Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Journal of the Southern African Institute of Mining and Metallurgy
versão On-line ISSN 2411-9717
versão impressa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.122 no.1 Johannesburg Jan. 2022
http://dx.doi.org/10.17159/2411-9717/1654/2022
PROFESSIONAL TECHNICAL AND SCIENTIFIC PAPERS
The concentration of rare earth elements from coal fly ash
G.B. Abaka-WoodI; J. Addai-MensahI, II; W. SkinnerII
IFuture Industries Institute, University of South Australia, Mawson Lakes Campus, Adelaide. G.B. Abaka-Wood https://orcid.org/0000-0002-2815-0022; J. Addai-Mensah: https://orcid.org/0000-0001-8381-2108
IIDepartment of Mining and Process Engineering, Namibia University of Science and Technology, Windhoek, Namibia. https://orcid.org/0000-0002-9606-023X
SYNOPSIS
Recently, coal fly ash has become a potential candidate as a secondary resource of rare earth elements (REE). In this investigation, we studied the recovery of REE from fly ash from a commercial power plant. The specific aim was to assess the technical feasibility of recovering REE from the coal fly ash using conventional preconcentration methods, including gravity separation, magnetic separation, and froth flotation. The experimental results revealed that flotation achieved major gains in REE recovery and upgrading. However, during gravity and wet magnetic separation tests, the bulk of REE reported to the tailings. The results showed significant variations in the performance of the various beneficiation methods investigated. This study has confirmed that existing physical separation methods could be used to recover REE from coal fly ash prior to hydrometallurgical and pyrometallurgical processing, although some challenges persist.
Keywords: rare earth elements, coal fly ash, physical separation, flotation.
Introduction
Over the past decade, rare earth elements (REE) have gained significant attention in the global market due to their industrial and technological applications. Although the demand for REE has increased tremendously, commercial or large-scale production is limited to a few countries. REE have been classified as critical and strategic materials due to their high supply risk and increased global demand, especially in the defence, energy, electronics, and automotive industries (Abaka-Wood et al., 2019a; Blissett, Smalley, and Rowson, 2014; Liu, Huang, and Tang, 2019). With the current depletion of high-grade resources globally, there has been a corresponding increase in attempts to exploit unconventional or secondary resources such coal fines, fly ash, permanent magnets, mining tailings, and phosphorus-based products as sources of REE (Abaka-Wood et al., 2019b, Liu, Huang, and Tang, 2019; Seredin et al., 2013).
Over the past two decades, coal deposits have been considered as alternative resource for REE (Hower et al., 2020; Pan et al., 2020; Sahoo et al., 2016; Seredin and Dai, 2012; Sis, Ozbayoglu, and Sarikaya, 2004; Zhang et al., 2015). On average, the REE content in coal is 68 ppm, and 404 ppm in coal ash (Ketris and Yudovich, 2009; Seredin et al., 2013; Zhang et al., 2015). Recent studies suggest that coal products contain sub-economic REE concentrations, which may be recovered and upgraded (Seredin et al., 2013).
Table I shows the total REE production in different countries along with estimated reserves (US Geological Survey, 2021). China controls the majority of REE resources and production, accounting for about 58-60% of documented global production in 2019-2020. The USA and Australia produce about 1518% and 7-15% of the global rare earth oxides (REO), respectively. The data also shows that China holds 41 Mt, representing approximately 37% of the economic demonstrated REE resources, followed by Brazil and Vietnam, with 21 Mt (18%) each, Australia (4.1 Mt, approximately 3%), and the USA (1.5 Mt, 1.2%).
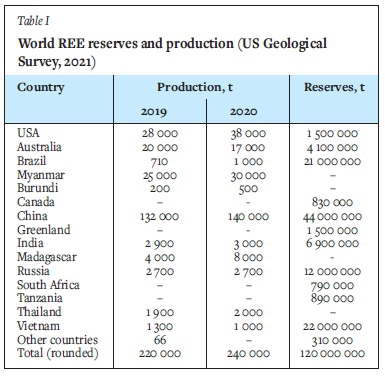
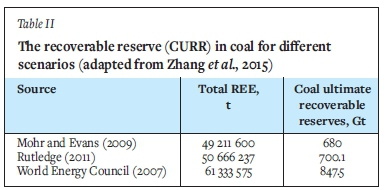
Zhang et al. (2015) suggest that the total amount of REE in coal could be about 50 Mt (Table II, which equates to about 42% of the total global traditional REE reserves. This implies that coal may be considered as an unconventional REE resource, subject to market circumstances and the development of efficacious beneficiation methods to recover the REE. However, Zhang et al. (2015) suggested that the complexity of the composition and distribution of REE in coal and its by-products have limited studies aimed at recovering REE from coal products.
