Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.121 n.9 Johannesburg Sep. 2021
http://dx.doi.org/10.17159/2411-9717/1487/2021
PAPERS OF GENERAL INTEREST
Decoupling the effects of alteration on the mineralogy and flotation performance of Great Dyke PGE ores
T. DzingaiI; B. McFadzeanI; M. TadieII; M. BeckerI
ICentre for Minerals Research, Department of Chemical Engineering, University of Cape Town, South Africa. M. Becker: http://orcid.org/0000-0002-7025-137X
IIDepartment of Process Engineering, University of Stellenbosch, South Africa. http://orcid.org/0000-0003-3111-5188
SYNOPSIS
Ores from a single deposit may exhibit extensive variability in their mineralogy and texture. The ability to quantify this variability and link it to mineral processing performance is one of the primary goals of process mineralogy. This study focuses on the effect of alteration in three platinum group element ore samples from the Great Dyke in Zimbabwe - two of which were more pristine compared to the third, which was locally classified as 'oxidized' ore. These ores are known to be characterized by varying degrees of alteration, resulting in numerous challenges in flotation and affecting both grade and recovery. Alteration, by near-surface oxidation, of the valuable base metal sulphides and platinum group minerals resulted in lower flotation recoveries of Cu, Ni, Pt, and Pd. Evidence of incipient oxidation was more readily observed in the base metal sulphide assemblage than the platinum group mineral assemblage, even though the loss in recovery (because of oxidation) was most significant for Pd. Alteration through hydration resulted in a significant increase in mass pull and dilution of concentrate grade through the inadvertent recovery of naturally floating gangue comprising composite orthopyroxene and talc particles. In this study, the amount of naturally floating gangue was more strongly correlated with the talc grain size distribution than the grade of talc in the flotation feed. The oxidation and hydration alteration reactions are not necessarily mutually exclusive, although one may be more dominant than the other, giving rise to ore variability.
Keywords: Process mineralogy; platinum ores; hydration; oxidation; alteration; naturally floating gangue.
Introduction
The ability to manage ore variability is a major challenge faced by the mining industry at present. Many existing plants cannot necessarily deal with ore variability since their design specifications have historically focused on averaged ore characteristics (e.g. Powell, 2013). As design flexibility is not always an option for existing plants, the need for optimization of these operations based on process mineralogy is increasing (Lotter et al., 2011, 2018; Baum, 2014). The major contributors to ore variability include hydrothermal alteration, oxidation, and weathering of a pristine ore deposit. The alteration of sulphide minerals by oxidation can lead to reduced recoveries of valuable minerals (Evans, 2002; Oberthür et al., 2013; Sefako, Sekgarametso, and Sibanda, 2017). There is, therefore, a need for an understanding of the mineralogical aspects of ore variability, especially those arising from alteration, and the consequent assessment of how it can be managed. Some of the ways of managing ore variability include ore sorting, stockpiling and campaigning, blending, or the use of tailored reagent suites based on the mineralogy of the run-of-mine ore.
Great Dyke PGE ores
The Great Dyke in Zimbabwe is, after the Bushveld Complex in South Africa, the world's second-largest resource of the platinum group elements (PGE - Pt, Pd, Ir, Ru, Rh, Os), containing an estimated 8 680 t 4E (Pt, Pd, Rh, and Au) (Mudd, 2012; Oberthür et al., 2013). The Great Dyke is a magmatic Ni-Cu-PGE stratiform intrusion stretching approximately 560 km in a NNE direction across Zimbabwe, with a width varying between 4 and 11 km. The Great Dyke is divided into two sections along strike, namely the North and South Chambers (see Wilson and Prendergast (2001) for a more comprehensive description of the Great Dyke). Economic PGE mineralization in the Great Dyke is stratabound and found in the Main Sulphide Zone (MSZ) and the Lower Sulphide Zone (Oberthür et al., 2003). Sulphide ores are mined underground and processed at the Ngezi, Unki, and Mimosa mines by conventional mineral processing and metallurgical methods which entail crushing and grinding, flotation, smelting, and matte production, as well as chemical refining (Jones, 2005; Oberthür et al., 2013). These mines have combined resources of approximately 2 000 Mt at an average PGE grade of 3.6 g/t (Mudd, 2012). The oxidized ores occur closer to the surface and contain possible resources up to 250 Mt (Oberthür et al., 2013). These oxidized ores are further divided into a spectrum according to the degree of oxidation from incipient to patchy to pervasively oxidized. The oxide ores are considered marginal to sub-economic and are not currently processed {e.g. Zimplats, 2019). Historical small-scale operations processing these oxidized ores by froth flotation were ultimately halted due to the low (less than 50%) PGE recoveries obtained (Evans, 2002). These oxide ores are therefore not being recovered to their full potential as oxidation and alteration present a 'mineralogical barrier' (Skinner, 1976).
In contrast to the majority of the ores from the Bushveld Complex in South Africa, which are relatively pristine with minor alteration, ores from the MSZ of the Great Dyke are characterized by more extensive alteration, resulting in numerous challenges in PGE recovery (Coghill and Wilson, 1993; Wilson and Prendergast, 2001; Oberthür et al., 2013; Sefako, Sekgarametso, and Sibanda, 2017).
Before exploring some of the challenges posed by these altered ores from the Great Dyke and Bushveld Complex, and potential remedies, it is important to ensure that the appropriate nomenclature is used for describing the changes these ores have undergone. We have found that the terms 'alteration', 'weathering', and 'oxidation' are sometimes used interchangeably, particularly when engaging with practicing industry professionals, and will define the terms used in this paper.
Alteration and its effect on mineral processing
There are several types of alteration reactions that can occur in rocks, some of which are oxidation, hydrolysis, hydration, silicification, and decarbonation, among others (Guilbert and Park, 1986; Robb, 2005). All these reactions are capable of producing variability in mineralogy and texture. Of interest as regards ores from the Great Dyke are the oxidation, hydration, and hydrolysis reactions and their products (Figure 1). Oxidation typically results in the formation of secondary sulphides such as violarite, millerite, covellite, and chalcocite, as well as oxides and sulphates from the former primary base metal sulphide (BMS) mineral assemblage of pentlandite, pyrrhotite, and chalcopyrite. Hydrolysis refers to the addition of H+, leading to the conversion of anhydrous silicates to hydrous ones, for example the conversation of plagioclase to muscovite. Hydration is the addition of water to the mineral, for example the alteration of orthopyroxene to talc, or olivine to serpentine. The latter reaction is known as serpentinization. Hydration and hydrolysis reactions can occur due to either hydrothermal alteration or deep weathering - in the case of hydrothermal alteration, the degree of alteration would increase with ore depth (like a typical porphyry ore), whereas weathering effects would decrease with ore depth.
