Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.121 n.9 Johannesburg Sep. 2021
http://dx.doi.org/10.17159/2411-9717/1510/2021
PAPERS OF GENERAL INTEREST
Investigation into the dephosphorization of ferromanganese alloys for the production of advanced high-strength steel
M.P. MaphuthaI, II; J.D. SteenkampI; P.C. PistoriusII, III
IMintek, Randburg, South Africa. J.D. Steenkamp: https://orcid.org/0000-0003-0635-7927
IIUniversity of Pretoria, South Africa. P.C. Pistorius: https://orcid.org/0000-0002-2966-1879
IIICarnegie Mellon University, Pittsburgh PA, United States of America
SYNOPSIS
Advanced high-strength steels (AHSS) are sophisticated materials being developed by the steel industry to mitigate challenges related to the performance of motor vehicles. To meet the requirements of AHSS, the ferromanganese alloys (FeMn) utilized in the production of the steel are required to contain acceptable levels of unwanted impurities, i.e. P, S, N, H, and C. The focus of the current study was to investigate dephosphorization of ferromanganese to produce a low-P alloy that could be effectively utilized in the production of AHSS. The study involved conducting laboratory-scale testwork to study the efficiency of CaO-based slag systems to dephosphorize FeMn alloys. The addition of Na2O, CaF2, and BaO to MnO-CaO-SiO2 slag was considered. The test work was carried out in a 25 kW induction furnace at temperatures of 1350°C, 1400°C, and 1450°C. The P partition coefficient (Lp) remained small at <1, which is an indication that dephosphorization had not been achieved. The baseline slag, comprising 40%CaO-40%SiO2-20%MnO, reported higher Lpvalues. Addition of Na2O and CaF2 did not show any further benefit. Substituting half of the CaO by BaO, resulted in similar Lpvalues to those of the baseline slag under conditions of 1350°C and 1450°C at 30 minutes. In summary, based on the Lpvalues obtained, the conditions investigated with the CaO-based slags appeared to have been unfavourable for dephosphorization of FeMn alloys, as most of this impurity element remained in the alloy.
Keywords: ferromanganese, dephosphorization, advanced high-strength steel.
Introduction
In the automotive industry the drive towards lightweight, high-strength steel grades to mitigate the challenges around the escalating energy crisis and environmental problems is a priority. It has been estimated that a 10% weight reduction in automobiles would reduce fuel consumption by between 3% and 7% (Demiri, 2013). This has led to the development of advanced high-strength steels (AHSS) (Baluch, Udin, and Abdullah, 2014).
AHSS steels are a group of special steel grades which offer excellent strength, allowing the use of thinner gauges to reduce the weight of vehicles. As the manganese (Mn) content in AHSS can be up to 25%, these steels could potentially be a significant market for manganese ferroalloys (Safarian and Kolbeinsen, 2013). The challenge lies in the maximum allowable phosphorus (P) content of <0.01% in the steel grades (Bernhard et al., 2019).
P has a detrimental effect on the strength, brittleness, ductility, and fracture toughness of steel. As a result, the quality of the steel could become compromised, especially in the case of AHSS where the Mn content can be up to 25%. As a consequence, FeMn alloys utilized in the production of AHSS are required to contain low P levels (You, Lee, and Pak, 1999; Chaudhary and Roy, 2001).
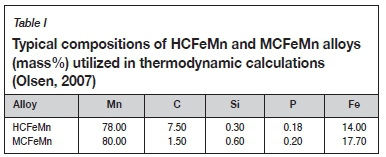
During carbonaceous reduction of Mn ores to produce FeMn alloys, almost all the P is reduced to the alloy phase. The P originates mainly from the Mn ores, and is intimately associated with iron (Fe) and Mn, making it difficult to remove by mineral processing routes (Chaudhary and Goel, 2007). South African Mn ores are, however, generally low in P compared to ores from other countries such as China. The P content in the typical South African ores, namely Gloria ore and Nchwaning ores, varies between 0.02% and 0.05% (Visser et al., 2013; Olsen et al., 2007). When using low-P ore, the P present in the slag and the alloy originates mainly from the carbonaceous reductants used (Olsen et al., 2007). To produce FeMn alloys containing P at levels acceptable to AHSS producers, methods have to be developed to lower the P levels in the alloy.
Background
Mechanisms of dephosphorization
Dephosphorization of the molten alloy under oxidizing conditions can be described by the following ionic reaction (Chaudhary Minj, and Goel, 2007; Nasaralla, Fruehan, and Min, 1991; Simeonov and Sano, 1985):
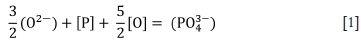
where:
[X]: species dissolved in the alloy
(Y): species dissolved in the slag.
The equilibrium constant can be expressed as follows:

Based on the above expression, it can be deduced that the removal of P from the alloy can be aided by the following (Chaudary and Goel, 1994):
> Higher oxygen activity in the alloy
> High activity of basic oxide (ao2-) in the slag
> Low activity coefficient of phosphate (ypo43-) in the slag
> High K values, which can be achieved at low temperatures since the reaction is of exothermic nature.
Slag basicity is associated with the degree of polarization of oxygen in the slag. Highly basic slag contains more free oxygen ions (O2-) and less bridging and terminal oxygen. Slag basicity can therefore be defined by the activity of oxygen ions in slags (Liu et al., 1998). As indicated by Equation [1], high concentrations of O2-, and therefore basic slags, are efficient for dephosphorization (Wagner, 1975).
