Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.121 n.9 Johannesburg Sep. 2021
http://dx.doi.org/10.17159/2411-9717/2021
PRESIDENTIAL ADRESS
Presidential Address: Thoughts on the value of history
I.J. GeldenhuysI, II
IMintek, Pyrometallurgy Division, South Africa. https://orcid.org/0000-0002-5995-2722
IIIsabel Geldenhuys Consulting, South Africa
SYNOPSIS
South Africa's mining and metals industries have an illustrious history dating back to the Late Iron Age. We live in a complex and data-intensive world that has already fundamentally changed how we work and live. The outbreak of the global COVID-19 pandemic in 2020 resulted in immeasurable suffering worldwide, but the crisis also accelerated many technological developments and altered the way we think about, and experience, technology and work. Problem-solving requires new and rapidly changing skills to manage the data tsunami. Whether at the rock face or on the processing plant, the fundamentals have not changed, although how we interact with minerals and their properties has changed dramatically. Sustainable processing and design are non-negotiable if we genuinely want to achieve the aim of the cradle-to-cradle principle. As we venture forth, what can scientists, engineers, technologists, and mathematicians learn from history?
History is data presented in a context; sometimes, the context is also important. We exist in an age of data, with most of the world's data created in only the past two years. History can enhance our understanding of the present and enhance our future outcomes. Lessons from history can help provide insights into making ethical and sustainable choices related to technology or engineering in the mining and metallurgical industry.
Shifting the perspective of mining and metallurgy professionals from a narrow focus on complex technical solutions towards a broader context for problem-solving and designs that includes the entire ecosystem is crucial. In other words, using or reflecting on historical process development is, at its core, systems thinking.
Keywords: titaniferous magnetite, open arc smelting, historical perspective, systems thinking, project development.
Introduction
South Africa's mining and metallurgical industry have an illustrious history dating back to the Late Iron Age. The mining and metallurgical professionals of today live in a complex and data-intensive world with challenging technical and ethical demands that increasingly require multi- and transdisciplinary approaches. The outbreak of the global COVID-19 pandemic In 2020 resulted in immeasurable suffering, but the crisis also accelerated the adoption of new ways of working, and changed how we think about, and even how we experience, technology and work. Amid overwhelming trends, events, challenges, and changes, what can we learn from history?
The emergence of Big Data and digitalization
The emergence of data as a megatrend has already fundamentally changed society, as we now easily access information digitally via smartphones and other devices. No longer is recorded knowledge found in a single location, such as the Library of Alexandria; today our information is increasingly decentralized, with digital information being created, analysed, and stored at an astonishing rate. Considering that 90% of the world's data has been produced in just the last two years, it is best described as an explosion of information, known as 'Big Data'. This trend is transforming the world around us, with data consisting of a greater variety, increased volume, and created at an increasing rate. As one of the megatrends of modern society, data is constantly changing our understanding of the world. Not only is new data created at an unprecedented rate, similarly changed is our ability to access historical data, as data and information storage is no longer confined to brick-and-mortar libraries.
In the context of the mining and metallurgy ecosystem, history is captured in publications such as journal and conference papers, textbooks, theses, and dissertations, and more recently recorded via webinars and digital and hybrid conferences. Recorded presentations will likely become a tangible and searchable part of our digital libraries and knowledge base. For now, we still have an enormous collection of valuable information to mine.
Relevant knowledge connects information to help us understand things. Connectedness is defined by the Oxford Dictionary as 'a state of being joined or linked'. Through a literature review dating back to the Late Iron Age, the history of processing titaniferous magnetite is presented here to illustrate the value of historical publications. An atypical literature review highlighting various case studies and historical perspectives within the context of the processing of titaniferous magnetite is used to illustrate the value of historical scientific publications.
Brief overview of the nature and occurrence of titaniferous magnetite
Titaniferous magnetite (also known titanomagnetite) deposits are numerous and significant in size. Titanomagnetite is generically defined as magnetite with more than 1% by mass titanium dioxide (TiO2) and is typically vanadium-bearing. The iron and titanium in titanomagnetite occur as a mixture of magnetite (Fe3O4) and ilmenite (FeTiO3) with magnetite intergrown or spatially associated with ilmenite, which prevents clean separation of the magnetite and ilmenite via physical beneficiation (Fischer, 1975; Rohrmann, 1985; Henry et al., 1987; Taylor et al., 2005; Peck and Huminicki, 2016).
