Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.121 n.11 Johannesburg Nov. 2021
http://dx.doi.org/10.17159/2411-9717/1608/2021
PROFESSIONAL TECHNICAL AND SCIENTIFIC PAPERS
http://dx.doi.org/10.17159/2411-9717/1608/2021
Behaviour of Cu, Fe, Ni, and PGMs during leaching of Ni-Fe-Cu-S converter matte
A.P. Van Wyk; G. Akdogan; S.M. Bradshaw
Department of Process Engineering, Stellenbosch University, South Africa. ORCID: A.P. Van Wyk: https://orcid.org/0000-0003-2006-8355; G. Akdogan: https://orcid.org/0000-0003-1780-4075; S.M. Bradshaw: https://orcid.org/0000-0001-6323-8137
SYNOPSIS
In the mineral processing industry, Ni-Cu-Fe-S converter matte is leached to remove base metals from a concentrate containing platinum group metals (PGMs). We investigated the atmospheric leaching process to develop a better understanding of the leaching behaviour of the base metals (Cu, Fe, and Ni) and the PGMs, in particular Ru, Ir, and Rh with respect to key process variables, acid concentration, and Cu concentration under oxidative and non-oxidative conditions.
With oxidative leaching, a higher initial acid concentration resulted in higher Cu, Ni, and Fe extractions, as well as faster leaching reaction rates. A higher initial acid concentration also resulted in faster precipitation of Cu, Ru, and Ir under oxidizing conditions.
Under non-oxidative conditions, Ni and Fe extraction was much slower, and the effect of initial acid concentration on Cu precipitation was less pronounced. The initial Cu concentration had a slightly smaller effect on the leaching behaviour of Fe and Ni, as well as precipitation of Ru, Ir and Rh. Moreover, a higher initial Cu concentration suppressed both Ni and Fe leaching and had a slight inhibiting effect on the precipitation behaviour of Cu, Ru, and Ir.
Keywords: platinum group metals, converter matte, leaching, precipitation.
Introduction
The largest platinum group metal (PGM) deposit in the world, located in the Bushveld Complex in South Africa, holds half of the world's platinum group elements (PGEs) and chromium. Smelting and processing of the PGM concentrates in this region is carried out by four major companies, namely Anglo American Platinum, Impala Platinum, Sibanye-Stillwater, and Northam Platinum (Jones, 2005).
Due to their unique physical and chemical properties such as good corrosion and oxidation resistance, high melting temperatures, good conductivity, and electronic and catalytic properties, the PGMs are used for their superior performance in certain industries and markets. These applications include (i) platinum or rhodium as autocatalysts to reduce greenhouse gas emissions produced by the combustion of fossil fuels, (ii) platinum and palladium jewellery, (iii) catalysts in the chemical sector, (iv) electrical equipment and electronic devices, (v) platinum used in the manufacturing of glass, and the (vi) production of drugs in the medical sector (Creamer, 2006). Moreover, platinum-catalysed hydrogen-powered fuel cells for electric vehicles (EVs) offer the most natural solution for emission-free vehicles, discharging only water and requiring negligible changes to current driving and refuelling habits (Theron-Ord, 2017).
The Sibanye-Stillwater (formerly Western Platinum) process involves milling, flotation, and smelting followed by Peirce-Smith converting to produce a Ni-Cu-Fe-S converter matte containing PGMs. The base metals and sulphur contained in the converter matte are removed through a multi-stage leaching process. The first stage leach, also known as the atmospheric leach, serves to remove Ni from the matte, while at the same time precipitating Cu and the PGMs from the leaching solution. Due to very little insight into the mechanisms of the atmospheric leaching process, studies conducted by Hofirek and Kerfoot (1992), Lamya and Lorenzen (2006), van Schalkwyk et al. (2011) and Snyders et al. (2018) were aimed at investigating the chemistry and mechanism of the process, as well as determining the effects of Fe endpoints, initial acid and Cu concentrations of the spent electrolyte solutions, and oxidative/non-oxidative conditions on the leaching behaviour of Ni-Cu-Fe-S converter mattes.