It is well documented that REE are mainly concentrated in bastnasite, allanite, monazite, xenotime, florencite, kimuraite, lanthanite, and zircon, and in some cases form composite particles with clay minerals such as kaolinite and illite (Dai et al., 2012; Gupta et al., 2017; Hood et al., 2017; Seredin and Dai, 2012; Sun et al., 2007; Zhang et al., 2015). Depending on the source and composition of the coal, the gangue components of coal fly ash include substantial amounts of quartz, calcite, siderite, rutile, and illite. The differences between physical and physicochemical characteristics (density, magnetic susceptibility, conductivity, surface hydrophobicity) of REE and gangue minerals may be exploited to recover and upgrade REE through gravity concentration, magnetic separation, froth flotation, and electrostatic separation (Zhang et al., 2015).
Lin et al. (2017) studied the enrichment of REE from coal and coal by-products using particle size, magnetic, and density separation methods. Particle size separation was conducted using 20-150 μm sieves mounted on a 3-inch shaker equipped with an electromagnetic vibrator. The experimental results indicated that the REE were enriched in the nonmagnetic products after separation. Elsewhere, Blissett, Smalley, and Rowson (2014) investigated coal fly ashes from the UK and Poland to evaluate any chance of recovering their REE content. The work demonstrated marginal REE upgrading after classification of the nonmagnetic inorganic content of the samples.
As a result of the increased use of biomass as a co-firing fuel in power plants, there is a corresponding increase in production of fly ash, with varying chemical properties which may not be suitable for traditional application in the cement/ concrete industry (Franus, Wiatros-Motyka, and Wdowin, 2014; Wdowin et al., 2014). Coal fly ash forms the bulk of the fly ash generated globally at an annual production rate of 750 Mt (Perämäki, Tiihonen, and Väisänen, 2019). Hence, it is important to investigate the recovery of REE as an alternative application for coal fly ashes. The aim of this work is to exploit the differences in the physical and physicochemical properties of REE and gangue minerals to recover and upgrade REE from a typical coal fly ash (CFA) produced from a commercial power plant.
Materials and methods
Materials
CFA samples from a coal-fired commercial power plant in Australia were used in this investigation. The samples were dried and split into representative subsamples and stored in vacuum in plastic bags for subsequent characterization and beneficiation studies. The elemental composition of the CFA was obtained using inductively coupled plasma mass spectroscopy (ICP-MS). At the end of each beneficiation test, the separation products were weighed, dried, and their chemical content determined by ICP-MS.
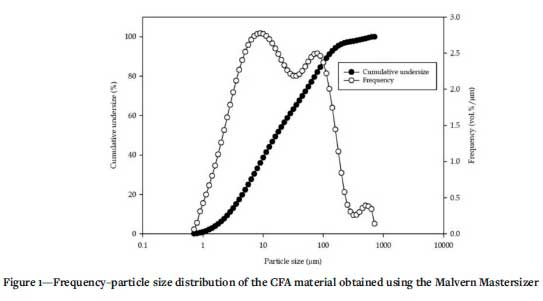
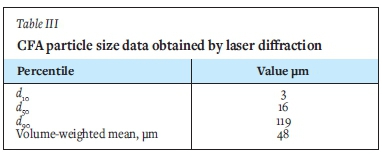
Particle size analysis was conducted using a Malvern Mastersizer 2000 laser-diffraction instrument with a measurement range of 0.02-2000 μm. A representative sample of CFA was prepared into a pulp (25 wt.%) and stirred at 800 r/min for 30 minutes, to de-agglomerate the particles. Subsamples were drawn and analysed using the Malvern Mastersizer 2000. The results are presented in Figure 1. The data obtained was used to estimate the 10th, 50th, and 90th percentile particle sizes, d10, d50, and d90, respectively, which are presented in Table III.