The oxidation of Great Dyke ores as described in studies by Evans (2002), Locmelis, Melcher, and Oberthür (2010), and Oberthür et al. (2013) affects both the BMS and platinum group minerals (PGMs) in the near-surface environment. Oxidation leads to partial or complete oxidation and disintegration of the PGMs and BMS. This affects the attachment of reagents to these altered minerals as flotation collectors are mineral-specific. Even when partial oxidation occurs, the surface of the mineral would not necessarily have the same physico-chemical properties as the unaltered sulphide, thus affecting collector attachment. In addition, the PGMs may no longer be texturally associated with the BMS, preventing their recovery as composite particles using traditional thiol collectors (Becker, Wiese, and Ramonotsi, 2014). The PGMs, especially the fine particles, therefore become even harder to recover physically because of the challenges associated with fine particle flotation (Farrokhpay, Filippov, and Fornasiero, 2020). Furthermore, the base metals and PGE may have been mobilized from the supergene environment or redistributed into the silicate mineral assemblage. Consequently, these metals may not occur in the same host minerals as in unoxidized ores. Pd, in particular, may be completely mobilized out of the supergene environment, and it has been estimated that up to 50% of the Pd may be lost (Evans, 2002; Oberthür et al., 2013). PGE-bearing phyllosilicate minerals such as serpentine, smectite, and chlorite have also been reported in the Great Dyke (Locmelis, Melcher, and Oberthür, 2010) as well as in the Bushveld Complex (Chetty et al., 2018). The combined effects of oxidation lead to a significant loss in pay metal recovery during processing. Altering the depressant type or dosage may not improve the recovery of the valuable minerals, but rather, more specific flotation collectors are needed to target these oxidized minerals (Becker, Wiese, and Ramonotsi, 2014; Sefako, Sekgarametso, ands Sibanda, 2017).
Hydration and hydrolysis reactions largely affect the primary silicate minerals in these ores, resulting in the formation of amphiboles as well as phyllosilicates such as mica, serpentine, talc, chlorite, and smectite (Locmelis, Melcher, and Oberthür, 2010; Chaumba, 2017). Talc, which is naturally hydrophobic, has a disproportionate effect on concentrate grade due to its inadvertent recovery in composite orthopyroxene-talc particles - these composites represent naturally floating gangue in these PGE ores (Becker et al., 2009). Due to the anisotropic surface charge on talc and other phyllosilicate minerals, they also have the potential to create rheological challenges (Burdukova et al., 2007; Becker et al., 2013; Ndlovu et al., 2014). Rheological complexity resulting from the higher amounts of phyllosilicate minerals in ores affects both the concentrate grade and the recovery (Bakker, Meyer, and Deglon, 2010; Patra, Nagaraj, and Somasundaran, 2011; Shabalala et al., 2011; Farrokhpay, 2012; Zhang and Peng, 2015). The presence of ultrafine phyllosilicate minerals such as serpentine may also result in slime coating of the valuable minerals, further affecting flotation recovery (e.g. Bremmell, Fornasiero, and Ralston, 2005). The effects of both oxidation and hydration were observed by Becker, Wiese, and Ramonotsi (2014) in their work on an altered PGM ore from the Bushveld Complex in which there were high amounts of oxides as well as talc in the feed; this implies that the different types of alteration are not mutually exclusive. Changing the depressant type and dosage could in this case improve the flotation performance, as this reduces the amount of the naturally floating gangue reporting to the concentrate. Apart from reducing the valuable mineral grades in the concentrate, the inadvertent recovery of Mg-bearing minerals such as talc, serpentine, and pyroxene to the concentrate may also lead to smelting penalties (Lotter et al., 2008). Overall, the alteration of the silicate minerals through hydration and hydrolysis reactions appears to have a greater effect on concentrate grade than on recovery (Figure 1).
Motivation and objective
Although current mining activities on the Great Dyke focus on sulphide ore, the run-of-mine ore may contain material with varying degrees of alteration. Recently, there has been renewed interest in the potential for the hydrometallurgical treatment of oxidized PGM ores, both in Zimbabwe and South Africa (Kraemer et al., 2015; Sefako, Sekgarametso, and Sibanda, 2017; Mpinga et al., 2018). However, modifying the operating conditions on an existing flotation plant presents an opportunity for managing ore variability when processing blends of pristine and oxidized ore, or even possibly for tailings retreatment. This could be followed by further downstream hydrometallurgical treatment for complex ores (Liddell and Adams, 2012; Sefako, Sibanda, and Sekgarametso, 2019).
In this paper we investigate the mineralogy of three different ores sampled across the Great Dyke, and compare their batch flotation responses. This information is used to develop a better understanding of the key mineralogical factors that affect the response of these ores to flotation, the effects of alteration, and the variability thereof. An improved understanding of these effects ultimately enables better management of processing performance.
Material and methods
Samples and sample preparation
Three 100-150 kg ore samples were obtained from different locations in the North and South chambers of the Great Dyke. Ores 1 and 2 were sampled from underground operations and represent sulphide ores that are currently processed. Ore 3 was obtained from a nearby open-pit source belonging to the operation from which ore 2 was sourced and represents what is classified as 'oxide ore'. Ores 1 and 2 were notably grey whereas ore 3 was a distinctive reddish colour, suggesting some degree of incipient oxidation. Samples were prepared on-site by crushing to 100% passing 3 mm and shipped to the Centre for Minerals Research laboratories at the University of Cape Town (UCT). Upon arrival, each sample was blended and split using a rotary riffle splitter into representative 1 kg portions.