Deposits of titanomagnetite are found in significant numbers throughout the world. The most important deposits are in the Panzhihua Complex in Sichuan Province, China (Pang et al., 2010), the Windimurra Complex in Australia (Ivanic et al., 2010), the Kachkanar deposit in Russia (Smirnov, Tret'yakov, and Gladyshev, 2000), and the vanadiferous titanomagnetite of the Bushveld Complex of South Africa. Chen and Chu (2014) report that titaniferous magnetite deposits account for about 90% of China's titanium reserves. However, during pyrometallurgical processing to extract iron and vanadium via blast furnace smelting the majority of the titanium is lost to the discarded slag. The Kachkanar deposit in Russia is also primarily exploited for its iron content using blast furnaces, with vanadium as a co-product (Badmatsyrenova and Orsoev, 2005).
South Africa's Bushveld Complex is uniquely rich in vanadium and titanium, and both pyrometallurgical means (smelting) and direct extraction processes (roast-leach) have been used to extract vanadium (Cawthorn et al., 2005; US Geological Survey, 2021). Detailed reviews of the various extractive practices for titaniferous magnetite, current and historical, are described by (Taylor et al. (2005) and Geldenhuys, Akdogan, and Reynolds (2021).
Connecting historical perspectives
The value of historical perspectives is presented as a connected journey through the history, challenges, and fundamental nature of smelting titaniferous magnetite. The review is drawn from a wide variety of scientific publications, illustrating the value of a multidisciplinary perspective. History provides us with a rich collection of case studies and options, and can greatly enhance our understanding of the present.
An introduction to Dr William Bleloch
In 2015, EVRAZ Highveld Steel and Vanadium (Highveld Steel) closed down after about 50 years of processing titaniferous magnetite to recover primarily iron and vanadium (EVRAZ Highveld Steel and Vanadium, 2015). The associated job losses were devastating for the economy of the region, but South Africa also lost a particularly unique smelting capacity. Highveld Steel was one of only two processing plants in the world where vanadium-bearing titaniferous iron ore was processed using electric smelting technology (Rohrmann, 1985; Steinberg, Geyser, and Nell, 2011). As reported by Steinberg, Geyser, and Nell (2011) the Highveld Steel process flow sheet was 'developed in the early 1960s, based on the research done by Dr William Bleloch in 1949. He showed that Bushveld complex magnetite ore could be melted using submerged-arc furnace (SAF) technology in a process that controls the carbon addition to selectively reduce vanadium and iron while leaving titania dissolved in the slag.' This brief historical perspective presented references the well-known paper by Rohrmann (1985), titled 'Vanadium in South Africa'. In this exceptional review paper, the history and development of Highveld Steel is described in more detail, referencing Dr Bleloch's 1949 paper 'The electric smelting of iron ores for production of alloy irons and steel and recovery of chromium and vanadium'. The work that led to the flow sheet being implemented at Highveld Steel in the 1960s spans a period of over 30 years (Bleloch, 1949),culminating in the establishment of Highveld Steel.
Dr Bleloch's role in the establishment of Highveld Steel cannot be overstated, and his work contributed greatly to the establishment of the South African submerged-arc furnace smelting industry. The research and development work on which Highveld Steel's process was based aimed to unlock the potential of the Bushveld Complex. Dr Bleloch's belief was that there is scope for the electric smelting process in Southern Africa because of the existence of workable reserves of chromium and vanadium bearing iron ores' (Bleloch, 1949), and he viewed electric smelting as a unique opportunity for the country to beneficiate its rich mineral wealth. Dr Bleloch was elected as the President of the South African Institute of Mining and Metallurgy (SAIMM) in 1956, and his Presidential Address was widely acclaimed for his visionary presentation of the prospects for electric smelting in South Africa. In his own words, an extract from his Brigadier Stokes Memorial Award citation in 1981: 'It is not really in the research laboratory that these problems are solved. It is in the minds of men that they are solved by the ability of mankind to think' SAIMM, 1981).
Dr Bleloch's accomplishments and his in-depth understanding of the fundamentals of smelting, techno-economics, and insights into the potential of South Africa's resources beyond mining are inspirational. His 1949 paper, despite being published over 70 years ago, is still a comprehensive and valuable reference point, which describes in detail all aspects of the journey that led to the establishment of Highveld Steel, more than a decade after its publication. The paper features aspects of fundamentals and thermodynamics, technology perspectives, as well as economic considerations, and continues to offer valuable insights into the mechanics of project development, showing that historical scientific publications can offer a wealth of knowledge to a willing reader.
The unusual history of Lowveld ironmaking
Archaeometallurgy is described by Killick and Finn (2012) as an interdisciplinary and international field of study that examines all aspects of the production, use, and consumption of metals in preindustrial societies. The story of titaniferous magnetite smelting in South Africa is an example of the value of interdisciplinary studies and offers great insights. The history of ironmaking in South Africa is particularly relevant and interesting, and an excellent reminder that the fundamental principles do not change with time.