The purpose of atmospheric leaching is to firstly leach the Ni from the matte, while at the same time rejecting the Cu from the solution, through metathesis reactions. Leaching takes place in five continuously stirred tank reactors (CSTRs) in series, with oxygen fed to the first three tanks only. The acid supplied to the first tank is spent electrolyte solution recycled from the copper electrowinning tankhouse and which contains 20-30 g/L Cu and 80-90 g/L sulphuric acid. Sulphuric acid make-up is only added during upset conditions on the plant. A Ni crystallizer bleed stream is added to the first stage leach in order to recovery any Cu that may have slipped through to the Ni crystallizer unit. The operating temperature of the first stage circuit is 85°C (Crundwell et al., 2011). The metathesis reactions serve to exchange Cu from the solution with Ni from the alloy and nickel sulphide phases in the matte. The PGMs present in the matte, along with the Cu, report to the leach residue (van Schalkwyk et al., 2011.)
In this project we investigated the first stage atmospheric leaching process of the converter matte, using base metal refinery (BMR) spent electrolyte from Sibanye-Stillwater, in order to develop a better understanding of the effect of the initial acid and Cu concentrations on the leaching behaviour of the base metals, in particular Fe and Ni, as well as Rh, Ir, and Ru, under oxidative and non-oxidative conditions. A better understanding of the process will assist in improving the process efficiency.
Experimental
Equipment
Atmospheric leaching tests were carried out in a 6 L stainless steel batch reactor with an active volume of 4 L. The reactor setup was geometrically scaled down from the atmospheric leaching reactors used at the Sibanye-Stillwater BMR. The temperature during the experiments was kept constant at 85°C by using a PID controller. Cooling coils which provided a constant flow of cooling water were mounted inside the vessel to remove the excess heat generated by the exothermic leaching reactions. To monitor the temperature, a thermocouple was placed inside the reactor. The vessel was fitted with a liquid sampling port to draw samples at set intervals. To ensure perfect mixing of the reactor contents a stirrer fitted with two agitation blades was used, and the reactor was fitted with four baffles to promote turbulent mixing. During oxidative leaching tests, oxygen was supplied from an oxygen cylinder and delivered to the reactor contents through a stainless-steel sparger. The flow rate of oxygen was regulated by a manual flow control valve and measured by a flow meter.
The sparger was designed in an 'L' shape, with small equally-spaced holes in the bottom to disperse small oxygen bubbles to the reactor contents. The oxygen entered the reactor vessel through a stainless steel tube with holes at the bottom to ensure that sufficient oxygen bubbles were provided to the reactor contents. A Liebig condenser was fitted to the reactor set-up to reduce the evaporation rate of the contents. Along with the Liebig condenser, a rubber O-ring was placed between the reactor vessel and reactor lid to ensure that vapours formed did not escape.
Materials
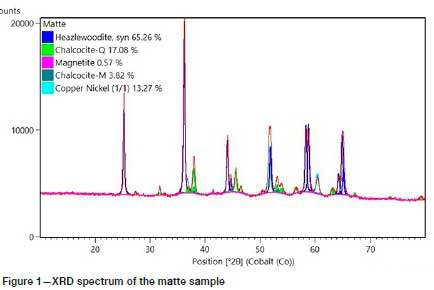
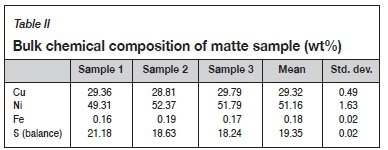
Granulated converter matte provided by Sibanye-Stillwater was analysed by quantitative XRD analysis to determine the mineral phases present. The results are shown in Table I and Figure 1. The bulk chemical composition is given in Table II.

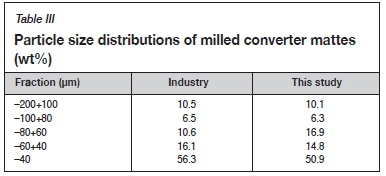
The converter matte was milled using a ball mill to a particle size distribution similar to that used by Sibanye-Stillwater so as to be in line with industrial practice. The size distributions are compared in Table III.