Each 1 kg sample was milled to 65% passing 75 mm (resulting in a similar particle size distribution) in a 1 kg stainless steel rod mill charged with 20 stainless steel rods of varying diameter (6 x 25 mm, 8 x 20 mm, and 6 x 16 mm), at 66 wt.% solids, using synthetic plant water (Wiese, Harris, and Bradshaw, 2005). All milling and batch flotation experiments were conducted using a standard synthetic plant water recipe made up from distilled water with the addition of various salts (supplied by Merck). The divalent cations in the plant water are essential for the effective adsorption of the depressants onto talc (Khraisheh et al., 2005).
Mineralological and chemical characterization
A combination of wet and dry screening was used to separate the mill product into various size classes (-25, +25 -53, +53 -75, and +75 mm). The discrete size fractions were split into smaller aliquots with a rotary microriffler and mixed with graphite before preparation as 30 mm diameter polished sections for measurement of their bulk mineralogical composition, sulphide liberation, and association by Quantitative Evaluation of Minerals by Scanning Electron Microscopy (QEMSCAN) using an FEI 650F instrument equipped with two Bruker 6130 silicon drift energy-dispersive spectrometers. All QEMSCAN analyses were run at 25 kV and 10 nA using the particle mineralogical analysis (PMA) and specific mineral search (SMS) routines to obtain the bulk mineralogy and sulphide liberation, respectively. The trace mineral search (TMS) routine was run on polished blocks of unsized sample for the PGM searches. The relative error of the mineral grades was calculated using the method of Van der Plas and Tobi (1965).
Aliquots of the bulk samples were also prepared for quantitative X-ray diffraction (XRD) with a McCrone micronizing mill, then analysed on a Bruker D8 Advance powder diffractometer with a Vantec detector and fixed divergence and receiving slits with Co-Ka radiation. The step size was 0.01° 26 per second over the range 10 to 90°, with a measurement run time of 90 minutes. Phase quantification was performed by Rietveld refinement with the Bruker Topas software. The goodness of fit and Rwp of the phase quantification were approximately 4.2% and 9%, respectively. X-ray fluorescence (XRF) spectrometry was performed using a Panalytical Axios wavelength-dispersive instrument with a 4 kW Rh tube. Calibration standards include natural element SARMs (South Africa Reference Materials) and USGS (US Geological Survey) standards. The QEMSCAN mineralogy of the various samples was validated by comparison with quantitative XRD, XRF, and the measured loss on ignition (LOI, performed in conjunction with the XRF analysis). (The full set of results of these analyses can be found in Dzingai (2017).
Batch flotation tests
Reagents were chosen based on those used at the Great Dyke PGE operations. The depressant of interest was characterized at the Centre for Minerals Research Polymer Characterization Laboratory at UCT. It is of high purity (97.6%), an intermediate degree of substitution (0.7), and intermediate molecular weight (251 430 g/mol). The depressant dosages used in the tests were 100 g/t (by active content). Collector (sodium isobutyl xanthate) and frother (SasFroth 200) dosages were 300 g/t and 75 g/t respectively, which were chosen based on the existing flow sheets from Great Dyke operations.
For batch flotation tests, the collector was added to the mill charge before milling. The milled slurry from the sample preparation stage was transferred to a Barker 3 L batch flotation cell and made up with synthetic plant water to a solids concentration of 35% by mass. The impeller speed was set at 1200 r/min, and the pulp level was controlled manually. Air supply was maintained at 7 L/min and the froth height was kept constant at 1 cm. A feed sample was taken before commencement of the flotation procedure. The depressant was added to the slurry in the flotation cell and a conditioning time of 2 minutes was allowed. The frother was then added and conditioning continued for 1 minute, after which the air supply was opened. Four concentrates were collected by scraping the froth every 15 seconds into a collecting pan at 2, 6, 12, and 20 minutes (C1, C2, C3, and C4 respectively). A tailings sample was also collected after each flotation test. The amount of water recovered was measured during each test.
The flotation feeds, concentrates, and tailings were filtered, dried, and weighed before analysis. Batch flotation tests at each condition were conducted in duplicate and the standard error calculated. The dried flotation feed, concentrate, and tailings samples were analysed for Cu, Ni, S, Pt, and Pd. Cu and Ni were measured on loose powders using a Bruker S4 Explorer XRF spectrometer, while S analysis was done using a LECO DR423 sulphur analyser. The average relative standard error of the recalculated Cu and Ni feed grades was 2.4 and 1.8%, respectively. All internal assays used a variety of standard reference materials, including SARM standards. Pt and Pd were concentrated by fire assay (Pb collection) and analysed by inductively coupled plasma optical emission spectrometry (ICP-OES) by an external accredited service provider.
The results of all batch flotation tests were analysed by comparing the solids mass and water recoveries, as well as Cu, Ni, S, Pt, and Pd grades and recoveries. Also, the amount of floating gangue was determined using the method described in Wiese (2009). In this method, it is assumed that the only material recovered at high depressant dosage (500 g/t in this study) is via entrainment, whereas at a standard depressant dosage (100 g/t in this study) the recovery of gangue is through both entrainment and true flotation. The cumulative mass of gangue at the 500 g/t depressant condition (that is, the full concentrate mass excluding PGMs and sulphide minerals) is then plotted against the amount of water recovered. The slope of the line is equivalent to an 'entrainability factor' which is then used to calculate the amount of entrained gangue at the standard depressant dosage conditions (see Equation [1]). The total mass of gangue less the mass of entrained gangue gives the amount of floating gangue (Equation [2]). Bulk mineralogy, liberation, and association analyses were carried out on the +38 -75 mm size fraction of the flotation concentrates and are considered representative of recovery by true flotation rather than recovery by entrainment (Savassi, 1998; Becker et al., 2009; Wang et al., 2015).

Results and discussion
Elemental and mineral grades of flotation feeds
The feed ore samples were assessed mineralogically and chemically (Tables I to III) to investigate what factors would contribute to differences in flotation responses, if any, given the use of a uniform reagent suite for all three ores.