The northern Lowveld of South Africa has a rich archaeological record of mining and smelting activities, with siteS estimated to date back to 350 CE. Evidence of ironmaking agriculturalists is found in the Tzaneen area. Around Phalaborwa, archaeologists have logged high concentrations of mining and smelting sites dating back to about 1000 CE, likely due to the nearly inexhaustible quantities of accessible magnetite outcrops found in this region. It is likely that these early settlers were not attracted to the region for the abundant iron deposits, but they needed iron and through experimentation adapted their bloomery smelting practices to use the unusual iron ore of the region to produce the metal they required (Miller, Killick, and van der Merwe, 2001; Killick and Miller, 2014).
Figure 1 shows a photograph of a typical bloomery furnace from the Lowveld region (around Phalaborwa) (Killick and Miller, 2014). In bloomery furnaces, the ironworkers converted iron ore directly into a bloom' of low-carbon iron, with the raw materials and fuel loaded into the furnace and a blast of air provided by some form of bellows. When the bowl was filled with slag, the process was halted and both the bloom of iron and slag were broken out (Brothers, 2001). The modern blast furnace still uses principally the same concept of blasting air through tuyeres to activate the fuel, typically coke. The scale and efficiency of modern blast furnaces are many orders of magnitude removed from the bloomery furnaces found in the Lowveld, but the principal metallurgical concepts are still valid.

Records of African bloomery smelters show that most smelting sites favoured lateritic iron ores, which are dominated by iron hydroxide minerals. Laterite ores are not found in the Lowveld, but the abundance of magnetite and titaniferous magnetite created a unique scenario for the ironworkers. Even in the global archaeological context, magnetite ores were rarely used in bloomery furnaces as the reduction of lumps of magnetite proceeds much slower than that of similarly sized lumps of hydroxide ores. For effective reduction of a magnetite ore in the short stack of the bloomery furnaces (typically only about 1 m high), smaller particle sizes were required. The Lowveld ironworkers adapted to their circumstances by grinding and crushing the massive magnetite ore found in the region. Evidence of crushing of the magnetite is scattered around Phalaborwa, where grinding hollows can be found from which magnetite grains can reportedly still be extracted with a magnet.
A unique characteristic of the magnetite from the northern Lowveld region is the high titania content, which would have also challenged the metallurgical talents of the bloomery ironworkers. Slags tested by archaeometallurgists contained from 12 to 25% TiO2 by mass, which is highly unusual for the time. The Lowveld ironworkers added fluxes to their furnaces to enable them to overcome the high titania contents of the slags, which would have been highly viscous. The bloomery ironworkers thus adapted their smelting technique to accommodate the strange iron ore of the region, demonstrating impressive metallurgical skills.
Everywhere else in the world where magnetite was processed in bloomery furnaces the magnetite was typically recovered from beaches and rivers. These 'black sands' result from natural erosion as the magnetite grains are liberated, and typically consist of granular particles smaller than 2 mm. This type of magnetite sand, also called 'ironsand', is still being processed to produce iron. A modern example of such a deposit is found in New Zealand. Magnetite sand is found on the black sand beaches of the west coast of the North Island in New Zealand. New Zealand Steel was established in 1965 by the New Zealand government to produce steel billet from the abundant titaniferous magnetite in the region. The iron concentrate processed by New Zealand Steel is mined from the Waikato North Head site, south of Auckland, as well as from the Taharoa deposit on the west coast of the North Island. The sands from the beaches are lower in titania and vanadium than the magnetite from the Bushveld Complex processed by Highveld Steel. With the closure of Highveld Steel in 2015, New Zealand Steel is currently the only facility in the world that employs electric smelting to recover iron and vanadium from titaniferous magnetite (Hukkanen and Walden, 1985; Kelly, 1993; New Zealand Steel, 2017, 2018).
The evolution of electric smelting of titaniferous magnetite
Highveld Steel and New Zealand Steel were both commissioned during the mid- to late 1960s. While both process flow sheets are embodiments of the rotary kiln-electric furnace (RKEF) process, they differ in concept and there are several interesting connections and historical perspectives.
Highveld Steel and Vanadium started smelting ore from the Mapochs Mine in 1968, following an implementation programme initiated in 1960 as described earlier (Bleloch, 1949; Rohrmann, 1985). The original RKEF process flow sheet for Highveld Steel comprised co-current rotary kilns for prereduction of the generally lumpy titaniferous magnetite concentrate. Steinberg, Geyser, and Nell (2011) report that there is no clear evidence for, or record of, the reasons for selected the co-current kiln designs.
The prereduced concentrate from the kilns was fed into submerged-arc furnaces together with fluxes and reductant, producing vanadium-bearing pig iron and waste slag. The use of conventional blast furnace technology had been regarded as being too high a risk due to the unusually high concentration of titanium in the Bushveld Complex ores.
