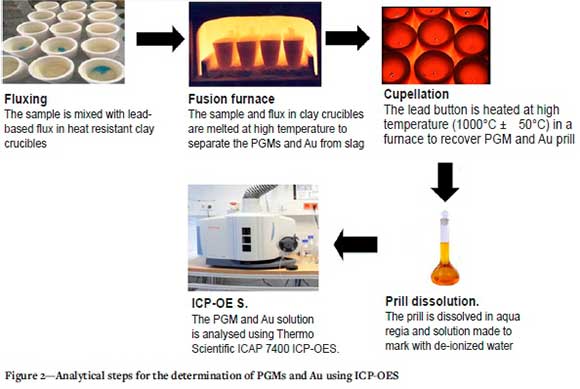
Dissolution of prill
The prill dissolution and instrumental analysis methods were adapted from Tao et al., (2017) with slight modifications. Briefly, a PGM and Au prill was placed in a 10 mL volumetric flask containing 1 mL of concentrated HNO3.The mixture was boiled until there was no effervescence, followed by the addition of 3 mL of concentrated HCl, Then, 2 mL of 50 mg/L yttrium (10 mg/L final concentration) was added to the mixture as an internal standard. The solution was diluted to the 10 mL mark using deionized water prior to analysis.
Preparation of calibration standards
The multi-element reference standards contained Pt (1000 mg/L), Pd (500 mg/L), Rh (200 mg/L), and Au (50 mg/L). Table II lists the concentrations of the standard solutions used to calibrate the ICP-OES instrument.
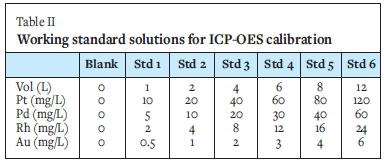
Yttrium was used as an internal standard (1 mL of 1000 mg/L stock solution). The working standards were made up to a 100 mL volume with deionized water. The control and geological exploration samples from each co-collector were analysed in 10 replicates.
The ICP-OES parameters as shown in Table III were adopted from Tao et al. (2017) with minor modifications.
Analytical performance
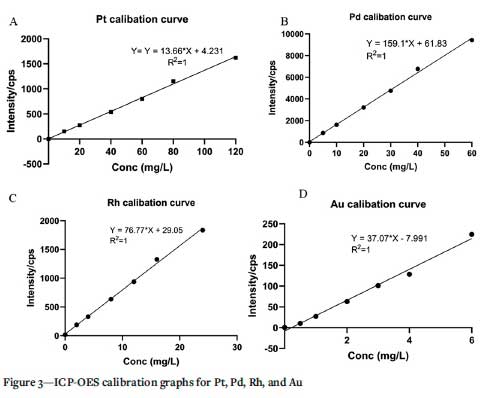
Using the optimum conditions, the intensity of each of the platinum group metals and gold was determined and quantified by the Thermofisher ICP-OES ICAP 7400 radial with yttrium (10 mg/L) as an internal standard. The analytical performance of the proposed fire assay method was validated by the linear range, the limit of detection (LOD), the correlation coefficient, and the relative standard deviation, as illustrated in Table IV. The linear range was between zero and 120 mg/L, with correlation coefficients from 0.997 to 0.999. Based on the 3-sigma blank approach, as recommended by IUPAC for spectrochemical measurements (Tao et al., 2017), the limits of detection of the proposed method for the target platinum group metals and gold in a 100 g sample were in the range of 0.001 mg/L to 0.047 mg/L. Figure 3 show the calibration curves for Pt, Pd, Rh, and Au. The analytical figures of merit by fire assay ICP-OES are also shown in Table IV.
Results
Table V show the average elemental compositions for the control sample and the geological exploration samples obtained using Fe, Co, and Cu co-collectors according to the experimental procedures described in Table I. Table V also shows the percentage relative standard deviation (RSD) and percentage recovery for PGMs and Au. The recoveries obtained from the Fe co-collector were 97.4% for Pt, 87.6% for Pd, 95.8% for Rh, and 104.9% for Au. In case of Co co-collector, the recoveries were 99.5% for Pt, 88.7% for Pd, 90.2% for Rh, and 97.2% for Au. Finally, the recoveries obtained from the Cu co-collector were 92.2% for Pt, 88.1% for Pd, 83.1% for Rh, and 88.1% for Au. These results demonstrate that Fe and Co co-collectors can be used for the determination of PGMs and Au. However, there is slight loss of analyte using Cu as co-collector for Pt and Pd, as indicated by the relatively low recoveries.