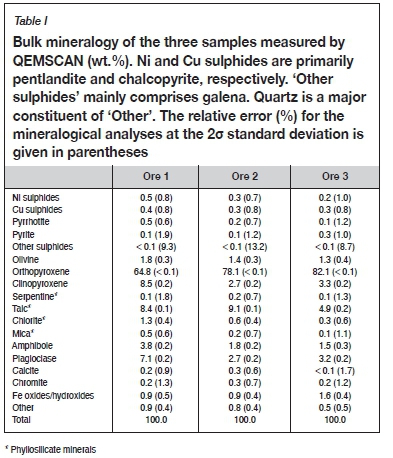
All three ore samples were pyroxene-rich (73-85 wt.% combined orthopyroxene and clinopyroxene, Table I), with lesser amounts of plagioclase (2.7-7.1 wt.%). Ores 1 and 2 had similar contents of phyllosilicate alteration minerals (approximately 10 wt.%) whereas ore 3 had the lowest phyllosilicate content (5.4 wt.%). In all three ores, talc was the most common phyllosilicate alteration mineral. High phyllosilicate concentrations (particularly talc, chlorite, and serpentine) are indicators of hydrothermal alteration in these magmatic Ni-Cu-PGE ores (Li et al., 2008; Chaumba, 2017).
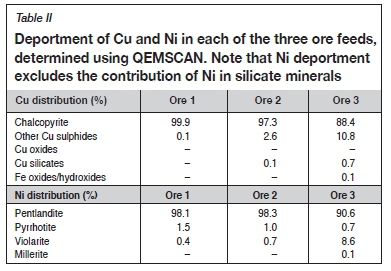
Ore 1 had the highest BMS content (1.5 wt.%), with ores 2 and 3 containing similar but lesser amounts (0.9 wt.%) as shown in Table I. One of the hallmarks of oxidation is the formation of secondary sulphides, hence the deportment of Cu and Ni was further investigated. Chalcopyrite was the major host of Cu in all three ores (Table II), although in ores 2 and 3 secondary sulphides comprising chalcocite and covellite hosted 2.6% and 10.8% of the Cu respectively. Pentlandite was the major host of nickel in all three ores, with some minor pyrrhotite-hosted Ni (Table II). Ore 3, however, notably contained some Ni hosted in the secondary sulphide violarite (8.6%). Pyrrhotite was the major Fe sulphide in ores 1 and 2, whereas pyrite was more common in ore 3 (Table I). The Fe oxide content of ore 3 was almost double that of the other two samples (Table I). This is consistent with the reddish colour of this sample, which suggests a degree of incipient oxidation.
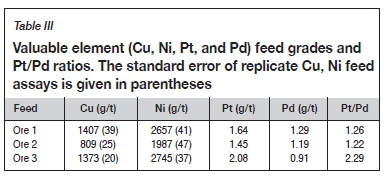
To further distinguish differences between the three samples their feed elemental Cu, Ni, Pt ,and Pd grades were analysed, as summarized in Table III. The Pt feed grade of ore 3 was higher than that of ores 1 and 2, whereas its Pd grade was lower, resulting in a Pt/Pd ratio of 2.3 for the former compared to approximately 1.2 for the latter samples. A higher Pt/Pd ratio is one of the distinguishing characteristics of an oxidized PGE ore due to the mobilization of Pd (Evans, 2002; Locmelis, Melcher, and Oberthür, 2010; Oberthür et al., 2013). The Pt/Pd ratio and composition of ore 3 are similar to those reported by Kraemer et al. (2015) and Oberthür et al. (2013) for oxidized PGE ores from the Great Dyke.
BMS and PGM mineralogy, liberation, and association
An understanding of the BMS and PGM mineralogy, liberation, and associations is an essential component in exploring the mineralogical variability of these ores, as well as facilitating the interpretation of the flotation behaviour. Liberation is defined here as particles comprising greater than 90% of the mineral of interest by particle area and is reported for the BMS as a group rather than for discrete Cu or Ni sulphides because of the bulk sulphide flotation process used. The liberation of BMS is similar for ores 1 and 2 (almost 90%) and only slightly lower in ore 3 (86% liberated, Figure 2). Unliberated BMS in all three ores show some association with pyroxene, the phyllosilicate alteration minerals, and Fe oxides. In ore 3 the association with Fe oxides is slightly greater (7%) than in ores 1 and 2 (approx. 2%), which is another indicator of incipient oxidation (Figure 2).
The mineralogy of the discrete PGMs analysed for these ores is summarized in Table IV, with results reported in terms of both the distribution by area and grain count percentage. The PGE bismuthotellurides are the dominant mineral group in all three ores, with moncheite-maslovite and kotulskite representing the main phases. Overall, the PGM mineralogy of ores 2 and 3 is more similar to one another than to ore 1 (especially with respect to the PGE-arsenides). This is likely because ores 2 and 3 were sampled from the same operation (and the same chamber of the MSZ). The presence of the PGE sulphides and PGE arsenides is also significant in ore 1 (5.2 and 31.7% respectively by area). For ore 2, gold and the PGE sulphides are more common (8.3 and 23.7% respectively by area). In ore 3, however, PGE alloys are the most dominant group after the PGE bismuthotellurides (25.1% by area) due to the presence of a single approx. 30 ym ferroplatinum particle skewing the distribution. On a grain count basis, however, the PGE sulphides, PGE arsenides, as well as the PGE alloys are the most common PGM groups in ore 3 after the PGE bismuthotellurides.
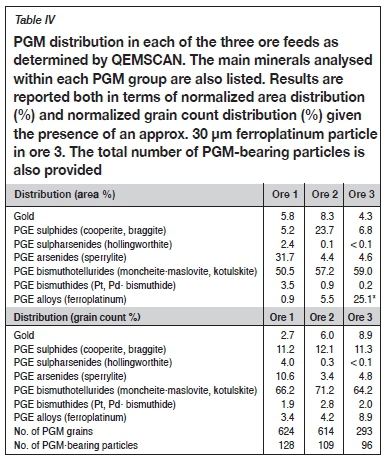
The PGMs in ores 1 and 2 are 55 and 48% liberated respectively, compared to ore 3 where the PGM liberation is 74% (Figure 3). PGM liberation is defined here as greater than 80% of the mineral of interest by area consisting of PGMS. In the case of ore 3, the data reported excludes a single liberated ferroplatinum nugget (that would increase the liberation up to 83%). When considering the effective PGM liberation (liberated PGMs plus PGMs in liberated BMS) the difference in the liberation between ores 1 and 2 compared with ore 3 is only around 14%. The balance of the unliberated PGMs are mostly associated with the silicates and phyllosilicates (talc, chlorite, serpentine) either as enclosed or attached grains. The relative differences in PGM grain size distribution are consistent with the trends in liberation data; the d50of ores 1, 2, and 3 being 8.1, 7.1, and 9.3 urn, respectively (the most liberated ore has the coarsest grain size distribution).