Dr Bleloch pioneered the recovery of iron from the Bushveld magnetite ore, proposing the use of South Africa's 'atypical iron ore' via 'electrometallurgy' - the use of electric arc furnaces to recover metals. His work was interrupted by the outbreak of World War II, but in the post-war years he turned his attention back to metallurgy and in particular electric smelting projects. in 1948 he oversaw the smelting of 100 tons of Bushveld magnetite in Norway and successfully demonstrated the production of pig iron and recovery of vanadium (Bleloch, 1949). Much like the adaptations of the Lowveld ironmaking of the Late Iron Age, the process required fluxing, and although the energy source would be supplied by electrical energy the same metallurgical principles were applied in the modern embodiment of processing the unusual iron ore from the Bushveld Complex.
The Highveld Steel flow sheet remained unchanged until the late 1990s, when a series of studies was conducted to identify opportunities to address operational problems and inefficiencies that had been part of the process since inception. As a result of successful testing on-site, Highveld Steel converted four furnaces from submerged arc to open arc mode over a period of about 6 years (starting in 2004), eliminating the dependence of the power input on the burden and slag composition. The conversion yielded several operational improvements as the open-arc smelting mode greatly improved metallurgical control and operational outcomes. The change to open-arc smelting improved vanadium recovery, lowered coal , electrode, and energy consumption, and could use less-reactive coal and process more fine ore directly in the smelting process. The benefits observed are typical and comparable to the principles of processing high-titania ores in an open-arc furnace (Jones and Geldenhuys, 2011; Geldenhuys, 2017). The overall metallurgical and operational performance at Highveld Steel improved substantially after the conversion, as reported by Steinberg, Geyser, and Nell (2011).
It is noteworthy that the only other RKEF operation processing vanadium-bearing titaniferous magnetite to produce iron and recover vanadium, namely New Zealand Steel, operated with a shielded open arc, similar to that used by ilmenite smelters such as Rio Tinto's Quebec Fer et Titane (formerly known as QIT) and Richards Bay Minerals (RBM) in South Africa (Hukkanen and Walden, 1985; Matyas et al., 1993).
According to Matyas et al. (1993), shielded-arc electric smelting furnace technology was developed by Falconbridge in the 1960s for the smelting of prereduced nickel laterite ores. However, in the 1950s, large electric furnaces were commissioned at Sorel (known today as Rio Tinto Fer et Titane) in Canada with the purpose of smelting the atypical ilmenite (FeTiO3) deposits in Quebec. These furnaces were designed to operate with open arcs, partially shielded by feed and without the use of slag modifiers, producing high-titania slags containing about 80% titania (Sorel slag) from hard-rock ilmenite with a starting grade of about 34% TiO2 (Habashi, 2010).
Matyas et al. (1993) illustrate the princlples of submerged arc (Figure 2a), shielded open arc (Figure 2b), and open arc smelting (Figure 2c). The representations are very useful for visualizing the differences between these three modes of electric smelting and are applicable to circular and rectangular furnaces as well as DC or AC power sources. The total number of electrodes may be configured depending on the power source with either 1, 2, 3, or 6 electrodes, depending on the electrical and mechanical configuration of the furnace. For a DC furnace, it is usual for the anode to be embedded in the hearth (not indicated in the graphics), but is it possible to configure a DC furnace as a dual-electrode furnace, with one electrode acting as the cathode and the second as the anode.
Figure 2a illustrate the original Highveld Steel smelting mode, and Figure 2c shows the evolution as implemented, and described by Steinberg , Geyser, and Nell (2011). The smelting furnaces at New Zealand Steel are shielded arc operations, as represented by Figure 2b. To operate with a shielded arc, feed ports are strategically located to manage the feed in such a way that the arc is always shielded by feed. There is clearly a continuum of modes of operation between shielded arc and open arc smelting, and it may be more accurate to describe shielded arc smelting and open bath smelting as sub-types of open arc smelting as the mechanism of heat transfer is fundamentally the same. There are clear benefits in covering at least part of the bath with feed, as the majority of heat radiation is from the molten slag bath surface, a common challenge for open arc open bath smelting operations.
These reports of shielded open arcs are a reminder of the fact that most scientific discoveries and inventions are made independently and more or less simultaneously, by multiple inventors (Kelly, 2011). The various historical references highlight that even in the relatively small industry of electric smelting, process developments across various commodities often occur in parallel or nearly simultaneously, within different commodity types. It is a reminder that great value is created through cross-pollination and is possibly one of the most valuable contextual lessons one can learn from historical publications.
Magnetite reduction perspectives
The prereduction technology at New Zealand Steel comprised coal-based direct reduction using rotary kilns to produce metallized iron, which was smelted in the electric furnaces. This technology, which is known as the SL/RN (Stelco-Lurgi/Republic Steel-National Lead) process, is widely used and is known for being able to process a wide range of iron-bearing materials, including lumpy ore, pellets, and finely sized iron-bearing feedstocks such as ilmenite. Carbon (in solid form) is fed together with the iron ore into a rotary kiln, where the coal is gasified and the iron ore is reduced. Notably, coal consumption is considerably higher than for a blast furnace, and the energy efficiency of individual plants depends on how efficiently the residual gas is utilized in the plant (Kelly, 1993; Lepinski, 2000).