Spent electrolyte solution, which is the return anolyte from the copper electrowinning cells, was received from the plant, and analysed by ICP-OES for base metals and PGMs. The concentrations are shown in Table IV.
The acid level was determined by precipitating out all the metals with a Na2CO3/NaHCO3 buffer and analysing the solution using high-performance liquid chromatography to determine the sulphate concentration. Four leaching solutions were prepared by diluting the concentrated spent electrolyte solution with an equal volume of distilled water. These four leaching solutions were used to investigate the effects of initial acid concentration or initial Cu concentration on the leaching kinetics of the base metals (Fe and Ni) and PGMs. For tests where a higher acid or Cu concentration was required, the desired concentrations were obtained by adding 98% sulphuric acid or copper sulphate pentahydrate crystals.
Experimental methods
The clean reactor vessel was weighed, 4 L of leaching solution added, and the vessel was weighed again to obtain the weight of the solution. The stirrer was started and set to a speed of 1100 r/min; simultaneously, the heating element was attached to the reactor vessel and the temperature on the PID controller set to 85°C. For oxidative leaching tests, the oxygen flow was turned on and the manual flow control valve set to a flow rate of 0.2 L/min. While the reactor contents were being heated to the set-point value of 85°C, the pH and Eh probes were calibrated using pH standards of 1.69 and 4.0. The Eh probe was a platinum pin double junction Ag/AgCl electrode calibrated with ORP Quinhydrone solutions. Eh-pH stability diagrams were also depicted in Ag/AgCl (mV) used in simulations. The milled converter matte was added to the reactor once the leach solution reached the set temperature. Liquid samples were continually taken throughout the leaching tests at the 15, 30, 60, 120, 150, and 180 minute marks using syringes and 0.45 μm syringe filters.
The four leaching solutions prepared were used to investigate the effect of the initial acid concentration and initial Cu concentration on leaching behaviour, under oxidative and non-oxidative conditions. Table V summarizes the initial Cu and acid concentrations of each of the four leaching solutions used. The experiments carried out are summarized in Table VI. Three repeat experiments were conducted for each set of conditions in order to establish confidence in the results produced. The standard deviations were less than 10.
The oxygen flow rate used in this study was geometrically scaled down from the flow rate used at the BMR. Since the active volume of the laboratory-scale reactor was 4 L, the required oxygen flow rate for the oxidative leaching tests was 0.2 L/min. Van Schalkwyk et al. (2011) investigated three different solids to liquid ratios, namely 80 g/L, 150 g/L, and 540 g/L. It was found that at a solid to liquid ratio of 80 g/L complete Cu removal was not possible and hence in this study a solids to liquid ratio of 150 g/L was used to investigate the possibility of maximum Cu and PGE precipitation with reasonable Ni extraction using solutions with different chemical compositions.
Sample analysis
Liquid samples taken during batch leaching tests were analysed immediately for pH and Eh to obtain data that was as close as possible to the conditions within the reactor. The instruments used were a HI 9321 microprocessor pH meter, capable of measuring pH at elevated temperatures, and a Eutech pH700 instrument with a platinum pin double junction Ag/AgCl electrode for Eh. Dissolved Cu, Ni, and Fe concentrations in the liquid samples from the leaching experiments, as well as in the original spent electrolyte solution, were analysed by means of atomic absorption spectroscopy (AAS) (Varian SpectrAA-250 Plus).
The dissolved PGE (Ir, Rh and Ru) concentrations in the liquid samples obtained from leaching experiments and in the original spent electrolyte solution were determined by ICP-MS. Pt and Pd were present at low concentration levels and were not considered in the study. For solid sample analysis, matte samples were digested in aqua regia for 24 hours to dissolve all the metals, and the resulting solutions analysed for Cu, Ni, and Fe by AAS.