The precision of PGMs and Au determinations was calculated as percentage relative standard deviation obtained from ten separate determinations of PGM concentrations in the reference material and geological exploration samples. The RSD values for the certified reference material were less than 7% for Pt and Pd using all three co-collectors, whereas for Rh the RSD was greater than 7% for Fe co-collector (10.97%) and less than 7% for Co and Cu co-collectors. In the case of Au, the RSD values for Fe and Co co-collectors were all greater than 7%. These values demonstrate that the determination was not highly precise for Au, but moderate for Rh and relatively precise for Pt and Pd using the three co-collectors. Also shown in Table V are the elemental compositions and RSD values of the geological samples. As shown in Table V, the RSDs for geological exploration samples obtained using experimental condition 2 in Table I for Fe, Co, and Cu co-collectors is less than 7%, a result that demonstrates that the procedure employed is relatively precise.
Tables VI and VII show statistical accuracy testing results for control and geological exploration samples performed using a Student's t-test. As shown in Table VI, the ρ-value for Pt (0.011) is less than the α-value (0.05) with the Fe co-collector, an indication that there is a significant difference between the mean Pt value and the certified reference value. Pd, Rh, and Au have p-values greater than α-value with the Fe collector, suggesting that there is no significant difference between the analysis results and the certified values. In case of Co, the p-value for Rh is less than the α-value (p value=0 vs α=0.05), whereas the p-values for Pt, Pd, and Au are greater than the α-value. These results demonstrate that for the Co co-collector, there is a significant difference between the mean Rh value and the certified reference value of the control sample. With Cu co-collection the results for Pt, Rh, and Au were significantly different from the certified values (p-values < α =0.05), and there was no significant difference in the Pd results.
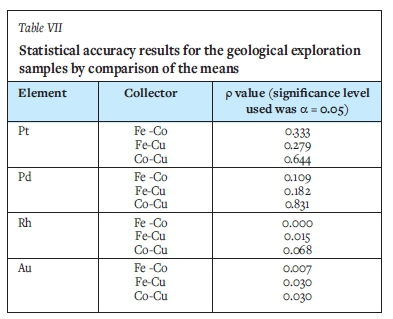
The statistical results for geological samples are shown in Table VII. The p-values for Pt and Pd are greater that the α-value for all the co-collectors, suggesting that Fe, Co, and Cu can be useful co-collectors for geological samples. For Au, the p-values are less than the α-value for all co-collectors, demonstrating that the mean values are significantly different from each other. The mean Rh results obtained from the Co and Cu co-collections showed no significant difference. However, the mean results from Fe/Co and Fe/Cu co-collection indicated a significant difference.
Table VIII summarizes the statistical analysis of variance for the certified reference material (control) and geological exploration samples. There is no significant difference in variance for the Pt results obtained from Fe and Co co-collectors for the control sample. The variances for the Pt results obtained from the Cu/Co and Cu/Fe co-collectors are statistically different. The variances of the Pd results obtained from all the co-collectors are not statistically different, indicating that the co-collecting capabilities of Pd are comparable. The Rh results from Co, Fe, and Co-Cu co-collectors show no difference. However, the Fe and Cu results are different. The Au results from all co-collectors were significantly different.
Table VIII also shows that the variances of Pt and Pd from all the co-collectors are statistically equal, but the Au results are statistically different since the ρ-values are all less than α. The Rh results from Co and Cu co-collection show no significant differences; however, the results of Fe, Co, and Fe-Cu co-collection indicate a significant difference.
Discussion
Accurate determination of the PGMs and Au is essential for geochemical and cosmochemical studies because these metals have significant economic values and can also provide important information about the origin, fractionation, and transportation of PGMs during geological processes (Qi et al., 2003). Generally, the determination of PGMs in geological materials is difficult because of their low crustal abundance (with background levels of a few nanograms per gram or less), heterogeneous distribution, and the complexity of sample preparation procedures. Accurate determination of these metals requires the use of highly sensitive analytical instruments. Because of its sensitivity and capability to measure traces, ICP-OES has been successfully employed to fully characterize PGMs and Au in geological materials and automotive catalysts (Senila et al., 2020). In addition, a variety of techniques have been employed for sample decomposition and preconcentration of PGMs prior to ICP-OES measurement. Nickel sulphide collection and sodium peroxide fusion followed by Te co-precipitation are a common method for PGM analysis (Balcerzak, 2002). The nickel sulphide fire assay method offers the advantage of accommodating a large sample mass, and all of the PGMs can be concentrated by this procedure. However, the disadvantages are the relatively large amounts of reagents used, often resulting in an analytical blank with a higher concentration of PGMs, introduction of Cu and Ni to the solutions, which often cause interference problems with the PGMs, and the requirement that the composition of the flux be changed according to the composition of the sample matrix (Qi et al., 2003).