New Zealand Steel initially fed green (unfired) titanomagnetite pellets directly to the rotary kiln while coal was injected into the kiln. Kelly (1993) describes the various process improvements that were required. It was found that the green pellets deteriorated significantly in the kiln, resulting in excessive fines generation. The waste gas handling system could not cope with the excess dust, which limited the throughput of the reduction step. Coal injection was found to be unsuccessful, with poor temperature control resulting in accretion problems in the kilns. In 1972, the plant abandoned pellet production and coal injection, and resorted to feeding iron concentrate directly together with the coal. Although the operation immediately improved, the plant was still operating below design capacity. The bottleneck remained the high volumes of waste gas from the coal. To address this issue, the plant implemented a coal charring step before feeding the reductant to the kiln. This led to the development of the multi-hearth furnaces currently in use at New Zealand Steel (Richards and Davies, 1980; Kelly, 1993).
Steinberg, Geyser, and Nell (2011) concluded that the Highveld Steel plant needed to improve the efficiency of prereduction, and that multi-hearth furnaces were being evaluated as an option. Perhaps if the process changes had been introduced earlier, Highveld Steel might still be in operation today. According to Kelly (1993), the energy released as part of the charring step is used to preheat the concentrate, and the overall productivity of the kilns improved about 35% as a result.
Prereduction of the iron prior to smelting in electric furnaces was a key process innovation for both New Zealand Steel and Highveld Steel. The benefits of prereduction in electric smelting are well-established. Highveld Steel's kiln operations were notoriously variable and ineffective, but unlike New Zealand Steel, no significant changes were ever implemented. During the submerged arc smelting era, fines were not well tolerated by the smelters, thus lumpy ore was favoured, which further compounded the problem at Highveld. As described earlier, bloomery furnaces generally processed magnetite only from black sands, where the magnetite was liberated through natural erosion. In the Lowveld slag samples from the many smelting sites around Phalaborwa have been recovered and studied by archaeologists and archaeometallurgists. An example of a slag from the region
Figure 3 (reproduced from Killick and Miller, 2014) shows a micrograph of a slag sample. The magnetite has been reduced to metallic iron (white) around its exterior, but the ulvöspinel laths were not reduced, and laths of Fe2TiO4 can be seen within the iron rim at the upper left of the frame. Manamela and Pistorius (2005) concluded that 'Ore size does affect direct reduction of titaniferous magnetite and that there is a limit to direct reduction for titania-rich ores. The ironworkers of the Late Iron Age already established that ore size for magnetite ores was crucial, and magnetite was generally processed only if naturally occurring as black sands or, as found in the Lowveld, innovatively crushed into smaller sizes. The overall conclusions of the ironworkers from the Lowveld and modern researchers are aligned, and the unusual minerals processing activities in the Lowveld demonstrates that the ironworkers also realized that particle size matters.

The exsolved ülvospinel (Fe2TiO4) in the metallic rim (Figure 3) clearly shows that during direct reduction of titanomagnetite in a bloomery furnace, Fe associated with the exsolved ülvospinel phase is essentially unavailable for direct reduction. In both papers (Manamela and Pistorius, 2005; Killick and Miller, 2014) the direct reduction mechanism of titaniferous magnetite is investigated, although from different perspectives and different disciplines: Killick and Miller (2014) via a postmortem study of the slag from bloomery furnace ironmaking in the Lowveld (archaeometallurgy perspective) and Manamela and Pistorius (2005) via a study of direct reduction of titaniferous magnetite in the context of reduction in the rotary kilns at Highveld Steel (metallurgical engineering perspective).
Manamela and Pistorius (2005) concluded that the particle size of ore matters, but they also quantified the degree of direct reduction that can be achieved. The Lowveld ironworkers relied on direct reduction in the short shafts of their bloomery furnaces, and the slag samples from the region show that only partial reduction was achieved. Manamela and Pistorius (2005) concluded that the direct reduction of titaniferous magnetite proceeds via a two-step process and that due to the nature of titaniferous magnetite, the theoretic maximum metallization for titaniferous ore depends on the Fe-to-Ti ratio.