Results and discussion
Previous research on the leaching behaviour of Ni-Cu-Fe-S mattes in sulphuric acid was conducted by Lamya and Lorenzen (2006), Fügleberg et al. (1995), Hofirek and Kerfoot (1992), Symens et al. (1979), and Llanos, Queneau, and Rickard (1974). Although these authors established the major features of the process, the results pertaining to the effects of different initial acid and Cu concentrations are sometimes contradictory or limited.
The Cu concentration is regarded as a leading indicator of PGE behaviour in plant operations, but this relationship has not been conclusively established. Van Schalkwyk, Eksteen, and Akdogan (2013) showed that the behaviour of Cu during oxidative leaching may possibly be used as an indicator of whether PGEs will precipitate, but during non-oxidative tests Cu precipitation was found to be a poor indicator. Batch oxidative and non-oxidative experiments similar to those by van Schalkwyk, Eksteen, and Akdogan (2013) were therefore performed with the main aim of investigating the link between PGE and Cu behaviour, as well as establishing the conditions that promote PGE precipitation in the first-stage atmospheric leach. Here, we report the results of laboratory-scale experiments on the leaching of converter matte at atmospheric conditions to elucidate the effects of oxygen, Cu, and acid concentration on the precipitation behaviour of Cu, PGEs, and Ni extraction.
Effect of oxygen, copper, and acid concentration on PGE behaviour
The effect of oxygen is shown in Figure 2, which indicates that oxidative conditions are conducive to the precipitation of Cu, Ru, Ir and Rh. This is similar to the findings of van Schalkwyk, Eksteen, and Akdogan (2013) with respect to low-Fe mattes. Pt and Pd concentrations in the spent liquor were very low, and although some Pt and Pd precipitated out of solution, the amounts were negligible compared to those for other PGEs and these elements are therefore not included in further discussions.
Figure 3 compares Cu and Ru precipitation out of solution under oxidative and non-oxidative conditions. As seen, both Cu and PGE precipitation exhibit faster kinetics at low Cu and high acid concentrations under oxidizing conditions.
Van Schalkwyk, Eksteen, and Akdogan (2013) argued that Rh, Ru, and Ir are cemented, similarly to Cu. Dorfling (2012) agreed that the precipitation of Rh, Ir, and Ru proceeds primarily via reactions similar to the cementation and metathesis reactions of Cu precipitates (Equation [1]).
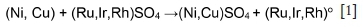
However, various studies propose different formulations for the precipitate, including Ru(OH)4, RuO2-mH2O and RuO(OH)2-H2O (Baes and Mesmer, 1976).
Figures 4 and 5 depict Eh-pH measurements, monitored for a duration of 180 minutes, from each group of tests under different conditions, superimposed onto stability diagrams for Cu and Ru, Ir, and Rh.
As can be seen from Figure 4, Cu precipitated as Cu2S in both oxidizing and non-oxidizing conditions. It is also observed that a low Cu concentration provided slightly better conditions for precipitation. Non-oxidizing conditions obviously shifted the ORP readings towards more reducing conditions closer to the Cu predominance field.
In Figure 5 one can clearly see that oxidizing conditions lead to the production of RuO2 rather than Ru metal, perhaps closer to the postulation by Baes and Mesmer (1976). This is in contrast to non-oxidizing conditions, in which the precipitation product was Ru metal due to the prevailing reducing conditions. Figure 5 also indicates that under all conditions, Ir and Rh should precipitate as the metals, which is in line with Dorfling's proposal (2012) summarized by Equation [1].
Effect of oxygen, copper, and acid concentration on Ni and Fe behaviour
It is generally accepted that in the presence of oxygen, leaching of the nickel sulphide phase proceeds according to Equation [2] (Llanos, Queneau, and Rickard, 1974; Plasket and Romanchuk, 1978; Hofirek and Kerfoot, 1992; Fugleberg et al., 1995).