Particle size separation
The distribution of REE in the CFA as a function of particle size was investigated by wet screening tests. A set of test sieves was selected and arranged in sequence 150, 106, 75, and 38 μm to screen approximately 150 g of representative CFA sample. The particles retained on the respective sieves were dried in an electric oven at 70°C for 12 hours, weighed, and analysed for their chemical content.
Gravity separation
Gravity separation tests were conducted using a Knelson concentrator (KC). The KC was operated at a feed flow rate of 150 g/min at a pulp density of 25 wt.% solids. During the tests, the pulped material was diluted with fluidization water at flow rate from 2.5 to 7.5 L/min, while the bowl rotation speed was set at 73 G. The concentrate and tailing fractions obtained were submitted for chemical analysis.
Magnetic separation
A wet high-intensity magnetic separator (Model L-4) was used to investigate the recovery of REE at different magnetic field intensities. For each test, 50 g of CFA sample was mixed with deionized water to yield a 17wt.% pulp (Abaka-Wood, Addai-Mensah, and Skinner, 2016, Abaka-Wood et al., 2019a). The pulp was thoroughly mixed and transferred with additional water at a rate of 1 L/min to minimize entrainment of nonmagnetic fractions on the matrix during operation. The magnetic field intensity was adjusted (0.11-1.74 T) by increasing the current from 1 to 21 A. The wet high-intensity magnetic separator (WHIMS) was fitted with a medium expanded metal (MEX) matrix.
Flotation separation
A 250 mL Polish microflotation cell (Instytut Metali Niezelaznych, Gliwice) was used in the flotation of CFA samples. During the tests, 50 g of dried CFA was transferred into the flotation cell along with distilled water. The impeller speed and air flow rate were set at 720 r/min and 1.5 L/min, respectively. The initial pH of the pulp was noted and adjusted to the desired value using HCl or NaOH. Oleic acid was used a collector. During the flotation tests, 1000 g/t oleic acid was added with 5 minutes conditioning time. Froths were collected every 15 seconds over 10 minutes. The flotation products were dried, weighed, and analysed by ICP-MS.
Results and discussion
Concentrations and recovery potential
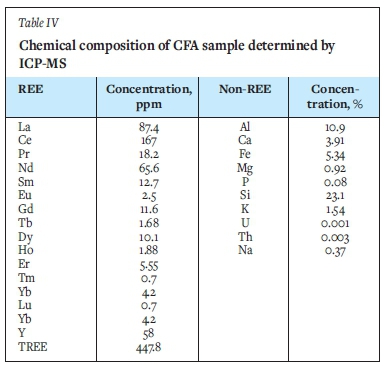
The ICP-MS analysis (Table IV) revealed that REE in the CFA material were dominated by cerium (Ce, 167 ppm), lanthanum (La, 87.4 ppm), neodymium (Nd, 65.6 ppm), and yttrium (Y, 58 ppm), which made up about 85% of the total REE (TREE) content. Aluminium (Al), silicon (Si), calcium (Ca), and iron (Fe) were the major gangue elements in the CFA sample. The outlook coefficient, which is the ratio of critical (Nd, Eu, Tb, Dy, Y, and Er) to excessive (Ce, Ho, Tm, Yb, and Lu) REE was proposed by Seredin (2010) to assess the market quality of REE deposits. REE resources with a high are regarded as profitable. The Koutlof the CFA material was calculated to be 0.82, which makes the CFA promising for potential economic extraction of REE, especially since no mining and comminution costs will be incurred. Specifically, the CFA sample contained approximately 32% critical REE. The critical REE content and Koutl are more comparable to those obtained for Finnish peat and biomass combustion fly ash, which contain 28-32% critical REE with Koutlvalues >0.7 (Perämäki, Tiihonen, and Väisänen, 2019). The TREE concentration in CFA (447.81 ppm) is similar to a typical coal fly ash, which has an average concentration of 404 ppm (Perämäki, Tiihonen, and Väisänen, 2019; Seredin and Dai, 2012). According to the data obtained, the CFA can be considered promising for recovery of REE, based on the outlook coefficient and critical REE content. However, the TREE concentration is less than 800 ppm, which has been suggested as the cut-off grade for coal seams thicker than 5 m (Seredin, (2010). It is worth noting that the CFA used in the present study is already of a suitable particle size for subsequent beneficiation.