Dephosphorization of FeMn can also be carried out with basic slag under reducing conditions to form a phosphide species. The reaction is shown below (Chaudhary and Goel, 2007):
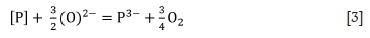
Based on Equation [3], it can be deduced that the removal of P from the alloy under these conditions can be aided by a high activity of basic oxide (a02-) in the slag. A relatively lower oxygen partial pressure is required for reducing conditions, and CO gas is typically used. Metallics such as Ca, typically added as calcium silicide or CaC2, would dissolve into the metal and react with P to form phosphide. The reducing reaction occurs through the reaction between CaO in the flux and Si in the melt (for SiMn alloy), and also through the transfer of Ca from the calcium silicide introduced into the melt. An example is shown below (Karbowniczek et al., 2014):
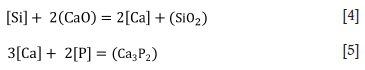
The reaction product from Equation [5] is phosphide, which in the presence of atmospheric moisture produces toxic phosphene. Dephosphorization of FeMn alloy under reducing conditions is therefore not desirable.
P removal in vapour form, as phosphine gas, by the reaction of P with hydrogen dissolved in the metal can occur (Chaudary and Goel, 1994):

The reaction product (phosphine) would not be environmentally tolerable when released into the atmosphere (Chaudary and Goel, 1994). Based on Equation [6], it can be deduced that the removal of P from the alloy under these conditions can be aided by higher hydrogen activity in the alloy. Dephosphorization under gaseous conditions may also result in loss of Mn, which has a higher vapour pressure than P. Application of the technique is deemed undesirable for dephosphorization of FeMn alloys (Chaudary and Goel, 1994).
Considering the abovementioned reaction mechanisms, dephosphorization of liquid FeMn alloy by basic slags for selective removal of P over Mn under oxidizing conditions is deemed the most viable route (Chaudary and Goel, 1994).
The study aimed at investigating the use of CaO-bearing, synthetic slag systems - similar to the systems applied in dephosphorisation of iron - for the dephosphorization of FeMn alloys commercially produced in South Africa.
Method
The study involved conducting preliminary thermochemical FactSage calculations followed by experimental laboratory tests to investigate the dephosphorization of South African FeMn alloys by different synthetic CaO-based slag systems, similar to the systems applied in dephosphorization of iron.
FactSage calculations
FactSage™ version 7.3 was utilized. The package is a fully integrated thermodynamic database computing system developed in 1976 by a joint research project between McGill University and École Polytechnique de Montreal (Canada). FactSage provides access to several databases, including pure substances, oxides, solutions, and alloy databases. The tool is widely applied to study thermochemistry for different chemical and metallurgical processes (Bale et al., 2016).
The calculations were conducted in the Equilib module. In all calculations, the pure substance database (FactPS), which is suitable for pure solids, liquids, and gases, was utilized. As solution databases, FToxid was utilized to describe the slag system and FSstel the alloy system. FToxid includes a wide range of components such as Al2O3, As2O3, B2O3, CaO, CoO, CrO, Cr2O3, Cu2O, FeO, Fe2O3, GeO2, K2O, MgO, MnO, Na2O, NiO, PbO, SiO2, SnO, TiO2, Ti2O3, ZnO, and ZrO2. Furthermore, FToxid contains many solid solution databases, which include those listed below (Bale et al., 2009):
> Wollastonite: CaSiO3 (+FeSiO3, MgSiO3, MnSiO3)
> Olivine: (Ca2+, CO2+, Fe2+, Mg2+, Mn2+, Ni2+, Zn2+)2SiO4
> a'-Ca2SiO4: a'-Ca2SiO4 (+Fe2SiO4, Mg2SiO4, Mn2SiO4)
> Monoxide: CaO-MgO-MnO-CoO-NiO-FeO (+Fe2O3-Al2O3-ZnO-Cr2O3)
> Corundum: Al2O3-Cr2O3-Fe2O3
FSstel is a steel database covering a wide range of compositions related to steelmaking processes (Bale et al., 2009).
Although thermodynamic data exists for HCFeMn, it does not contain equilibrium data on P (Tang and Olsen, 2006). FSstel has been proven in the past to represent equilibrium conditions in SiMn production, and as it contained data on P, was selected for the calculations presented here (Steenkamp, Pistorius, and Tangstad, 2015).
As pure species, all liquids and solids were selected except the Mn phosphide phases. As pure ideal gas species, only O2 and Ar were selected. For solution systems, all solution phases were selected, except in cases where more than one option existed. Solution phases that were suppressed were SlagD Slag? MeOB, MeP_? cPyrB, and cPyr?. SlagA model was selected over Slag? model because SlagA provides much more reasonable LPvalues for steelmaking than does Slag?, which overestimates P removal. Since MeOB, does not include BaO as a solute, this solution phase was not selected. MeP? cPyrB, and cPyr? were not selected because more than one option existed.
The calculations were done to investigate the phosphorus partition coefficient (LP) between slag and alloy. The expression for LPis

The typical HCFeMn and MCFeMn compositions obtained from the literature (see Table I) were utilized for the dephosphorization thermochemical calculations.