Batch flotation performance
The flotation performance of the ores was assessed by comparing the Cu, Ni, Pt' and Pd recoveries with the mass recovery of solids. This was followed by an assessment of the mass of naturally floating gangue as well as the key indicators of valuable mineral recoveries using Cu, Ni, Pt, and Pd.
Grade and recovery
The Cu, Ni, Pt, and Pd recoveries from the batch flotation tests at 100 g/t depressant are shown in Figure 4. Grades and recoveries at 500 g/t depressant are not reported here, since these high depressant dosages were used purely for the calculation of floating gangue and are not representative of actual plant operating conditions (where such high depressant dosages are extremely unlikely to be used on a single rougher bank, which the batch flotation test represents). The highest Cu and Ni recoveries were obtained for ore 1 (87.3 and 67.0% respectively), whereas for Pt and Pd, the recoveries were similar for ores 1 and 2 (approx. 86 and 85% respectively). Consistently lower recoveries were obtained in the flotation of ore 3 for all four metals (Figure 4). This is despite ore 3 having the highest head grades of Pt and Ni (Table II) - it is generally accepted that flotation recovery increases with increasing head grade (Napier-Munn, 1998).
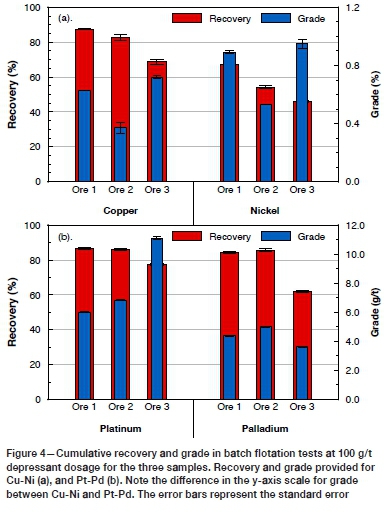
These differences in recovery can be correlated with the observations from the characterization of the flotation feed material, which indicated the incipient oxidation of the BMS in ore 3 - a red colour coupled with the slightly higher Fe oxide/hydroxide content in the feed as well as slightly higher association of unliberated BMS with the Fe oxide/hydroxides. Although the association of the BMS with the Fe oxide/ hydroxides may quantitatively be very low, the possibility that surface rims may occur as nano-coatings on the sulphide minerals (not visible using QEMSCAN) significantly reducing floatability, should not be discounted. The presence of secondary Cu minerals (chalcocite, covellite) and Ni sulphides (violarite) is another indicator of the incipient oxidation of ore 3. These secondary copper minerals are not readily floatable with the current SIBX collector (Grobler, Sondashi, and Chidley, 2005; Lee et al., 2009; Lotter, Bradshaw, and Barnes, 2016).
In the PGM mineralogical characterization, however, the indications of oxidation were not as clear in ore 3 (other than the increased Pt/Pd ratio of the feed, Table II) as they were for the BMS. The PGM mineralogy reported here (Table IV) for all three ores is more similar to that reported for pristine sulphide ores (where the PGE bismuthotellurides are the dominant phases) than for the oxidized ores considered by Locmelis et al. (2010) and Oberthür et al. (2013), in which PGE oxides/hydroxides were recognized. The PGE deportment of the Great Dyke ores, however, is known not to be limited to discrete PGMs, and Oberthür et al. (2013) estimated that approximately 95% of the Pt budget is hosted by discrete PGMs, with the balance in solid solution with the BMS in pristine sulphide ores. For Pd however, only approximately 20% of the Pd was estimated to be hosted in discrete PGMs, and the balance in solid solution in pentlandite. In this case, the comparative decoupling of the trends in Pt and Ni recovery for ore 3 relative to the behaviour of ores 1 and 2 may be a further indication of incipient oxidation and mobilization of Pd in ore 3. In terms of PGM liberation, the fact that the PGM grain size and liberation were greatest in ore 3, yet the Pt and Pd recoveries were the lowest, also suggests that some of the PGE may be hosted in some of the more exotic phases (e.g. PGE oxides/hydroxides) that are not recoverable with thiol collectors. For ores 1 and 2 however, the similarity in Pt-Pd recoveries was consistent with the liberation data when considering the combined categories of liberated PGMs and PGMs hosted by liberated BMS (Figures 3, 4).
Ore 3, however, yielded the highest flotation concentrate grades for Cu, Ni, and Pt. Little difference in Pd concentrate grade was observed between the three ores (Figure 4). The associated increase in grade that usually accompanies a decrease in mass pull is noted in Figures 4 and 5, resulted in dilution of the concentrate grade for ores 1 and 2. A second point worth noting is the differences in the mass pull between the ores for the same cell operating and reagent conditions (Figure 5). This suggests that if operating conditions were optimized to increase the mass pull for ore 3, increases in recovery may be realized, although this would be at the expense of grade. To further understand the mechanisms for concentrate dilution in these ores, the role of the naturally floating gangue should be explored.
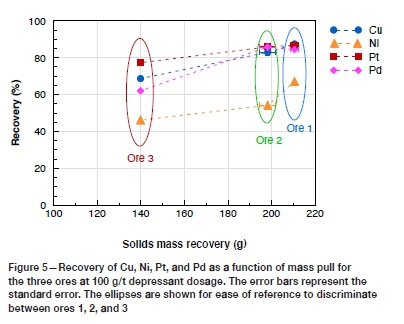
Naturally floating gangue
Naturally floating gangue in PGE ores ideally represents only hydrophobic minerals such as talc which are expected to be recovered during flotation. However, in practice, naturally floating gangue in these ores typically consists of composite particles of talc and orthopyroxene. These particles were formed through the hydration of orthopyroxene to talc. Ore 1 had the greatest amount of naturally floating gangue, followed by ore 2 and ore 3 respectively (Figure 6). This was also the order of decreasing solids recovery. Based on comparison of the feed bulk mineralogy, the initial expectation would be that ore 2 contained the greatest amount of naturally floating minerals due to its higher orthopyroxene content (with a talc content comparable to that of ore 1), although this was not the case.