Particle size separation
Wet sieve analysis was conducted to study REE distribution in the various size fractions. The results are summarized in Figure 2, which shows the REE concentration, REE distribution, and mass yield of the respective particle size fractions. The results indicate variable REE concentrations in the respective size fractions, with +150 pm having the least TREE concentration of 441 ppm, whereas the highest TREE concentration was measured in the -75 +53 pm and -150 +106 pm size fractions. However, the largest proportion of REE in the CFA was contained "within the -38 pm size fraction, which accounted for 67% and 69% of the TREE and feed mass, respectively.
Gravity separation
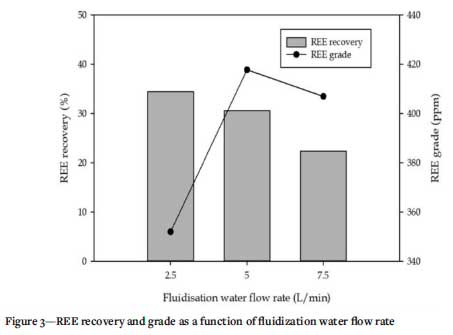
Gravity separation tests were conducted using a laboratory-scale KC. During operation, the concentrating beds formed within the KC are prevented from consolidating by means of a water current which flows through the fluidization holes, leading to the formation of fluidized beds within each ring (Abaka-Wood et al, 2019c). The fluidization water flow rate was varied from 2.5- 7.5 L/ min in this investigation.
The results obtained have been summarized in Figure 3 and Table V. It can be seen that REE recovery and upgrade is significantly affected by changes in the fluidization water flow rate. An increase in the water flow rate from 2.5 L/min to 7.5 L/ min saw a decrease in REE recovery from 34% to 22%, with a corresponding increase in REE grade from 352 ppm to 407 ppm. REE minerals are known to have specific gravities typically >4.0 (Subbarao, 1980), hence they were expected to report with the KC concentrate. As shown in Table V, the bulk of REE reported with the KC tailings along with Si particles. Notably, about 53% of the Fe was recovered into the KC concentrate at 2.5 L/min water flow rate. This decreased significantly as the fluidization water flow rate was increased. The results suggest that the recoveries of both Fe and Si followed the same trend as the REE.
Magnetic separation
The influence of magnetic field intensity on REE recovery was studied using a laboratory-scale WHIMS. In this test, the magnetic field intensity was increased from 0.11 T to 1.74 T. The cumulative recoveries of REE, Fe, and Si at the applied field intensities are shown in Figure 4. REE recovery increased from 11% to 37% with an increase in applied field intensity from 0.11 T to 1.74 T, with a corresponding increase in grade from 328 ppm to 516 ppm. The bulk of the REE in the CFA reported to the nonmagnetic fraction, which accounted for 63% and 60% of the TREE and mass, respectively, with an REE grade of 490 ppm.
The high REE content in the nonmagnetic fraction can be attributed to the fact that most of the REE particles may be associated with the nonmagnetic minerals such as quartz and other silicates. This is shown by the high Si content (64%) in the nonmagnetic fraction. Furthermore, REE recovery follows both the Si recovery and mass yield trends. Another significant observation was the high Fe content (56%) in the concentrate obtained at an applied field intensity of 0.11 T, and the low Fe content (28%) in the nonmagnetic fraction. The results suggest that half of Fe content in the CFA could be rejected at low magnetic field intensity, while upgrading REE in the corresponding nonmagnetic fraction along with silicate minerals.
Flotation
The effects of changes in pulp pH on REE recovery and upgrade were investigated during the flotation experiments. The pulp pH was decreased from the pristine CFA pulp value of 11 ± 0.5 to 7. Dilute solutions of HCl and NaOH were used as pH modifiers. The results presented in Figure 5 show that a decrease in pulp pH from 11 to 9 caused a significant decrease in REE recovery from 65% to 13%, and a corresponding increase in REE grade from 498 ppm to 678 ppm. A further decrease in pH from 9 to 7 resulted in an increase in recovery to 37% at a concentrate grade of 633 ppm. The results suggest that the recovery and upgrade of REE from CFA is pulp pH-dependent. In terms of REE recovery, the best result was achieved at pulp pH 11. This is consistent with flotation tests carried out by Satur et al. (2016), where fatty acids were used to achieve the highest REE recovery from a silicate-haematite ore at pH 11.