In the absence of oxygen, dissolution of the Ni from the sulphide phase takes place according to Equation [3] (Lamya, 2007);

Chalcocite (Cu2S) leaching under atmospheric oxidative conditions proceeds by Equation [4] (Plasket and Romanchuk, 1978):

Being more noble than Ni, Cu from solution can exchange with Ni in the nickel sulphide matrix by metathesis (Equations [5], [6]) or with Ni from the alloy by cementation (Equation [7].

Although present in relatively small concentrations, Fe has been reported to play an important role as a catalyst and oxygen carrier to enhance the rates of the leaching reactions (Burkin, 2001; Mulak, 1987).
Figures 6 and 7 illustrate the leaching behaviour of Fe and Ni in oxidative and non-oxidative conditions in the presence of Cu, and PGEs. It is clear from these figures that both Fe and Ni undergo greater dissolution from the matrix in the presence of high acid concentrations in an oxidative environment than in the absence of oxygen. Fe leaching proceeds much faster than Ni under both oxidative and non-oxidative conditions in low Cu-high acid conditions. However, the increase in Fe extraction and Cu precipitation coincides with diminishing Ru concentration in solution towards 20 mg/L.
(Hofirek and Kerfoot, 1992). At a low pH, ferrous ions are oxidized to ferric according to Equation [8].
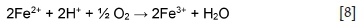
Ferric ions can act as an oxidant in the leaching of heazlewoodite, which leads to ferrous ions being continuously regenerated (Equation [9]).

The pH and Eh diagrams for Ni and Fe under both the oxidative and non-oxidative tests are shown on the Pourbaix diagrams in Figures 8 and 9. Ni and Fe are dissolved as Ni2+ and Fe2+, while Cu is precipitated as Cu2S (refer to Figure 4).
Conclusions
The relationship between Cu, Ni, Fe, and PGE behaviour was investigated through a series of batch leaching tests replicating the first-stage atmospheric leach in a base metal refinery. A low-Fe converter matte consisting mainly of heazlewoodite, chalcocite, Cu-Ni alloy, and minor magnetite was leached in a batch reactor under both oxidative and non-oxidative conditions at various acid and Cu concentrations.
The results revealed a higher degree of precipitation for Cu, Ru, Ir, and Rh under oxidative conditions, which agrees with the findings of van Schalkwyk, Eksteen, and Akdogan (2013) and Snyders et al. (2018).The precipitation behaviour of Ru and Ir closely followed that of Cu. A high acid concentration had a positive effect on Cu, Ru and Ir precipitation, as well as Fe and Ni dissolution.
For oxidative leaching experiments it was found that a higher initial acid concentration resulted in higher Ni and Fe extractions, as well as faster leaching rates. A high initial acid concentration also resulted in faster precipitation of Cu, Ru, and Ir.
The effect of initial acid concentration on the leaching behaviour of Ni and Fe, as well as on the extent of Cu precipitation, was much less marked under non-oxidative conditions. A high initial Cu concentration had a negative effect on the leaching of Ni and Fe, as well as the precipitation of Cu and PGEs, under non-oxidative conditions.
Acknowledgements
The support by Sibanye-Stillwater (formerly Western Platinum Ltd) is gratefully acknowledged.