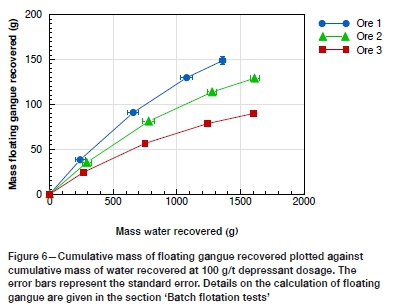
To further understand why ore 1 yielded the greatest mass of floating gangue, a mineralogical analysis of the naturally floating material in the +38 -75 μm size class of the concentrate was carried out. In order to aid the interpretation, the results reported in Table V are shown as absolute concentrate masses obtained during flotation tests on the 1 kg feed sample. It is noteworthy that the mass of talc recovered from ore 1 was the lowest, despite this ore containing the highest mass of naturally floating gangue. Analysis of the mineral liberation and association showed that the talc in the ore 1 concentrate was mostly locked (less than 30% liberated, Figure 7) and strongly associated with pyroxene (Figure 8). The associated talc grain size distribution within the same size fraction showed that the talc in the ore 1 concentrate was more finely disseminated than in ore 2 (Figures 9, 10).
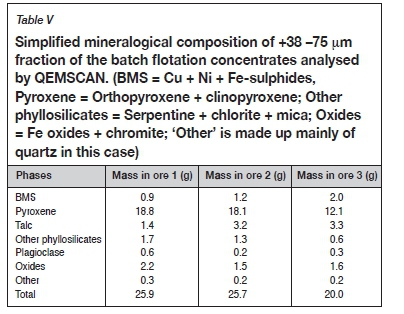
This difference is likely due to the difference in the extent of the hydrothermal hydration (alteration) reaction, with coarser grained talc indicating more extensive alteration (Figure 10). The results indicate that even low amounts of talc finely dispersed in pyroxene particles lead to the inadvertent flotation and recovery of the pyroxene resulting in greater amounts of naturally floating gangue. In this case, a simple upfront quantification of talc mineral content may not necessarily be entirely sufficient to predict the amount of naturally floating gangue. Mineral textural information that quantifies the talc liberation, grain size distribution, and relationship to orthopyroxene will be more revealing.
Conclusions
The primary objective of this investigation is to understand the effects of mineralogical variability due to alteration on the flotation response of three Great Dyke PGE ore samples. To do so involves decoupling the different types of alteration that these ores have undergone, as well as articulating what the indicators of alteration are through quantitative mineralogical analysis. Although on first inspection mineralogical analyses did not reveal any major differences between the three ores studied, closer investigation allowed those subtle differences that have a significant effect on flotation performance to be further identified.
Alteration through oxidation of the valuable BMS and PGMs resulted in significantly lower flotation recoveries of Cu, Ni, Pt, and Pd in ore 3 compared to ores 1 and 2. Evidence of incipient oxidation was more readily observed in the BMS assemblage (association of unliberated BMS with Fe oxide/hydroxides, presence of minor secondary Cu and Ni-sulphides) than the PGM assemblage. Further investigation of the PGM assemblage with improved sampling statistics and instrumental analysis would ideally be required to visualize the effect of the incipient oxidation and confirm the PGE distribution in both conventional and unconventional phases (e.g. PGE oxides/hydroxides or PGE silicates).
The alteration of orthopyroxene to talc through hydration resulted in significant differences in mass pull and dilution of concentrate grade through the inadvertent recovery of naturally floating gangue comprising composite orthopyroxene and talc particles. The greatest amount of naturally floating gangue was recovered from ore 1, despite it not having the highest amount of talc in the feed. However, talc in ore 1 was the least liberated, with the finest grain size distribution (more finely disseminated). In this case quantification of the talc mineral textural characteristics would be required to predict which ore types will need active grade management through the use of depressant.
Oxidation and hydration reactions are not necessarily mutually exclusive in these magmatic Ni-Cu-PGE ores, although one may be more dominant, giving rise to ore variability. Continual assessment of the mineralogy of both the valuable and gangue fractions of the ore is needed to enable a better understanding of the repercussions and application of the appropriate measures to ensure consistent grades and recoveries. Such measures could include, but are not limited to, ore blending strategies, use of different reagent suites, optimized mass pull, and even the design of reagents that target the more oxidized valuable minerals.
Acknowledgements
Our appreciation goes to Senmin (Pty) Ltd, the National Research Foundation (NRF) of South Africa, and the University of Cape Town for their support and funding of this research. Our thanks go to Mimosa Mining Company (Pvt) Ltd and Zimplats Holdings Ltd for the ore samples. Useful discussions with Thomas Oberthür are also acknowledged. Grateful thanks to staff in the Centre for Minerals Research for their assistance, especially Gaynor Yorath and Keshree Pillay. This work is based on the research supported in part by the National Research Foundation of South Africa (Grant Number 86054). Any opinions, findings, and conclusions or recommendations expressed in any publication generated by NRF-supported research is that of the authors, and the NRF accepts no liability whatsoever in this regard.
Author contributions
Conceptualization - MB, BM; Methodology - DZ, BM, MT, MB; Formal analysis - DZ, BM, MT, MB; Writing of original draft - DZ, MB; Review and editing - BM, MT, MB; Supervision - BM, MT, MB; Funding acquisition - MB.