In the first step, titaniferous magnetite is converted to a mixture of wüstite (FeO) and ülvospinel. This reaction appears to occur uniformly throughout the ore particles and is not significantly affected by particle size. In the second reduction step the wüstite formed during the first step is reduced to metallic iron, but for larger ore particles, metallization is confined to the outer regions of the particles, with an unreduced core consisting of a mixture of wüstite and ülvospinel. This would suggest that gaseous diffusion through the product layer limits the rate of reduction (Manamela and Pistorius, 2005). Figure 4 shows a partially reduced ore sample, and the similarities with Figure 3 are clear. Once fully reduced, the titaniferous magnetite ore would consist of a mixture of metallic Fe and ülvospinel, and as is clear from Figure 3, the product of direct reduction will contain metallic Fe together with Fe trapped in as ülvospinel as no further reduction of the ülvospinel occurs.
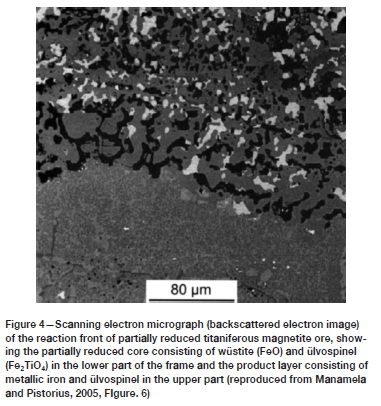
Manamela and Pistorius (2005) explain that for a titaniferous magnetite ore with an Fe-to-Ti ratio of 6 (i.e., for every six moles of Fe there is one mole of Ti), out of every six moles of iron in the original ore, approximately two moles would be trapped in the ülvospinel phase (from the chemical formula Fe2TiO4) and a maximum of four moles of iron could be reduced to the metal. For ore with an Fe-to-Ti ratio of 6, the theoretical maximum reduction would be about 66%.
Project development lessons from history
The journey from ore to metal is often fraught with challenges. New projects are typically beset by commissioning delays, ramp-up challenges, and financial constraints, but usually the operation achieves the desired outputs. In general, mining and metallurgical projects have a poor track record of delivering on time or budget. The McNulty curves (McNulty, 1998; McNulty, 2004)are a series of graphs which predict the level of pain that companies will endure when ramping up a project, based on historical case studies. An excellent example of how history can add value to current and future projects in a systematic and scientific sense. The work by McNulty clearly demonstrates the challenges associated with developing and implementing a complex mining and mineral process. Pyrometallurgical processes are extremely prone to difficulties in ramping up, despite significant development efforts and careful planning. McNulty published an update of the earlier works in 2014 in a paper titled 'Plant ramp-up profiles an update with emphasis on process development. In the paper he indicates that 'some very carefully developed and executed projects can ramp-up quickly and that owners of new projects should avoid being too pessimistic... if they are certain that they have done their homework (McNulty, 2014).
The case of the Albemarle Iron Works
According to available records, the Albemarle Iron Works, consisting of a cold blast furnace, was completed in early 1772, but closed in September of 1772 with no useable iron ever produced. Cold blast furnaces in ironmaking are differentiated from modern blast furnaces as cold air was blown into the furnace - an evolution of the bloomery iron furnace.
In an affidavit by one of the investors about the management problems experienced during the short life of the project the following statement stands out, namely that the Albemarle Iron Works 'were carried on at a very great expense and never produced any profit (Brothers, 2001).
From the very beginning, Albemarle was in trouble because of perceived management problems and during a short period in 1771-1772, three of the five partners in the company tried to run the iron works. At that time, the cause of the failure was poorly understood, but certainly, management challenges contributed. Following the closure in 1772, many subsequent attempts were made to produce iron at Albemarle, but without success (Brothers, 2001).
The historical perspectives and the postmortem investigation into the failure of the Albemarle Iron Works are documented by Brothers (2001). The author presents some valuable insights into the true cause of the failure and the investigations highlight the complexities encountered when starting up a project. In the 18th century, iron blast furnaces were among the most expensive and complex businesses of their time and from just the smelting perspective, not much has changed in the 21st century (as demonstrated by the McNulty curves).
The following quote from Brothers (2001) summarizes the challenges faced by the investors well: 'The 18th century iron blastfurnaces of the British North American Colonies... required an enormous capital investment both for construction and acquisition of land, required large crews of skilled and unskilled labor, and operated continuously for months at a time. They consumed tons of raw materials, which had to be delivered to a relatively tight schedule, and produced tons of cast iron a day:
At the time, blast furnaces were owned by groups of investors, not craftsmen, an thus relied heavily on a diverse group with specialist skills to manage and operate them. Despite the abject failure of the Albemarle project, the investors appeared to have done their homework before setting up their business venture. For example, the site selection for Albemarle Iron Works took two of the partners over seven years to finalize, and on the surface they appeared to have done an excellent job. The selected site was near a river for the water wheels, situated in a well-wooded area for the charcoal, with abundant beds of iron ore in the area. Despite the apparent attention to detail, the company failed during its first year of operation and never produced any usable iron.