In this work, we developed a new low-cost technique to accurately determine PGMs and Au using Fe, Co, and Cu co-collectors during sample decomposition and preconcentration prior to ICP-OES. This technique is relatively simple, with higher PGM and Au recoveries. As the results demonstrate, better recoveries for PGMs and Au are obtained using Fe and Co as co-collectors. However, with Cu as a co-collector, the recoveries for Pt and Pd were lower than those obtained with Fe and Co collectors. Since co-collectors are used in the preconcentration of PGMs and Au, the decrease in recovery with Cu co-collector is likely due to losses of PGMs and Au into the slag. Generally, concentrates from UG-2 ores contain much higher levels of chromium, which results in the formation of chromium-rich spinels during melting (Nell, 2004). During the converting process care is needed to avoid excessively oxidizing conditions, which result in cobalt, nickel, and copper losses to the slag (Nell, 2004). This could also be the reason for the lower recoveries using Cu as a co-collector.
Amassé (1998) successfully developed a method for determination of PGMs by employing selenium and tellurium as carriers in the presence of a potassium iodide catalyst. The extraction yields obtained using this method were between 95% and 100% for PGMs and around 80% for gold (Amassé, 1998). Jin and Zhu (2000) determined Pt, Pd, Rh, and Au in geological samples by nickel sulphide (NiS) fire assay and co-collecting using Te before ICP-MS analysis, and showed that the recovery of Au was enhanced from 65%. to 80%. In the present study the Au recovery was above 90%, indicating that co-collection with lead fire assay can be a reliable technique for Au recovery. Wang and Brindle (2014) digested 0.5-2.0 g of the sample in a solution of 1% (m/v) L-cysteine and 1% HCl (v/v), for 10-12 minutes, and recovered between 94% and 100% gold according to the the ICP-MS results. In the current work, the recoveries from the Fe and Co co-collectors were in a similar range. This indicates that the method can be an alternative for the determination and quantification of Au. Zhang et al. (2014) determined Pt, Pd, Ru, Rh, and Ir in ultrabasic rock from the Great Dyke of Zimbabwe using lead fire assay laser ablation and ICP-OES. The recoveries of Pt, Pd, Ru, Rh, and Ir were 92.5%, 91.25%, 91.25%, 92.5%, and 93.75%, respectively. Consequently, the method of using Fe, Co, and Cu as co-collectors can be a suitable alternative for the analysis of PGMs and Au in large sample volumes.
Conclusion
In this work we developed a new preconcentration technique for the determination of Pt, Pd, Rh, and Au in control and geological samples by the addition of Fe, Co, and Cu co-collectors. Fire assay was used for the preconcentration process. The samples were digested in aqua regia and quantification was done using ICP-OES. The method was validated using the AMIS 0426 certified reference material. Based on percentage recoveries, we showed that Cu is not a good co-collector for Au and Rh determination, presumably due to loss of analyte in the slag, whereas Fe and Co are better co-collectors for the determination of PGMs and Au. Together, this study and the methodology developed for the determination of PGMs and Au can present a novel means to quantify PGMs and Au in control and geological exploration samples.
Acknowledgements
The authors thank the staff of the Geology Department of the Siyanda Union Mine for their assistance with the sampling. We also thank the Siyanda Union laboratory for sample preparation and analysis by ICP-OES.