Supplementary data
The raw data is available at: https://doi.org/10.25375/uct.13580378
References
Bakker, C.W., Meyer, C.J., and Deglon, D.A. 2010. The development of a cavern model for mechanical flotation cells. Minerals Engineering, vol. 23, no. 11-13. pp. 968-972. doi: 10.1016/j.mineng.2010.03.016 [ Links ]
Baum, W. 2014. Ore characterization, process mineralogy and lab automation: A roadmap for future mining. Minerals Engineering, vol. 60. pp. 69-73. doi: 10.1016/j.mineng.2013.11.008. [ Links ]
Becker, M., Harris, P.J., Wiese, J.G., and Bradshaw, D.J. 2009. Mineralogical characterisation of naturally floatable gangue in Merensky Reef ore flotation. International Journal of Mineral Processing, vol. 93, no. 3-4. pp. 246-255. doi: 10.1016/j.minpro.2009.10.004 [ Links ]
Becker, M., Yorath, G., Ndlovu, B., Harris, M., Deglon, D., and Franzidis, J.P. 2013. A rheological investigation of the behaviour of two Southern African platinum ores. Minerals Engineering, vol. 49. pp. 92-97. http://www.sciencedirect.com/science/article/pii/S0892687513001489 [ Links ]
Becker, M., Wiese, J., and Ramonotsi, M. 2014. Investigation into the mineralogy and flotation performance of oxidised PGM ore. Minerals Engineering. vol. 65. pp. 24-32. http://www.sciencedirect.com/science/article/pii/S0892687514001198 [ Links ]
Bremmell, K.E., Fornasiero, D., and Ralston, J. 2005. Pentlandite-lizardite interactions and implications for their separation by flotation. Colloids and Surfaces A: Physicochemical and Engineering Aspects, vol. 252, no. 2-3. pp. 207-212. doi: http://dx.doi.org/10.1016/j.colsurfa.2004.10.100 [ Links ]
Burdukova, E., Becker, M., Bradshaw, D.J., and Laskowski, J.S. 2007. Presence of negative charge on the basal planes of New York talc. Journal of Colloid and Interface Science, vol. 315. pp. 337-342. [ Links ]
Chaumba, J.B. 2017. Hydrothermal alteration in the Main Sulfide Zone at Unki Mine, Shurugwi Subchamber of the Great Dyke, mineral chemistry. Minerals, vol. 7. p. 127. doi: 10.3390/min7070127 [ Links ]
Chetty, D., Carelse, C., Mogoru, J., Pebane, M., and Ngoasheng, M. 2018. Mineralogical factors affecting platinum group element recovery from oxidised chromite of the middle group, western Bushveld Complex. Proceedings of Process Mineralogy '18, Cape Town. Minerals Engineering International, Falmouth, UK. [ Links ]
Coghill, B.M. and Wilson, A.H. 1993. Platinum-group minerals in the Selukwe Subchamber, Great Dyke, Zimbabwe: Implications for PGE collection mechanisms and post-formational redistribution. Mineralogical Magazine, vol. 57. pp. 613-634. [ Links ]
Dzingai, T. 2017. A process mineralogical study on the effect of alteration on the flotation of Great Dyke platinum group element (PGE) ores. MSc thesis, University of Cape Town. [ Links ]
Evans, D. 2002. Potential for bulk mining of oxidized platinum-group element deposits. Applied Earth Science, vol. 111. pp. 81-86. [ Links ]
Evans, D.M., Buchanan, D.I., and Hall, G.E.M. 1994. Dispersion of platinum, palladium and gold from the Main Sulphide Zone, Great Dyke, Zimbabwe. Transactions of the Institution of Mining and Metallurgy. Section B: Applied Earth Science. vol. 103 (Jan-Apr). pp. 57-67. [ Links ]
Farrokhpay, S. 2012. The importance of rheology in mineral flotation: A review. Minerals Engineering, vol. 36-38. pp. 272-278. doi: 10.1016/j.mineng.2012.05.009 [ Links ]
Farrokhpay, S., Filippov, I., and Fornasiero, D. 2020. Flotation of fine particles: A review. Mineral Processing and Extractive Metallurgy Review. doi: 10.1080/08827508.2020.1793140 [ Links ]
Grobler, W.A., Sondashi, S., and Chidley, F. 2005. Recent developments in flotation reagents to improve base metal recovery. Proceedings of the 3rd Southern African Base Metals Conference. Kitwe, Zambia. Southern African Institute of Mining and Metallurgy, Johannesburg. pp. 185-190. [ Links ]
Guilbert, J. and Park, C. 1986. The Geology of Ore Deposits. W.H. Freeman, New York. [ Links ]
Jones, R. 2005. An overview of Southern African PGM smelting. Proceedings of Nickel and Cobalt2005: Challenges in Extraction and Production, Calgary, Alberta, 21-24 August. Donald, J. and Shoneville, R. (eds). Canadian Institute of Mining, Metallurgy and Petroleum, Montreal. pp. 147-178. [ Links ]
Khraisheh, M., Holland, C., Creany, C., Harris, P., and Parolis, I. 2005. Effect of molecular weight and concentration on the adsorption of CMC onto talc at different ionic strengths. International Journal of Mineral Processing. vol. 75. pp. 197-206. [ Links ]
Kraemer, D., Junge, M., Oberthür, T., and Bau, M. 2015. Hydrometallurgy improving recoveries of platinum and palladium from oxidized platinum-group element ores of the Great Dyke, Zimbabwe, using the biogenic siderophore Desferoxamine B. Hydrometallurgy. vol. 152. pp. 169-177. doi: 10.1016/j.hydromet.2015.01.002 [ Links ]
Lee, K., Archibald, D., McLean, J., and Reuter, M. 2009. Flotation of mixed copper oxide and sulphide minerals with xanthate and hydroxamate collectors. Minerals Engineering, vol. 22. pp. 395-401. [ Links ]
Li, C., Ripley, E.M., Oberthür, I, Miller, J.D., and Joslin, G.D. 2008. Textural, mineralogical and stable isotope studies of hydrothermal alteration in the main sulfide zone of the Great Dyke, Zimbabwe and the precious metals zone of the Sonju Lake Intrusion, Minnesota, USA. Mineralium Deposita, vol. 43. pp. 97-110. [ Links ]
Liddell, K.S. and Adams, M.D. 2012. Kell hydrometallurgical process for extraction of platinum group metals and base metals from flotation concentrates. Journal of the Southern African Institute ofMining and Metallurgy, vol. 112, no. 1. pp. 31-36. [ Links ]
Locmelis, M., Melcher, F., and Oberthür, T. 2010. Platinum-group element distribution in the oxidized Main Sulfide Zone, Great Dyke, Zimbabwe. Mineralium Deposita, vol. 45. pp. 93-109. doi: 10.1007/s00126-009-0258-y [ Links ]