In 1882. analysis of ore from the Betsy Martin Mine that supplied the site of iron ore to the ill-fated iron works revealed a high titanium content, the first indications of the possible reasons for the failure of the venture. In 1977 it was established that the ore also contained ilmenite, apatite, and even rare earth oxides. In 1999, slag samples (Figure 5) were recovered from the Albemarle site and evaluated, revealing that the primary cause for Albemarle's failure was indeed the titania content of the iron, which showed there had been a true mismatch between process and due diligence done by the partners and investors (Brothers, 2001; Brothers, Grime, and Swann, 2002). The high levels of titania would have resulted in highly viscous slags with up to 40% titania and relatively high levels of phosphorus. Sadly, even if the plant had adapted their operation like our bloomery ironworkers from the Lowveld, by adding fluxes, the cast iron would have been too brittle due to the high phosphorous content of the ore.

The outcomes of the Albemarle Iron Works project, and the subsequent historical and archaeological assessment, comprise an excellent case study of the pitfalls of project development.
Data is not knowledge, and knowledge is not wisdom
Data sciences can be described as a way of knowing. All data does not necessarily lead to knowledge; for data to become useful it needs to be organized or categorized in an appropriate or relevant manner to transform the data into useful information. Information in turn, enables knowledge, of which learning is the primary protagonist.
Relevant knowledge creates connectivity leading to true understanding and insight, which enables the application of data towards achieving a goal or the desired impact. The DIKW framework or pyramid is widely used to represents the relationship between data (D), information (I), knowledge (K), and wisdom (W), and while there are many alternative models or frameworks, the DIKW framework offers a useful way to represent the relationships, and as a result is still widely used. In the DIKW model, each stage is a step towards a higher level, starting with data, and ending with wisdom. Each step answers different questions about the data, increasing the value along the way. The more we enrich data with meaning and context, the more knowledge, and insights we get out of it. Figure 6 shows an expanded version of the DIKW framework and the relationship between data and impact, illustrating the various stages and the relationships well, as visualized by Gapingvoid.
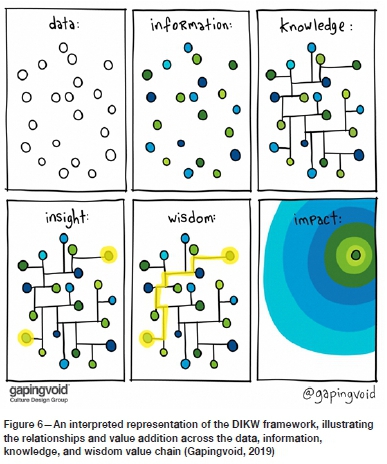
Historical perspectives or narratives, in the context of publications found in technical journals, conference proceedings, theses, and dissertations, form part of the value chain described in the DIKW model. Technical publications can provide us with a rich collection of case studies, and through the experiences and results described by authors, the good, the bad, and even the ugly, we can enhance our understanding of the nature of current challenges or trends. The words of author Anais Nin: 'We don't see things as they are, we see things as we are', highlight the importance of perspective and context. Both aspects are crucial ingredients of wisdom and insight.
While knowledge ages quickly, wisdom is more unyielding and steadfast, and for now, wisdom is still a uniquely human skill. The pace of innovation has accelerated dramatically over the past 100 years, and the challenges faced by the mining and metals industry require a multidisciplinary and transdisciplinary systems approach. At the rock face or on the processing plant, the fundamentals have not changed. The minerals and the physical properties of the materials we process are the same, but our technologies and our understanding of some of the interactions have advanced considerably.
We sometimes confuse the ability to generate data with generating information and knowledge. If we design our model according to physical properties that are not accurately known or well understood, our models can generate masses of data with little or no value. There are many representations, possible solutions, frameworks, definitions, approaches, and techniques available. Historical insights are one avenue that can help us connect and interpret the appropriate dots, help identify gaps in knowledge, and importantly, help eliminate options from the tsunami of alternatives.
This paper draws not only on publications from the expected fields or disciplines associated with mining, minerals processing, and extractive metallurgy. Publications across various multi- and transdisciplinary fields added significant perspectives to the case studies. This diversity is well aligned with the ever-increasing awareness that problem solving and innovation in industry requires a systems thinking approach. because silo thinking limits potential.
History can offer inspiration via 'an inventory of alternatives' and a future-focused perspective by demonstrating which elements of our present are transient and which are more enduring (Gaynor and Crebbin, 2013).
Conclusions
History can play an important role in how we categorize or organize data at various stages in the value-adding processes when converting data into knowledge and, ultimately, wisdom. History can benefit our understanding of the present and thus enhance future outcomes.
Technical publications can illuminate our thoughts, change our perspectives, or even help us to discover new or alternative ways of thinking which can be used to transform data, information, and knowledge into insight and wisdom.