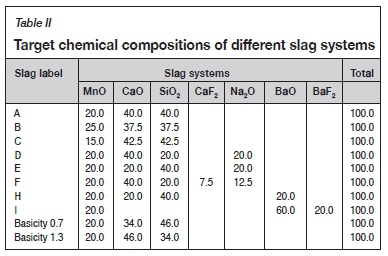
The slag systems in Table II were utilized. To determine the LPfor the different slag-alloy systems, 100 g of the alloy was reacted with 10 g of slag, i.e. the slag-to-alloy mass ratio was maintained constant at 0.1 (but equilibrium is essentially independent of the relative masses, other than the effect of equilibration of Mn and Si between metal and slag). A small amount of Ar (0.01 g) was also added to allow for reactions to converge (Steenkamp, Pistorius, and Tangstad, 2015). The temperature range considered was 1300-1700°C at 50°C intervals.
Materials and equipment
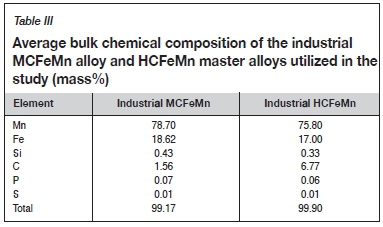
Two FeMn alloys were utilized for the dephosphorization experiments, namely MCFeMn and HCFeMn alloys. The MCFeMn and HCFeMn alloys were sourced from an industrial producer in South Africa. HCFeMn was utilized in only a few cases to investigate experimentally which of the two alloys would be easier to dephosphorize. Table III summarizes the average chemical composition of the MCFeMn alloy and HCFeMn master alloy. Because a graphite crucible was used for the experiments, these alloys converged on a similar carbon concentration after the reaction.
The slag reagents utilized in the study were sourced from the commercial suppliers Sigma Aldrich and ACE chemicals. Analytical grade CaO, SiO2, MnO2, CaF2, BaCO3, Na2CO3, and BaF2 were utilized. Different synthetic slags were prepared. The slag components, i.e. CaO, SiO2, Na2CO3, and BaCO3, were calcined separately at 1000°C for 2 hours in air to remove volatile matter. Decomposition of the carbonaceous matter for Na2CO3 and BaCO3 was, however, not successful at 1000°C. The calcined materials were then weighed and blended in a ring mill to generate a homogeneous mixture and charged into a graphite crucible. The crucible charges were subsequently heated in an induction furnace at 10°C/min to 1600°C and maintained at the temperature for 1 hour under an inert atmosphere using Ar gas. The molten materials were allowed to cool to room temperature under an inert atmosphere. The slags were then retrieved from the crucibles, crushed, milled, and decarburized at 1200°C for 2 hours in air, using alumina crucibles to burn off the residual carbon. The decarburized slags were analysed to determine their elemental compositions.
The MnO2 reagent was not added during the melting stage of the synthetic slags to avoid reaction with the graphite crucibles. The component was incorporated after the decarburizing stage. BaCO3 was also only added to the decarburized slags. The inclusion of BaCO3 during the melting stage resulted in the erosion of the graphite crucibles.
Equipment
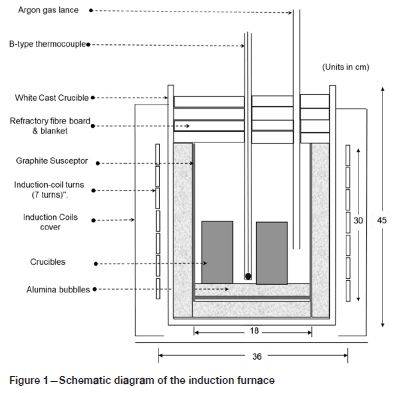
The dephosphorization tests were conducted in a 25 kW induction furnace Figure 1 presents the schematic diagram of the furnace. The furnace operates by radiation heating from a graphite susceptor heated by magnetic induction created by a water-cooled copper coil. The furnace power is switched on by using an on/off button on the control panel. The power was adjusted manually; the temperature was controlled by manually increasing the power setpoint at an interval of 30 minutes until the target temperature was reached. As it is generally difficult to maintain a constant furnace temperature, a range of 10°C above and 10°C below the target temperature was maintained. Increasing the power setpoint at an interval of 30 minutes is the standard approach to ensure that the operating temperature is not overshot excessively.
Recipe design
Baseline CaO-based slag (Slag A)
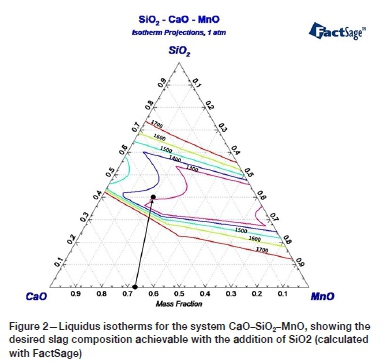
In the current study, the slag system comprising 20%MnO-40%CaO-40%SiO2 was adopted as a baseline slag. The basic oxide CaO was selected to provide free oxygen (O2-) when dissolved in the liquid slag (refer to Equation [1]). MnO was added to provide the oxidizing condition in the system. The acidic SiO2 was selected as a flux to reduce the melting point of the high-melting, basic MnO-CaO binary system. Figure 2 presents the MnO-SiO2-CaO phase diagram, showing the fluxing strategy chosen and the expected change in slag liquidus temperature at 40% SiO2 addition. As shown in Figure 2, the baseline slag has a liquidus temperature of 1320°C.