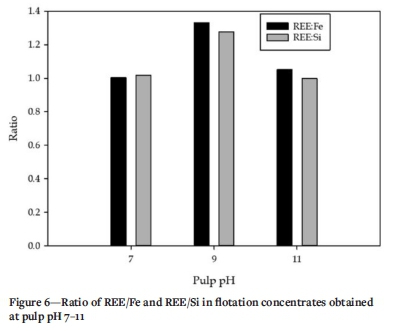
Figure 6 presents the ratios of REE content to Si and Fe in the flotation concentrates obtained at the respective pulp pH levels tested. The results suggest significant proportions of both Fe and Si in the froths recovered. However, lower quantities of Fe and Si reported to the concentrates obtained at pH 9, which explains the highest REE concentrate grade achieved. It can be noticed that the recoveries of Fe and Si follow those of mass yield and REE recovery, respectively. It has been demonstrated that Fe can be recovered using oleic acid at mild to strong alkaline pulp pH values (Abaka-Wood, Addai-Mensah, and Skinner, 2017a, 2017b; Abaka-Wood et al., 2019b; Joseph-Soly, Quast, and Connor., 2015; Quast, 2000, 2006). The high Si recoveries could be attributed to entrainment due to the presence of a high level of 'fine' particles in the feed (Duarte and Grano, 2007; Leistner Peuker,and Rudolph, 2017).
Implications and future work
The CFA used in the present study had a TREE grade of 447.8 ppm, with about 32% critical REE and an outlook coefficient of 0.82. This places the CFA within a potentially economic range if feasible beneficiation methods could be developed to selectively recover and upgrade REE values. The present study indicates that significant REE recovery could be achieved by flotation. However, during both magnetic and gravity separation, the bulk of the REE reported with the tailings fractions, which suggests that both these physical separation methods may be combined with flotation as an appropriate beneficiation strategy in future investigations.
For instance, either gravity or magnetic separation may be optimized to remove Fe-bearing particles prior to concentrating REE via froth flotation. Depressants could be used to minimize the recovery of silicate gangue minerals during REE beneficiation. For example, sodium silicate, disodium sulphide, starch, ammonium lignosulfonate, and sodium oxalate have been shown to be effective depressants of various gangue minerals in REE flotation (Abaka-Wood, Addai-Mensah, and Skinner, 2017b; Chelgani et al., 2015; Satur et al., 2016; Zhang and Anderson, 2017). A combination of the respective separation methods will help achieve a concentrate recovery and grade for subsequent REE extraction by hydrometallurgical or pyrometallurgical processes. Elsewhere, Abaka-Wood et al. (2019b), Jordens et al. (2016), Yang et al. (2015), and Xiong et al. (2018) have demonstrated the feasibility of achieving significant REE recovery and upgrade through the combination of selected physical separation methods and flotation. These previous investigations will serve as a guide in future studies which will target producing richer REE concentrates from the CFA.
However, prior to any further studies with the view of improving the results obtained in the present work, a detailed mineralogical study of the CFA to determine the true chemical and physical deportment of the REE and gangue species will be carried out. Mineralogical studies including quantitative X-ray diffraction (QXRD) and Quantitative Evaluation of Minerals by Scanning Electron Microscopy (QEMSCAN) analyses have been demonstrated to be crucial in establishing REE and gangue minerals deportment in different ores (Edahbi et al., 2018; Smythe et al., 2011; Van Rythoven et al., 2020). Smythe, Lombard, and Coetzee (2013) pointed out that QEMSCAN analysis provides useful information in estimating elemental recoveries and selecting potential beneficiation techniques for recovering minerals of interest. Furthermore, it was suggested that QEMSCAN analysis gives a good indication of the textural relationships, mineral association, and liberation characteristics between the mineral of interest and gangue minerals, which is crucial in selecting concentration processes for recovering REE.
Conlusions
The current study highlights the potential for CFA from a commercial power plant to contain significant REE reserves for subsequent beneficiation. Chemical characterization of representative CFA samples showed a concentration of 447.8 ppm TREE. The bulk of the REE in the CFA sample was concentrated in the fine fraction (< 38 μm), which contained more than half of the TREE. Furthermore, the varying distribution of REE between the respective size fractions indicates that both the coarse and fine fractions of CFA can be considered for REE beneficiation. The high outlook coefficient, coupled with the fact that CFA carries no mining and comminution costs, suggest that the recovery of REE could present a potential economic advantage.