References
Amassé, J. 1998. Determination of platinum-group elements and gold in geological matrices by inductively coupled plasma-mass spectrometry (ICP-MS) after separation with selenium and tellurium carriers. Geostandards Newsletter, vol. 22, no. 1. pp. 93-102. https://doi.org/10.1111/j.1751-908X.1998.tb00548.x [ Links ]
Balcerzak, M. 2002. Sample digestion methods for the determination of traces of precious metals by spectrometric techniques. Analytical Sciences, vol. 18, no. 7, pp. 737-750. https://doi.org/10.2116/analsci.18.737 [ Links ]
Bayrak, H.E., Bulut, V.N., Tüfekci, M., Bayrak, H., Düran, C., and Söylak, M. 2017. Determination of Au (III) and Pd (II) ions by flame atomic absorption spectrometry in some environmental samples after solid phase extraction. Toxicological & Environmental Chemistry, vol. 99, no. 4. https://doi.org/10.1080/02772248.2016.1212351 [ Links ]
Berezhnaya, E.D. and Dubinin, A.V. 2016. Determination of platinum-group elements and gold in ferromanganese nodule reference samples. Geostandards and Geoanalytical Research, vol. 41, no. 1 pp. 137-145. https://doi.org/10.1111/ggr.12130 [ Links ]
Carelse, C., Manuel, M., Chetty, D., and Corfield, A. 2020. Au and Ag distribution in alloys produced from the smelting of printed circuit boards -An assessment using SEM-EDS , EPMA , and LA-ICP-MS analysis. Journal of the Southern African Institute of Mining and Metallurgy, vol. 120, no. 3. pp. 203-210. doi: 10.17159/2411-9717/698/2020 [ Links ]
Creech, J.B., Baker, J.A., Handler, M.R., and Bizzarro, M. 2014. Platinum stable isotope analysis of geological standard reference materials by double-spike MC-ICPMS. Chemical Geology, vol. 363. pp. 293-300. https://doi.org/10.1016/j.chemgeo.2013.11.009 [ Links ]
Díaz, D., Hahn, D.W., and Molina, A. 2017. Quantification of gold and silver in minerals by laser-induced breakdown spectroscopy. Spectrochimica Acta Part B: Atomic Spectroscopy, vol. 136. pp. 106-115. https://doi.org/10.1016/j.sab.2017.08.008 [ Links ]
Hinds, M.W. and Burgess, R.W. 2014. The non-destructive determination of Pt in ancient Roman gold coins by XRF spectrometry. Journal of Analytical Atomic Spectrometry. pp. 1799-1805. https://doi.org/10.1039/c4ja00170b [ Links ]
Hughes, H.S.R., McDonald, I., and Kerr, A.C. 2015. Platinum-group element signatures in the North Atlantic Igneous Province: Implications for mantle controls on metal budgets during continental breakup.. Lithos, vol. 233. pp. 89-110. https://doi.org/10.1016/j.lithos.2015.05.005 [ Links ]
Jansen, M., Aulbach, S., Hauptmann, A., Heidi, E.H., Klein, S., Krüger, M., and Zettler, R.L. 2016. Platinum group placer minerals in ancient gold artifacts - Geochemistry and osmium isotopes of inclusions in Early Bronze Age gold from Ur / Mesopotamia. Journal of Archaeological Science, vol. 68 (April). pp. 12-23. https://doi.org/10.1016/j.jas.2016.02.004 [ Links ]
Jin, X. and Zhu, H. 2000. Interlaboratory Note. Journal of Analytical Atomic Spectrometry. pp. 747-751. https://doi.org/10.1039/b000470g [ Links ]
Liipo, J., Hicks, M., Takalo, V., Remes, A., Talikka, M., Khizanishvili, S., and Natsvlishvili, M. 2019. Geometallurgical characterization of South Georgian complex copper-gold ores. Journal of the Southern African Institute of Mining and Metallurgy, vol. 119, no. 4. pp. 333-338. [ Links ]
Makua, L., Langa, K., Sagürü, C., and Ndlovü, S. 2019. PGM recovery from a pregnant leach solution using solvent extraction and cloud point extraction: A preliminary comparison. Journal of the Southern African Institute of Mining and Metallurgy, vol. 119, no. 5. pp. 453-458. [ Links ]
Moroelli, C.P.R., Figueiredo, A.M.G., Enzweiler, J., Sarkis, J.E.S., Jorge, A.P.S., and Kakazu, M. 2004. Determination of platinum-group elements in geological reference materials by high resolution-ICP-MS after nickel sulfide fire-assay collection and Te Co-precipitation. Geostandards and Geoanalytical Research, vol. 28, no. 2. pp. 305-310. https://doi.org/10.nn/j.1751-908X.2004.tb00745.x [ Links ]