Na2O-CaF2 addition (Slag D, Slag E, and Slag F)
The addition of Na2O has proven to enhance the ability of CaO-based slags to dephosphorize liquid iron (van Niekerk and Dippenaar, 1998; Nassaralla and Fruehan, 1992). CaF2, on the other hand, is added during hot metal treatment to maintain the fluidity of lime-based slags. Both additives have been reported to influence the phosphate capacities of basic slags (Fujita et al., 1988) and were therefore investigated in the current study. CaO was partially replaced with Na2O and CaF2, while the MnO content was maintained constant.
BaO addition (Slag H)
Chaudhary and Roy (2001) found the BaO-BaF2-MnO slag system to be effective for the dephosphorization of FeMn alloys, as well as liquid iron when BaO was utilized. The effect of BaO addition was investigated in the current study by replacing a fraction of the CaO with BaO.
BaO-BaF2 addition (Slag I)
Replacing both the lime and silica by BaO and BaF2 flux was also investigated. This slag system has been reported to be effective for dephosphorization of high-carbon FeMn alloy (Chaudary, Goel, and Minz, 2008; (Fujita et al., 1988; (Dashevski et al., 1998). The use of BaO-based slag without CaO posed numerous challenges during the current test work. The slag was very wetting towards the graphite crucible and spilled from the crucible due to what is assumed to have been the Marangoni effect. This slag could therefore not be investigated further.
Basicity changes to baseline CaO-based slag
The effect of changes in basicity, %CaO/%SiO2, on the P partition coefficient was also investigated. The basicities investigated were 0.7, 0.9, and 1.3 using baseline slag A with the MnO content constant.
Table IV presents the chemical compositions of the prepared master slags. The results indicate that FeO, MgO, S, P, and Al2O3 were the major impurities. The slag reagents used contained significant amounts of impurities. The FeO was introduced mainly by the MnO2 reagent. The target MnO, SiO2, and CaO contents in the master slags were not entirely attained, owing mainly to the FeO content in the slag. The total chemical compositions of the slags are also generally lower than 100%. This was attributed to the expression of Mn as MnO instead of MnO2 and the presence of carbonaceous matter introduced by BaCO3 and Na2CO3. These slags were utilized in the dephosphorization tests.
Dephosphorization test procedure
All tests used the same test preparation method, which entailed weighing the required amounts of the slag and alloy, followed by homogenization of the feed charges in a ring mill for about 30 seconds. About 10 g of both the starting alloy and slag was used for the experiments. The charge was then packed into the graphite crucible. Packing was done such that a dense, compact solid charge was formed to promote the interaction of solid particles in the crucible. Graphite crucibles sere used to avoid attack of the crucible by the different slag systems. The crucibles were also selected with the intention of maintaining the slag chemistries during the tests without interference by the crucible.
The charged crucibles were weighed and placed in the furnace chamber. The furnace chamber was covered with high-temperature refractory blankets (Fiberfrax) to prevent air ingress and preserve heat in the chamber. An alumina tube piped argon gas into the furnace chamber to create an inert environment for the duration of each test. An alumina sheath encased the Type B control thermocouple. The same thermocouple monitored the sample temperature.
The furnace was heated gradually by incrementally increasing the power input until the target test temperature was reached. The typical heating rate was 8-10°C/min under an inert atmosphere. The argon flow rate was maintained at around 2 L/min for the duration of the test. The samples were maintained at the target temperature for retention times of 30 minutes and 60 minutes. A lower retention time of 5 minutes was also investigated, but the analysis of the samples could not be completed due to poor separation of the slag and alloy at 1350°C. Upon completion of each test, the furnace power was switched off and the refractory blankets slightly moved to allow removal of the graphite crucibles from the furnace chamber. The hot crucibles were removed from the furnace using steel tongs and immediately quenched in a water-and-ice bath to ensure rapid cooling of the molten slag and alloy and maintain the phase chemistry in both the slag and alloy at the specific test conditions. The quenched crucibles were then dried at 105°C overnight in a drying oven. The dried sample was weighed and broken to retrieve the slag and alloy.
The alloy and slag were separated manually, weighed, and pulverized for analysis. The bulk chemical compositions of the feed materials and products were determined by Inductively coupled plasma-optical emission spectroscopy (ICP-OES). C and S were determined by combustion (LECO). P was determined using wet chemical analysis by ICP. Table V represents a summary of the completed test matrix. Test work using slag D was unsuccessful because the slag has a high liquidus temperature of about 1800°C and separation of slag and alloy was not possible at the experimental temperatures. This slag was therefore not investigated further. Slag I was aggressive towards the graphite crucible, resulting in crucible erosion and leakage of the contents.
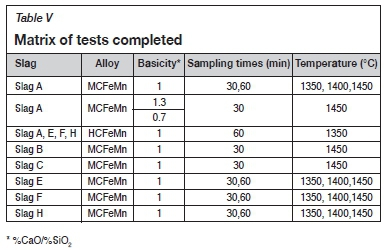
Results and discussion
FactSage calculations
Phosphorus partition coefficient
The Lpvalues obtained from the thermochemical calculations are presented in Figure 3 for HCFeMn and Figure 4 for MCFeMn. The calculations generally predict that dephosphorization of both alloys will not be possible with the CaO slags as the Lp values obtained were well below unity. The results generally showed an increase in Lpfor most of the slag-alloy systems as the temperature is increased. The increase in Lpwith increasing temperature was unexpected, as the dephosphorization reaction is exothermic (Chaudary and Goel, 1994; Simeonov and Sano, 1985).