Based on the results obtained, the KC does not appear to be a suitable gravity concentration method for concentrating REE from the CFA used in the present study. This is shown by the low recoveries and poor upgrades in the KC concentrates produced. However, WHIMS tests on CFA showed appreciable REE upgrade in magnetic concentrates produced at 1.08-1.74 T, although the corresponding recoveries were low. The results point out that the bulk of the REE were concentrated in the nonmagnetic fraction. Flotation using oleic acid resulted in higher REE concentrations in the froths produced than in the tailings. The pulp pH affected the recovery and upgrade of REE, with the highest recovery achieved at pH 11, whereas the best REE upgrade occurred at pH 9, where the lowest recovery was observed. Overall, the study suggests that beneficiation processes combining the methods employed in the present study may achieve enhanced REE recoveries and upgrades. This will be investigated in future work, along with hydrometallurgical or pyrometallurgical separation processes.
Acknowledgements
This work was supported by the Australian Government Research Training Program Scholarship and Future Industries Institute of the University of South Australia (Adelaide, Australia).
References
Abaka-Wood, G.B., Addai-Mensah, J., and Skinner, W. 2016. Magnetic separation of monazite from mixed minerals. Proceedings of Chemeca 2016: Chemical Engineering-Regeneration, Recovery and Reinvention. Engineers Australia, Melbourne. pp. 596-604. [ Links ]
Abaka-Wood, G.B., Addai-Mensah, J., and Skinner, W. 2017a. A study of flotation characteristics of monazite, hematite, and quartz using anionic collectors. International Journal of Mineral Processing, vol. 158. pp. 55-62. [ Links ]
Abaka-Wood, G.B., Addai-Mensah, J., and Skinner, W. 2017b. Selective flotation of rare earth oxides from hematite and quartz mixtures using oleic acid as a collector. International Journal of Mineral Processing, vol. 169. pp. 60-69. [ Links ]
Abaka-Wood, G.B., Zanin, M., Addai-Mensah, J., and Skinner, W. 2019a. Recovery of rare earth elements minerals from iron oxide-silicate rich tailings-Part 1: Magnetic separation. Minerals Engineering, vol. 136. pp. 50-61. [ Links ]
Abaka-Wood, G.B., Zanin, M., Addai-Mensah, J., and Skinner, W. 2019b. Recovery of rare earth elements minerals from iron oxide-silicate rich tailings - Part 2: Froth flotation separation. Minerals Engineering, vol. 142. pp. 105888. [ Links ]
Abaka-Wood, G.B., Quast, K., Zanin, M., Addai-Mensah, J., and Skinner, W. 2019c. A study of the feasibility of upgrading rare earth elements minerals from iron-oxide-silicate rich tailings using Knelson concentrator and Wilfley shaking table. Powder Technology, vol. 344. pp. 897-913. [ Links ]
Blissett, R., Smalley, N., and Rowson, N. 2014. An investigation into six coal fly ashes from the United Kingdom and Poland to evaluate rare earth element content. Fuel, vol. 119. pp. 236-239. [ Links ]
Chelgani, S.C., Rudolph, M., Leistner, T., Gutzmer, J., and Peuker, U.A. 2015. A review of rare earth minerals flotation: Monazite and xenotime. International Journal of Mining Science and Technology, vol. 25, no. 6. pp. 877-883. [ Links ]
Dai, S., Jiang, Y., Ward, C.R., Gu, L., Seredin, V.V., Liu, H., Zhou, D., Wang, X., Sun, Y., Zou, J., and Ren, D. 2012. Mineralogical and geochemical compositions of the coal in the Guanbanwusu Mine, Inner Mongolia, China: Further evidence for the existence of an Al (Ga and REE) ore deposit in the Jungar Coalfield. International Journal of Coal Geology, vol. 98. pp. 10-40. [ Links ]