Lotter, N.O., Bradshaw, D.J., Becker, M., Parolis, L.A.S., and Kormos, L.J. 2008. A discussion of the occurrence and undesirable flotation behaviour of orthopyroxene and talc in the processing of mafic deposits. Minerals Engineering, vol. 21, no. 12-14. doi: 10.1016/j.mineng.2008.02.023 [ Links ]
Lotter, N.O., Kormos, I., Oliveira, J., Frangomeni, D., and Whiteman, E. 2011. Modern process mineralogy: Two case studies. Minerals Engineering, vol. 24. pp. 638-650. [ Links ]
Lotter, N.O., Bradshaw, D.J., and Barnes, A.R. 2016. Classification of the major copper sulphides into semiconductor types, and associated flotation characteristics. Minerals Engineering. vol. 96-97. pp. 177-184. doi: 10.1016/j.mineng.2016.05.016 [ Links ]
Lotter, N.O., Baum, W., Reeves, S., Arrué, C., and Bradshaw, D.J. 2018. The business value of best practice process mineralogy. Minerals Engineering, vol. 116, April. pp. 226-238. doi: 10.1016/j.mineng.2017.05.008 [ Links ]
Mpinga, C.N., Eksteen, J.J., Aldrich, C., and Dyer, I. 2018. Atmospheric leach process of high-chromitite PGM-bearing oxidized mineralized ore through a singlestage and two-stage techniques. Minerals Engineering, vol. 125. pp. 165-175. doi:10.1016/j.mineng.2018.05.007. [ Links ]
Mudd, G.M. 2012. Key trends in the resource sustainability of platinum group elements. Ore Geology Reviews, vol. 46. pp. 106-117. doi: 10.1016/j.oregeorev.2012.02.005 [ Links ]
Napier-Munn, T.J. 1998. Analysing plant trials by comparing recovery-grade regression lines. Minerals Engineering, vol. 11. pp. 949-958. [ Links ]
Ndlovu, B., Forbes, E., Farrokhpay, S., Becker, M., Bradshaw, D., and Deglon, D. 2014. A preliminary rheological classification of phyllosilicate group minerals. Minerals Engineering, vol. 55. pp. 190-200. http://www.sciencedirect.com/science/article/pii/S0892687513001921 [ Links ]
Oberthür, T., Weiser, T.W., Gast, I., and Kojonen, K. 2003. Geochemistry and mineralogy of platinum-group elements at Hartley Platinum Mine, Zimbabwe: Part 2: Supergene redistribution in the oxidized Main Sulfide Zone of the Great Dyke, and alluvial platinum-group minerals. Mineralium Deposita, vol. 38, no. 3. pp. 344-355. doi: 10.1007/s00126-002-0337-9 [ Links ]
Oberthür, T., Melcher, F., Buchholz, P., and Locmelis, M. 2013. The oxidized ores of the Main Sulphide Zone, Great Dyke, Zimbabwe: Turning resources into minable reserves-mineralogy is the key. Journal of the Southern African Institute ofMining and Metallurgy, vol. 113. pp. 191-201. [ Links ]
Patra, P., Nagaraj, D.R., and Somasundaran, P. 2011. Impact of pulp rheology on selective recovery of value minerals from ores. Proceedings of the XII International Seminar on Mineral Processing Technology (MPT-2011), Udaipur, India. pp. 1223-1231. http://eprints.nmlindia.org/4122 [ Links ]
Powell, M.S. 2013. Utilising orebody knowledge to improve comminution circuit design and energy utilisation. Proceedings of the 2nd AUSIMM International Geometallurgy Conference, Brisbane, Australia. Australasian Institute of Mining and Metallurgy, Melbourne. pp. 27-35. [ Links ]
Robb, I. 2005. Introduction to Ore Forming Processes. Blackwell, Malden. [ Links ]
Savassi, O.N. 1998. Direct estimation of the degree of entrainment and the froth recovery of attached particles in industrial flotation cells. PhD thesis, University of Queensland. [ Links ]
Sefako, R., Sekgarametso, K., and Sibanda, V. 2017. Potential processing routes for recovery of platinum group metals from southern African oxidized PGM ores: A review. Journal of Sustainable Metallurgy, vol. 3, no. 4. pp. 797-807. doi: 10.1007/s40831-017-0146-0 [ Links ]
Sefako, R., Sibanda, v., and Sekgarametso, K. 2019. PGM extraction from oxidized ores using flotation and leaching. Journal of the Southern African Institute of Mining and Metallurgy, vol. 119, no. 11. pp. 929-936. doi: 10.17159/24119717/287/2019 [ Links ]
Shabalaia, N.Z.P., Harris, M., Leal Filho, LS., and Deglon, D.A. 2011. Effect of slurry rheology on gas dispersion in a pilot-scale mechanical flotation cell. Minerals Engineering, vol. 24, no. 13. pp. 1448-1453. doi: 10.1016/j.mineng.2011.07.004 [ Links ]
Skinner, B. 1976. A second Iron Age ahead? The distribution of chemical elements in the earth's crust sets natural limits to man's supply of metals that are much more important to the future of society than limits on energy. American Scientist, vol. 64, no. 3. pp. 258-269. [ Links ]
Van der Plas, I. and Tobi, A. 1965. A chart for judging the reliability of point counting results. American Journal of Science, vol. 263. pp. 87-90.x [ Links ]
Wang, I., Peng, Y., Runge, K., and Bradshaw, D. 2015. A review of entrainment: Mechanisms, contributing factors and modelling in flotation. Minerals Engineering, vol. 70. pp. 77-91. doi: 10.1016/j.mineng.2014.09.003 [ Links ]
Wiese, J. 2009. Investigating depressant behaviour on the flotation of selected Merensky ores. MSc thesis, University of Cape Town. [ Links ].
Wiese, J.G., Harris, P.J., and Bradshaw, D.J. 2005. The influence of the reagent suite on the flotation of ores from the Merensky Reef. Minerals Engineering, vol. 18. pp. 189-198. [ Links ]
Wilson, A.H. and Prendergast, M.D. 2001. Platinum-group element mineralisation in the Great Dyke, Zimbabwe, and its relationship to magma evolution and magma chamber structure. South African Journal of Geology, vol. 104. pp. 319-342. [ Links ]
Zhang, M. and Peng, Y. 2015. Effect of clay minerals on pulp rheology and the flotation of copper and gold minerals. Minerals Engineering, vol. 70. pp. 8-13. doi: 10.1016/j.mineng.2014.08.014. [ Links ]
Zimplats. 2019. Integrated Annual report. [ Links ]
 Correspondence:
Correspondence:
M. Becker
Email: megan.becker@uct.ac.za
Received: 18 Jan. 2021
Revised: 15 Jul. 2021
Accepted: 22 Jul. 2021
Published: September 2021