References
Baes, C.F. and Mesmer, R.E. 1976. The Hydrolysis of Cations. Wiley, New York. [ Links ]
Burkin, A.R. 2001. Chemical Hydrometallurgy: Theory and Principles. Imperial College Press, London. 414 pp. [ Links ]
CoETZEE, R., Dorfling, C., and Bradshaw, S.M. 2018. Precipitation of Ru, Rh and Ir with iron ions from synthetic nickel sulphate leach solutions. Hydrometallurgy, vol. 175. pp. 79-92. [ Links ]
Creamer, M. 2006. The uses of platinum-group metals. Mining Weekly. http://www.miningweekly.com/article/the-uses-of-platinumgroup-metals-2006-11-10 [ Links ]
Crundwell, F.K., Moats, M.S., Ramachandran, v., Robinson, T.G., and Davenport, W.G. 2011. Overview of extraction of platinum-group metals. Extractive Metallurgy of Nickel, Cobalt and Platinum-Group Metals. Elsevier. pp. 411-413. [ Links ]
Dorfling, C. 2012. Characterisation and dynamic modelling of the behaviour of platinum group metals in high pressure sulphuric acid/oxygen leaching systems. PhD thesis, Stellenbosch University, South Africa. [ Links ]
Fügleberg, S., Hultholm, S.E., Rosenback, I., and Holohan, T. 1995. Development of the Hartley Platinum leaching process. Hydrometallurgy, vol. 39. pp. 1-10. [ Links ]
Hofirek, z. and Kerfoot, D. 1992. The chemistry of the nickel-copper matte leach and its application to process control and optimization. Hydrometallurgy, vol. 29. pp. 357-381. [ Links ]
Jones, R. 2005. An overview of Southern African PGM Smelting. Nickel and Cobalt 2005: Challenges in Extraction and Production. Proceedings of the 44th Annual Conference of Metallurgists, Calgary, Alberta, Canada, 21-24 August 2005. Canadian Institute of Mining, Metallurgy and Petroleum, Montreal. pp. 147-178. [ Links ]
Lamya, R.M. 2007. A fundamental evaluation of the atmospheric pre-leaching section of the nickel-copper matte treatment process, PhD thesis, Stellenbosch University, South Africa. [ Links ]
Lamya, R. and Lorenzen, I. 2006. Atmospheric acid leaching of nickel-copper matte from Impala Platinum Refineries. Journal of the Southern African Institute of Mining and Metallurgy, vol. 106. pp. 385-396. [ Links ]
Llanos, z.R., Queneau, P.B., and Rickard, R.S. 1974. Atmospheric leaching of matte at the Port Nickel Refinery. CIM Bulletin, vol. 67. pp. 74-81. [ Links ]
Mulak, W. 1987. The catalytic action of cupric and ferric ions in nitric acid leaching of Ni3S2. Hydrometallurgy, vol. 17. pp. 201-214. [ Links ]
Plasket, R.P. and Romanchuk, S. 1978. Recovery of nickel and copper from high-grade matte at Impala Platinum by the Sherrit process. Hydrometallurgy, vol. 3. pp. 135-151. [ Links ]
Snyders, C.A., Akdogan, G., Thompson, G., Bradshaw, S.M., and van Wyk, A.P. 2018. Investigating the behaviour of PGEs during first-stage leaching of a Ni-Fe-Cu-S converter matte. Journal of the Southern African Institute of Mining and Metallurgy, vol. 118. pp. 353-360. [ Links ]
Symens, R.D., Queneau, P.B., Chou, E.C., and Clark, F.F. 1979. Leaching of iron-containing copper-nickel matte at atmospheric pressure. Canadian Journal of Metallurgy and Materials Science, vol. 18. pp. 145-153. [ Links ]
Theronord, A. 2017. Hydrogen to drive the energy revolution. https://www.esi-africa.com/regional-news/international/hydrogen-drive-energy-revolution/ [ Links ]
Van Schalkwyk, R.F., Eksteen, J.J., Petersen, J., Thyse, E.L., and Akdogan, G. 2011. An experimental evaluation of the leaching kinetics of PGM-containing Ni-Cu-Fe-S Peirce Smith converter matte, under atmospheric leach conditions. Minerals Engineering, vol. 24. pp. 524-534. [ Links ]
Van Schalkwyk, R.F., Eksteen, J.J., and Akdogan, G. 2013. Leaching of Ni-Cu-Fe-S converter matte at varying iron endpoints; Msineralogical changes and behaviour of Ir, Rh and Ru. Hydrometallurgy, vol. 136. pp. 36-45. [ Links ]
 Correspondence:
Correspondence:
A.P. Van Wyk
Email: apvanwyk@sun.ac.za
Received: 2 Feb. 2021
Revised: 1 Apr. 2021
Accepted: 13 Oct. 2021
Published: November 2021