Duarte, A.C.P. and Grano, S.R. 2007. Mechanism for the recovery of silicate gangue minerals in the flotation of ultrafine sphalerite. Minerals Engineering, vol. 20, no. 8. pp. 766-775. [ Links ]
Edahbi, M., Benzaazoua, M., Plante, B., Doire, S., and Kormos, L. 2018. Mineralogical characterization using QEMSCAN® and leaching potential study of REE within silicate ores: A case study of the Matamec project, Quebec, Canada. Journal of Geochemical Exploration, vol. 185. pp. 64-73. [ Links ]
Franus, W., Wiatros-Motyka, M.M., and Wdowin, M. 2015. Coal fly ash as a resource for rare earth elements. Environmental Science and Pollution Research, vol. 22, no. 12. pp. 9464-9474. [ Links ]
Gupta, T., Ghosh, T., Akdogan, G., and Srivastava, V.K. 2017. Characterizing rare earth elements in Alaskan coal and ash. Minerals & Metallurgical Processing, vol. 34, no. 3. pp. 138-145. [ Links ]
Hood, M.M., Taggart, R.K., Smith, R.C., Hsu-Kim, H., Henke, K.R., Graham, U.M., Groppo, J.G., Unrine, J.M., and Hower, J.C. 2017. Rare earth element distribution in fly ash derived from the Fire Clay coal, Kentucky. Coal Combustion and Gasification Products, vol. 9, no. 1. pp. 22-33. [ Links ]
Hower, J.C., Groppo, J.G., Joshi, P., Preda, D.V., Gamliel, D.P., Möhler, DT., Wiseman, J.D., Hopps, S.D., Morgan, T.D., Beers, T., and Schröck, M. 2020. Distribution of lanthanides, yttrium, and scandium in the pilot-scale beneficiation of fly ashes derived from eastern Kentucky coals. Minerals, vol. 10, no. 2. p. 105. https://doi.org/10.3390/min10020105 [ Links ]
Jordens, A., Marion, C., Grammatikopoulos, T., Hart, B., and Waters, K.E. 2016. Beneficiation of the Nechalacho rare earth deposit: Flotation response using benzohydroxamic acid. Minerals Engineering, vol. 99. pp. 158-169. [ Links ]
Joseph-Soly, S., Quast, K., and Connor, J.N. 2015. Effects of Eh and pH on the oleate flotation of iron oxides. Minerals Engineering, vol. 83. pp. 97-104. [ Links ]
Lin, R., Howard, B.H., Roth, E.A., Bank, T.L., Granite, E.J., and Soong, Y. 2017 Enrichment of rare earth elements from coal and coal by-products by physical separations. Fuel, vol. 200. pp. 506-520. [ Links ]
Liu, P., Huang, R., and Tang, Y. 2019. Comprehensive understandings of rare earth element (REE) speciation in coal fly ashes and implication for REE extractability. Environmental Science & Technology, vol. 53, no. 9. pp. 5369-5377. [ Links ]
Leistner, T., Peuker, U.A., and Rudolph, M. 2017. How gangue particle size can affect the recovery of ultrafine and fine particles during froth flotation. Minerals Engineering, vol. 109. pp. 1-9. [ Links ]
Ketris M.P. and Yudovich, Y.A.E. 2009. Estimation of clarkes for carbonaceous britholites: World average for trace element contents in black shales and coals. International Journal of Coal Geology, vol. 78. pp. 135-148. [ Links ]
Mohr, S.H., and Evans, G.M. 2009. Forecasting coal production until 2100. Fuel, vol. 88. pp. 2059-2067. [ Links ]
Pan, J., Nie, T., Hassas, B.V., Rezaee, M., Wen, Z., and Zhou, C. 2020. Recovery of rare earth elements from coal fly ash by integrated physical separation and acid leaching. Chemosphere, vol. 248, p. 126112. [ Links ]
Quast, K. 2000. A review of hematite flotation using 12-carbon chain collectors, Minerals Engineering, vol. 13, no. 13. pp. 1361-1376. [ Links ]
Quast, K. 2006. Flotation of hematite using C6-C18 saturated fatty acids. Minerals Engineering, vol. 19, no. 6-8. pp. 582-597. [ Links ]
Perämäki, S.E., Tiihonen, A.J., and Väisänen, A.O. 2019. Occurrence and recovery potential of rare earth elements in Finnish peat and biomass combustion fly ash. Journal of Geochemical Exploration, vol. 201. pp. 71-78. [ Links ]