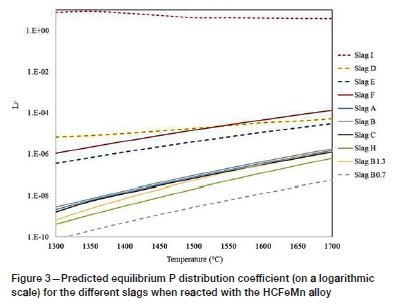
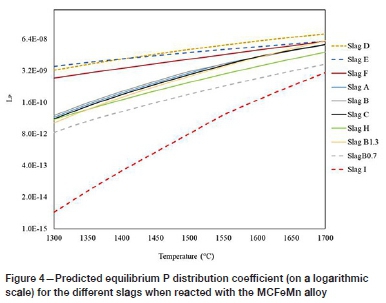
The addition of BaO in conjunction with CaO caused a detrimental effect, with lower predicted Lpvalues obtained as indicated by slag H. Replacing both CaO and SiO2 with BaO and BaF2 flux in slag I resulted in higher LPvalues, above unity. The FactSage predictions concerning the BaO-BaF2-MnO slag system agree with the observations made by various researchers (Fujita, et al., 1988;Chaudhary, Minj, and Goel, 2007; Dashevski et al., 1998). The effect of temperature on LP for slag I is small compared to the opposite effect for most of the other slags. Comparing the results obtained from the two alloys, the prediction by FactSage show that the BaO-based slag will not dephosphorize the MCFeMn alloy, and lower Lpvalues were obtained with MCFeMn alloy.
In conclusion, the results obtained from the FactSage calculations predicted that none of the CaO-based slags would yield significant dephosphorization. The Lppredictions for CaO-based slags were very low and not in agreement with findings in the literature that Lpdecreases as temperate is increased (Chaudhary and Goel, 2007; Fujita et al., 1988). Based on the results, it appears that FactSage does not accurately predict dephosphorization ratios for FeMn calculations. Experimental test work was thus necessary to serve as a check on the FactSage results.
Dephosphorization test work
Phosphorus partition coefficient: effect of temperature and reaction time
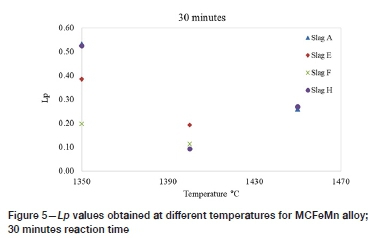
Figures 5 and 6 show the Lpvalues obtained from the different slags at different temperatures for reaction times of 30 minutes and 60 minutes, respectively. The results show that the baseline slag CaO-SiO2-MnO slag (slag A) appeared to yield higher Lp values than the other slags at the temperatures and reaction times investigated. The results also show that at higher temperatures, generally lower Lpvalues were obtained. Slag H, however, gave slightly higher values at 1450°C. This anomaly may be attributed to the analytical errors for samples from either the 1400°C or 1450°C experiments. Liu et al. (1995) similarly observed lower Lpvalues as the temperature was increased during dephosphorization of FeMn alloy with BaO-containing slags.
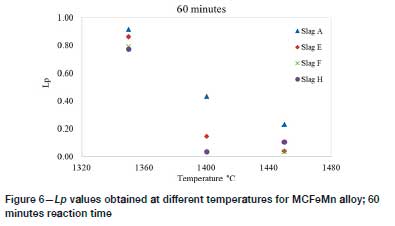
Effect of changing MnO content of baseline slag
Figure 7 shows the Lpvalues obtained at the three initial MnO slag contents at 1450°C, 30 minutes reaction time. Figure 8 represents the Lp versus the final MnO slag contents, which increase in the final slags. The loss of Mn from the alloy is influenced by the oxygen partial pressure in the system. Factors that may have contributed to the loss of Mn include possible air ingress during the tests and the exposure of the alloys to oxygen during quenching, as Mn has a high affinity for oxygen. Liu et al. (1995) observed that the use of CaO, SiO2, and CaF2 to dephosphorize ferromanganese melts resulted in increased Mn losses to the slag. Watanabe et al. (1993) reported that MnO in basic slag acts as a diluent which may lessen the basic effect of the slag, reducing the capacity of the slag to remove P from the alloy. The presence of MnO in the slag is important to provide the required oxygen, although high MnO content can lead to low dephosphorization (Chaudhary and Roy, 2001).
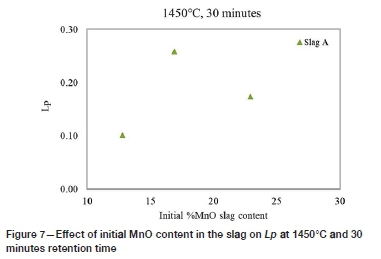
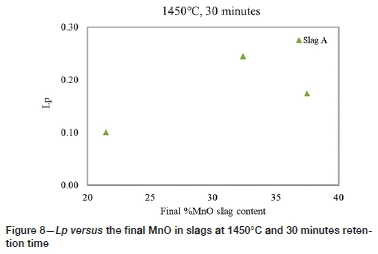
The Lpresults show that there was an increase in Lpfrom 15% to 20% MnO, followed by a reduction in the partition ratio. High amounts of MnO may affect the activity of the basic oxides such as CaO and BaO in the slag and can reduce the capacity of the slag to absorb P. Chaudhary, Minj, and Goel (2007) observed that an increase in the MnO content to above 20% (in BaO-containing slag) resulted in poor P removal. In the HCFeMn dephosphorization studies by Liu et al. (1995), it was observed that an increased concentration of MnO in slag had a negative influence on the phosphate capacity of BaO-MnO-BaCl2 slag. It was, therefore concluded from the investigations that an MnO content above a certain value is detrimental to the capability of the slag to dephosphorize FeMn alloys. In the current study, the high MnO slag contents may have adversely affected the P capacity of the slags.