Ndovorwi, F. 2014. Determination of platinum , palladium , rhodium and gold in ores and concentrates using iridium and ruthenium as co-collectors by fire assay. MSc thesis, University of Zimbabwe. [ Links ]
Nell, J. 2004. Melting of platinum group metal concentrates in South Africa. Journal of the South African Institute of Mining and Metallurgy, vol. 104, no. 7. pp. 423-428. [ Links ]
Qi, L., Gregoire, D.C., Zhou, M.F., and Malpas, J. 2003. Determination of Pt, Pd, Ru and Ir in geological samples by ID-ICP-MS using sodium peroxide fusion and Te co-precipitation. Geochemical Journal, vol. 37, no. 5. pp. 557-565. https://doi.org/10.2343/geochemj.37.557 [ Links ]
Rodríguez-Rodríguez, Y. and Miguel, O. 2018. Determination of gold in geological samples combining the fire assay and ultraviolet visible spectrophotometry techniques. Academia Journal of Scientific Research, vol. 6, no. 1. pp. 27-33. doi: 10.15413/ajsr.2017.0112 [ Links ]
Senila, M., Cadar, O., Senila, L., Böringer, S., Seaudeau-Pirouley, K., Ruiu, A., and Laoroix-Desmazes, P. 2020. Performance parameters of inductively coupled plasma optical emission spectrometry and graphite furnace atomic absorption spectrometry techniques for Pd and Pt determination in automotive catalysts. Materials, vol. 13, no. 22. pp. 1-13. https://doi.org/10.3390/ma13225136 [ Links ]
Serbin, R., Bazel, Y., and Ru, S. 2017. Speciation of platinum by GFAAS using various possibilities of analytical signal enhancement. Talanta, vol. 175 (June). pp. 46-52. https://doi.org/10.1016/j.talanta.2017.06.078 [ Links ]
Suominen, M., Kontas, E., and Niskavaara, H. 2004. Comparison of silver and gold inquarting in the fire assay determination of palladium, platinum and rhodium in geological samples. Geostandards and Geoanalytical Research, vol. 28, no. 1. 131-136. https://doi.org/10.1111/j.1751-908X.2004.tb01049.x [ Links ]
Tanner, D., Modonald, I., Harmer, R.E.J., Muir, D.D., and Hughes, H.S.R. 2019. A record of assimilation preserved by exotic minerals in the lowermost platinum-group element deposit of the Bushveld Complex: The Volspruit Sulphide Zone. Lithos, vol. 324-325. pp. 584-608. https://doi.org/10.1016/j.lithos.2018.10.032 [ Links ]
Tao, D., Guo, W., Xie, W., Jin, L., Guo, Q., and Hu, S. 2017. Rapid and accurate determination of gold in geological materials by an improved ICP-MS method. Microchemical Journal, vol. 135. pp. 221-225. https://doi.org/10.1016/j.microc.2017.09.014 [ Links ]
Vanhaeoke, F., Resano, M., Kooh, J., MoIntosh, K., and Günther, D. 2010. Femtosecond laser ablation-ICP-mass spectrometry analysis of a heavy metallic matrix: Determination of platinum group metals and gold in lead fire-assay buttons as a case study. Journal of Analytical Atomic Spectrometry, vol. 25, no. 8. pp. 1259-1267. https://doi.org/10.1039/c002746d [ Links ]
Volzhenin, A.V, Petrova, N.I., Medvedev, N.S., and Saprykin, A.I. 2018. Multiple probe concentrating of Au and Pd in geological samples for atomic absorption determination with two-stage probe atomization. Microchemical Journal, vol. 138. pp. 390-394. https://doi.org/10.1016/j.microc.2018.01.037 [ Links ]
Wang, Y. and Brindle, I.D. 2014. Rapid high-performance sample digestion for ICP determination by ColdBlockTM digestion. : Part 2: gold determination in geological samples with memory e ff ect elimination. Journal of Analytical Atomic Spectrometry, vol. 29. pp. 1904-1911. https://doi.org/10.1039/c4ja00189c [ Links ]
Zhang, N., Ma, Y., Shen, Y., and Gao, X. 2014. Determination of platinum , palladium , ruthenium , rhodium , and iridium in ultrabasic rock from the Great Dyke of Zimbabwe by inductively coupled plasma - optical emission spectrometry. Analytical Letters, vol. 47, no. 12 pp. 2072-2079. https://doi.org/10.1080/00032719.2014.893441 [ Links ]
 Correspondence:
Correspondence:
M. Mupa
Email: mathewmupa@buse.ac.zw
Received: 23 May 2021
Revised: 23 Nov. 2021
Accepted: 29 Nov. 2021
Published: January 2022