Rutledge, D. 2011. Estimating long-term world coal production with logit and probit transforms. International Journal of Coal Geology, vol. 85. pp. 23-33. [ Links ]
Sahoo, P.K., Kim, K., Powell, M.A., and Equeenuddin, S.M. 2016. Recovery of metals and other beneficial products from coal fly ash: A sustainable approach for fly ash management. International Journal of Coal Science & Technology, vol. 3, no. 3. pp. 267-283. [ Links ]
Satur, J.V., Calabia, B.P., Hoshino, M., Morita, S., Seo, Y., Kon, Y., Takagi, T., Watanabe, Y., Mutele, L., and Foya, S. 2016. Flotation of rare earth minerals from silicate- hematite ore using tall oil fatty acid collector. Minerals Engineering, vol. 89. pp. 52-62. [ Links ]
Seredin, V.V. 2010. A new method for primary evaluation of the outlook for rare earth element ores. Geology of Ore Deposits, vol. 52. pp. 428-433 [ Links ]
Seredin, V.V. and Dai, S. 2012. Coal deposits as potential alternative sources for lanthanides and yttrium. International Journal of Coal Geology, vol. 94. pp. 67-93. [ Links ]
Seredin, V.V., Dai, S., Sun, Y., and Chekryzhov, I.Y. 2013. Coal deposits as promising sources of rare metals for alternative power and energy-efficient technologies. Applied Geochemistry, vol. 31. pp. 1-11. [ Links ]
Sis, H., Ozbayoglu, G., and Sarikaya, M. 2004. Utilization of fine coal tailings by flotation using ionic reagents. Energy Sources, vol. 26, no. 10. pp. 941-949. [ Links ]
Smythe, D.M., Lombard, A., and Coetzee, L.L. 2013. Rare earth element deportment studies utilising QEMSCAN technology. Minerals Engineering, vol. 52. pp. 52-61. [ Links ]
Subbarao, E.C. 1980. Science and Technology of Rare Earth Materials. Academic Press, New York. [ Links ]
Sun, Y., Lin, M., Qin, P., Zhao, C., and Jin, K. 2007. Geochemistry of the barkinite liptobiolith (Late Permian) from the Jinshan mine, Anhui Province, China. Environmental Geochemistry and Health, vol. 29, no. 1. pp. 33-44. US Geological Survey. 2021. Rare earths. https://pubs.usgs.gov/periodicals/mcs2021/mcs2021.pdf [accessed 8 May 2021]. [ Links ]
Van Rythoven, A.D., Pfaff, K., and Clark, J.G. 2020. Use of QEMSCAN® to characterize oxidized REE ore from the Bear Lodge carbonatite, Wyoming, USA. Ore and Energy Resource Geology, vol. 2. 100005. [ Links ]
Wdowin, M., Franus, M., Panek, R., Badura, L., and Franus, W. 2014. The conversion technology of fly ash into zeolites. Clean Technologies and Environmental Policy, vol. 16, no. 6. pp. 1217-1223. [ Links ]
World Energy Council. 2007. 2007 survey of energy resources. https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.478.9340&rep=rep1&type=pdf [Accessed 10 September 2021]. [ Links ]
Xiong, W., Deng, J., Chen, B., Deng, S., and Wei, D. 2018. Flotation-magnetic separation for the beneficiation of rare earth ores. Minerals Engineering, vol. 119. pp. 49-56. [ Links ]
Yang, X., Satur, J.V., Sanematsu, K., Laukkanen, J., and Saastamoinen, T. 2015. Beneficiation studies of a complex REE ore. Minerals Engineering, vol. 71. pp. 55-64. [ Links ]
Zhang, Y. and Anderson, C. 2017. A comparison of sodium silicate and ammonium lignosulfonate effects on xenotime and selected gangue mineral microflotation. Minerals Engineering, vol. 100. pp. 1-8. [ Links ]
Zhang, W., Rezaee, M., Bhagavatula, A., Li, Y., Groppo, J., and Honaker, R. 2015. A review of the occurrence and promising recovery methods of rare earth elements from coal and coal by-products. International Journal of Coal Preparation and Utilization, vol. 35, no. 6. pp. 295-330. [ Links ]
 Correspondence:
Correspondence:
G.B. Abaka-Wood
Email: george.abaka-wood@unisa.edu.au
Received: 15 Jun. 2021
Revised: 1 Sep. 2021
Accepted: 3 Sep. 2021
Published: January 2022