Effect of adding Na2O and CaF2
Na2O is a more basic oxide than CaO, so it was expected that dephosphorization would be improved by Na2O additions. The results represented in Figures 5 and 6 generally show that the addition of Na2O, indicated by slag E, generally did not provide any added benefit as low Lpvalues were still obtained. The literature indicates that the addition of Na2O to CaO-based slags improves the dephosphorization of liquid iron; however, Na2O can be reduced by either C or Mn, producing CO/MnO. The instability of Na2O can therefore have a detrimental effect on the dephosphorization of FeMn alloys due to loss of Mn (Tabuchi and Sano, 1985). In the current study, the loss of Mn from the alloy was observed even with the Na2O-free slags, so it is not possible to establish the effect of Na2O on Mn loss. Fujita et al. (1988) also observed that low degrees of dephosphorization were achieved when using Na2CO3 flux on FeMn alloy.
The fluoride ion (F-) from CaF2/BaF2 is reported to stabilize the phosphate ion (PO43-) during the ionic dephosphorization reaction in molten slag under oxidizing conditions, and thus contribute positively to dephosphorization (Liu et al., 1998). However, Ca2+ binds fluoride ions much more strongly than Ba2+ does, because of the difference in the ionic radius of the two cations. Therefore, fewer free fluoride ions would be available when using CaF2 to enhance dephosphorization (Liu et al., 1998). Liu et al. observed that the addition of CaF2 to MnO-BaO-BaF2 slag reduced Lpand increased the Mn capacity of the slag, i.e. Mn losses to the slag. In the current study, the addition of CaF2 (slag F) showed no improvement in terms of Lp.
Effect of adding BaO
The use of BaO in conjunction with CaO (slag H), also resulted in no improvement. Similar Lpvalues to those of slag A were obtained under conditions of 1350°C and 1450°C at 30 minutes. Slag A was, however, not outperformed by slag H under any of the other conditions. Nasaralla, Fruehan, and Min (1991) investigated the use of BaO as an additive in CaO-based flux for dephosphorizing Fe-C-P alloy. It was observed that amounts of BaO < 40% had no effect on the phosphate capacity of the slag. A larger increase in the phosphate capacity was only observed after increasing the BaO content of the CaO-CaF2 slag to above 40%. The researchers also later observed that the Lpincreased significantly, by a factor of 4.6, when a high BaO content of 30% was utilized in the CaO-Al2O3-CaF2 slag system (Nassaralla and Fruehan, 1992). During the current study, a BaO content of 20% was utilized. The lower Lpvalues obtained could be attributed to the low concentration of the basic oxide and uncertainties in the analysis of P, as well as the dilution of the slag by MnO.
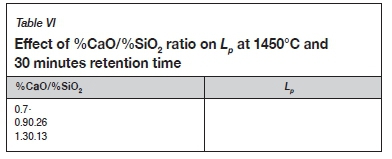
Effect of slag basicity
Increasing the slag basicity (%CaO/%SiO2) to 1.3 caused the slag liquidus temperature to increase to about 1500°C. This led to difficulties in melting the slag and resulted in poor slag separation during the experiment at 1450°C, due to the alloy being entrained in the slag. This had a detrimental effect on the ability of the slag to remove P. As the reaction occurs in a liquid phase, a lower Lpwas obtained by increasing the basicity to 1.3, as shown in Table VI. At the lower %CaO/%SiO2 ratio of 0.7, the P concentration in the slag was below the detection limit (<0.005%) and Lpcould not be calculated. The results, therefore, show an initial increase in Lpvalue from a basicity of 0.7 to 1.0, followed by a slight reduction in Lp.
Effect of initial alloy composition
Figures 9 and 10 show the Lpvalues obtained with HCFeMn at 1350°C and 1400°C respectively. Dephosphorization of HCFeMn by the respective slags was also not significant, as lower Lpvalues of <1 were generally obtained.
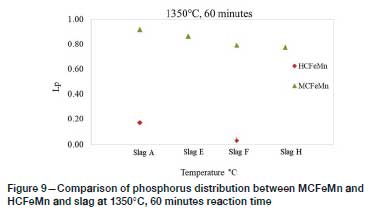
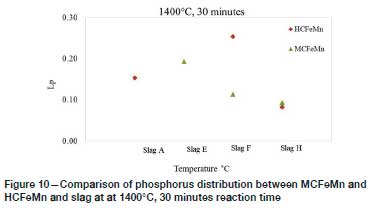
Comparison between the results shows that at 1350°C, relatively higher Lpvalues were obtained from MCFeMn, and lower values at 1400°C. As noted earlier, both the HCFeMn and the MCFeMn alloy would have reached carbon saturation during the experiments. The studies on the effect of initial C, Mn, and Si content on dephosphorization have shown that a high Mn in the alloy decreases the activity coefficient of P, and Lpincreases with increasing initial C content until the C saturation point. High initial Si (>0.2%) is not favourable for dephosphorization as the Si is oxidized during dephosphorization, leading to increased SiO2 in the slag, which consumes the high basic oxides and ultimately reduces the capacity of the slag to remove P (Chaudary, Goel, and Minz, 2008; Bhardwaj, 2014). During the current study, the molten MCFeMn absorbed C from the crucible, forming HCFeMn, and the final average compositions of the alloys appeared to be similar after dephosphorization. The initial Si in both alloys was similar at about 0.4%, which is reported to be undesirable for dephosphorization of FeMn alloys.
Conclusion
The FactSage calculations indicated that none of the CaO-based slags would result in significant dephosphorization.
The results generally showed that Lpremained small (<1): a significant proportion of P remained in the alloy, and thus dephosphorization was not favoured. The CaO-SiO2-MnO (slag A) slag system yielded higher values of Lp. The addition of Na2O (slag E) generally did not show any added benefit. Substituting half of the CaO by BaO (slag H) resulted in similar Lpvalues to those of slag A under conditions of 1350°C and 1450°C at 30 minutes. Slag H did not outperform slag A under any of the other conditions. Increasing the temperature generally resulted in lower Lpvalues. This may be attributed to the exothermic nature of the reaction which should be favourable at lower temperatures. Increasing the %CaO/%SiO2 ratio of the starting slag gave an initial increase in Lpvalue from a basicity of 0.7 to 1.0, followed by a slight reduction in the partition ratio. The latter results were not expected, as a higher basicity is expected to improve the P capacity of the slag. Increasing the basicity increased the slag liquidus temperature, which negatively affected dephosphorization.
In summary, based on the Lpvalues obtained, the conditions investigated are unfavourable for the removal of P from South African MCFeMn and HCFeMn industrial alloys, as significant amounts of P remained in the alloy. The experimental results are in line with FactSage predictions that the CaO slags are not suitable for dephosphorization of FeMn alloys.
Below is a summary of the shortcomings of the current study and recommended future work (Table VIII).
Due to the unsuccessful removal of P from the FeMn alloys by the CaO-based slag systems, it is recommended that further investigations be considered on the use of BaO-based slags with no CaO addition. BaO slags are reported in the literature to be effective for dephosphorization of FeMn alloys. However, during the current study experimental challenges were encountered with the BaO-based slag. Further test work should be conducted with highly pure slag reagents that do not introduce P as an impurity in the master slags. It is also recommended that various other slags be investigated, with which higher basicity can be achieved at lower temperatures.
Acknowledgment
The paper is published with the permission of Mintek. The authors wish to thank Mintek for financial support.
References
Bale, C., Bélisle, E., Chartrand, P., Decterov, S.A., Eriksson, G., Hack, K., Jung, I.-H., Rang, Y.-B., Melanoon, J., Pelton, A.D., Robelin, C., and Petersen, S. 2009. FactSage thermochemical software and database-recent development. Calphad, vol. 33, no. 2. pp. 295-311. [ Links ]
Bale, C. Bélisle, E., Chartrand, P., Decterov, S., Eriksson, G., Gheribi, A., Hack, K., Jung, I., Rang, Y-N., Melancon, J., Pelton, A., Petersen, S., Robelin, C., Sangster, J., Spencer, P., and van Ende, M-A. 2016. Reprint of: FactSage thermochemical software and databases, 2010-2016. Calphad, vol. 55, no. 1. pp. 1-19. [ Links ]
Baluch, N., Udin, Z.M., and Abdullah, C.S. 2014. Advanced high strength steel in auto industry: An overview. Engineering Technology and Applied Science Research, vol. 4, no. 4. pp. 686-689. [ Links ]
Bernhard, C., Linzer, B., Presoly, P., Watzinger, I., and Watzinger, J. 2019. Evaluation of AHSS concepts with a focus on the product properties and appropriate casting characteristics of Arvedi ESP thin slab casters. IOP Conference Series - Materials Science and Engineering, vol. 529, no. 1. Joint 5th International Conference on Advances in Solidification Processes (ICASP-5) & 5th International Symposium on Cutting Edge of Computer Simulation of Solidification, Casting and Refining (CSSCR-5), Salzburg, Austria, 17-21 June 2019. pp. 1-10. [ Links ]
Bhardwaj, B.P. 2014. Production of ferroalloys. The Complete Book on Ferroalloys. NCPS, Delhi, India. pp. 300-309. [ Links ]
Chaudhary, P.N. and Goel, R.P. 1994. Dephosphorization of liquid ferromanganese. 4th Refresher Course on Ferro Alloys, Jamshedpur, India, 12-14 January 1994. National Metallurgical Laboratory, Jamshedpur. pp. 1-11. [ Links ]
Chaudhary, P.N., Goel, R.P., and Minz, R.K. 2008. An improved process for dephosphorization of high carbon ferromanganese. Patent no. 217812. Council of Scientific and Industrial Research, New Delhi. [ Links ]
Chaudhary, P.N., Minj, R.K., and Goel, R.P. 2007. Development of a process for dephosphorisation of high carbon ferromanganese. INFACON 8. Proceedings of the 11th International Ferroalloys Congress, New Delhi. pp. 288-295. https://www.pyrometallurgy.co.za/InfaconXI/288-Chaudhary.pdf [ Links ]
Chaudhary, P.N. and Roy, G.G. 2001. Dephosphorization of HCFeMn using BaCO3 based fluxes. Ionmaking & Steelmaking, vol. 29, no. 5. pp. 396-403. [ Links ]
Dashevskii, V.Y.. Kashin, V.l., Lyakishev, N.P., Velichko, B.F., and Koval, A.V. 1998. Desphosphorization of manganese ferroalloys with CaO and MnO based slag-forming mixtures. Proceedings of INFACON VIII, Beijin, China. pp. 300-301. https://www.pyrometallurgy.co.za/InfaconVIII/300-Dashevskii.pdf [ Links ]
Fujita, M., Katayama, H., Yamamoto, A., and Mutsuo, M. 1988. Dephosphorization of Fe-Mn-C alloy with BaCO3. Tetsu-to-Hagane, vol. 74, no. 5. pp. 816-822. [ Links ]
Karbowniczek, M., Michaliszym, A., Wcislo, Z., and Slezak, W. 2014. Dephosphorization of ferromanganese. Acta Metallurgica Slovaca-Conference, vol 4. pp. 138-144. doi:10.12776/amsc.v4i0.252 [ Links ]
Liu, X., Wijk, 0., Selin, R., and Edstrom, J.O. 1998. Effects of additives in BaO-BaF2- MnO slags on phosphate and manganese capacities. ISIJ International, vol. 38, no. 1. pp. 36-45. [ Links ]
Liu, X., Wijk, 0., Selin, S.R., and Edstrom, J.O. 1995. Phosphorus equilibrium between BaO-BaF2-MnO fluxes and ferromanganese melts. Steel Research, vol. 66, no. 3. pp. 96-102. [ Links ]
Nasaralla, C., Fruehan, R.J., and Min, D.J. 1991. A thermodynamic study of dephosphorization using BaO-BaF2, CaO-CaF2, and BaO-CaO-CaF2 systems. Metallurgical Transactions B, vol. 22, no. 1. pp. 33-38. [ Links ]
Nassaralla, C. and Fruehan, R.J. 1992. Phosphate capacity of CaO-AI2O3 slags containing CaF2, BaO, Li2O, or Na2O. Metallurgical Transaction B, vol. 23, no. 2. pp. 117-123. [ Links ]
Olsen, S.E., Olsen, S., Tangstad, M., and Lindstad, T. 2007. Manganese ores. Production of Manganese Ferroalloys. Tapir Academic Press, TTondheim, Norway. pp. 19-26. [ Links ]
Safarían, J. and Kolbeinsen, I. 2013. Purity requirements for Mn-alloys for producing high manganese TRIP and TWIP steels. Proceedings of the Thirteenth International Ferro-Alloys Congress, Almaty, Kazakhstan, 9-13 June 2013. pp. 175-183. https://pyrometallurgy.co.za/InfaconXIII/0175-Safarian.pdf [ Links ]
Simeonov, S.R. and Sano, N. 1985. Phosphorus equilibrium distribution between slags containing MnO, CaF2 and Na2O and carbon-saturated iron for hot metal pretreatment. Transactions of the Iron and Steel Institute of Japan, vol. 25, no. 10. pp. 1031-1035. [ Links ]
Steenkamp, J.D. BamII; W.G., Ringdalen, E., MushwanaI; M., Hockaday, S.A.C., and Sithole, N.A. 2018. Working towards an increase in manganese ferroalloy production in South Africa - A research agenda. Journal of the Southern African Institute of Mining and Metallurgy, vol. 118, no. 6. pp. 645-654. [ Links ]
Steenkamp, J.D., Pistorius, P.C., and Tangstad, M. 2015. Chemical wear analysis of a tap-hole on a SiMn production furnace. Journal of the Southern African Institute of Mining and Metallurgy, vol. 115, no. 3. pp. 199-208. [ Links ]
Tabuchi, S. and Sano, N. 1985. Thermodynamics of phosphate and phosphide in CaO-CaF2 melts. Metallurgical Transactions B, vol. 15, no. 2., pp. 351-356. [ Links ]
Tang, K. and Olsen, S.E. 2006. Computer simulation of equilibrium relations in manganese ferroalloy production. Metallurgical ad Materials Transactions B, vol. 37, no. 4. pp. 599-606. [ Links ]
Van Niekerk, W.H. and Dippenaar, R.J. 1998. Phosphorus distribution between carbon-saturated iron at 1350°C and lime-based slags containing Na2O and CaF2. Metallurgical and Materials Transactions B, vol. 29, no. 1. pp. 147-153. [ Links ]
Visser, M., Smith, H., Ringdalen, E., and Tangstad, M. 2013. Properties of Nchwaning and Gloria ores in the production of Mn Ferro-alloys. Proceedings of the Thirteenth International Ferroalloys Congress, Almaty, Kazakhstan, 9-13 June 2013. pp. 553-566. https://www.pyrometallurgy.co.za/InfaconXIII/0553-Visser.pdf [ Links ]
Wagner, C. 1975. The concept of the basicity of slags. Metallurgical Transactions B, vol. 6, no.3. pp. 405-409. [ Links ]
Watanabe, Y. Kitamura, K., Rachev, I.P., Tsukihashi F., and Sano, N. 1993. Thermodynamics of phosphorus and sulfur in the BaO-MnO flux system between 1573 and 1673 K. Metallurgical Transactions B, vol. 24, no. 2. pp. 339-347. [ Links ]
You, B., Lee, B., and Pak, J. 1999. Manganese loss during the oxygen refining of high-carbon ferromanganese melts. Metals and Materials, vol. 5, no. 5. pp. 497-502. [ Links ]
 Correspondence:
Correspondence:
M.P. Maphutha
Email: Patriciam@mintek.co.za
Received: 9 Feb. 2021
Revised: 25 Aug. 2021
Accepted: 26 Aug. 2021
Published: September 2021