The growing awareness around ethical and socially responsible processing in the mining and metallurgical industry can be greatly enhanced through the lenses of historical case studies. From successes and failures of the past, it is possible to identify which of the options or choices are the more ethical and socially responsible. Lessons from history can help provide insights into making ethical and sustainable choices in the mining and metallurgical industry.
References
Badmatsyrenov A, R. and Orsoev, D. 2005. Origin of titanomagnetite-ilmenite mineralization, Arsentyev gabbro-syenite massif, Transbaikalia, Russia. Mineral Deposit Research: Meeting the Global Challenge. Springer, Berlin, Heidelberg. pp. 725-727. doi: 10.1007/3-540-27946-6_184 [ Links ]
Bleloch, W. 1949. The electric smelting of iron ores for production of alloy irons and steels and recovery of chromium and vanadium. Journal of the Chemical, Metallurgical and Mining Society of South Africa. March. pp. 363-402. [ Links ]
Brothers, J.H. 2001. 'Carried on at a very great expense and never produced any profit': The Albemarle Iron Works (1770-72). doi: 10.21220/s2-psqr-4m85 [ Links ]
Brothers, J.H., Grime, G.W., and Swann, CP. 2002. Albemarle Iron Works (17711772): Why did this operation fail? Nuclear Instruments and Methods in Physics Research, Section B: Beam Interactions with Materials and Atoms, vol. 189, no. 1-4. pp. 340-343. doi: 10.1016/S0168-583X(01)01083-7 [ Links ]
Cawthorn, R.G., Barnes, S., Ballhaus, C., and Malitch, K.N. 2005. Platinum group element, chromium and vanadium deposits in mafic and ultramafic rocks. Economic Geology 100th Anniversary Volume. V. 100th Anniversary of the Society of Economic Geologists. pp. 215-249. [ Links ]
Chen, S. and Chu, M. 2014. A new process for the recovery of iron, vanadium, and titanium from vanadium titanomagnetite. Journal of the Southern African Institute of Mining and Metallurgy, vol. 114, no. 6. pp. 481-487. [ Links ]
Evraz Highveld Steel and vanadium. 2015. Business tescue. http://www.evrazhighveld.co.za/businessrescue.asp [accessed 18 February 2017]. [ Links ]
Fischer, R.P. 1975. Vanadium resources in titaniferous magnetite deposits. US Geological Survey Professional Paper. 926-B:1-9. [ Links ]
Gapingvoid. 2019. Data-Information-Knowledge-Insight-Wisdom. https://www.gapingvoid.com/content/uploads/2019/03/data-information-knowledge-insight-wisdom-impact.jpg [accessed 9 September 2021]. [ Links ]
Gaynor, A. and Crebbin, G. 2013. What can engineers learn from the past? A potential role for history in engineering education. International Journal of Engineering, Social Justice, and Peace, vol. 2, no. 2. pp. 43-54. doi: 10.24908/ ijesjp.v2i2.3512 [ Links ]
Geldenhuys, I.J. 2017. The exact art and subtle science of DC smelting: Practical perspectives on the hot zone. JOM. vol. 69, no. 2. pp. 343-350. doi: 10.1007/ s11837-016-2171-z [ Links ]
Geldenhuys, I.J., Akdogan, G., and Reynolds, Q.G. 2021. Towards sustainable processing of vanadium-bearing titaniferous magnetite deposits - an overview of barriers and opportunities. Proceedings of IMPC2020. pp. 18-22. http://wwww.mintek.co.za/Pyromet/Files/2021Geldenhuys.pdf [ Links ]
Habashi, F. 2010. A short history of electric furnaces in iron and steel making. Part 2 Induction and smelting. Steel Times International. vol. 34, no. 7. p. 48. [ Links ]
Henry, J., Stephens, W., Blue, D.. and Maysilles, J. 1987. Bureau of Mines development of titanium production technology. http://trove.nla.gov.au/work/152481664 [ Links ]
Hukkanen, E. and Walden, H. 1985. The production of vanadium and steel from titanomagnetites. International Journal of Mineral Processing, vol. 15, no. 1-2. pp. 89-102. doi: 10.1016/0301-7516(85)90026-2 [ Links ]
Ivanic, T.J., Wingate, M.T.D., Kirkland, CL., van Kranendonk, M.J., and Wyche, S. 2010. Age and significance of voluminous mafic-ultramafic magmatic events in the Murchison Domain, Yilgarn Craton. Australian Journal of Earth Sciences. vol. 57, no. 5. pp. 597-614. doi: 10.1080/08120099.2010.494765 [ Links ]
Jones, R.T. and Geldenhuys, I.J. 2011. The pros and cons of reductive matte smelting for PGMs. Minerals Engineering, vol. 24, no. 6. pp. 495-498. doi: 10.1016/j.mineng.2011.03.007 [ Links ]
Kelly, B.F. 1993. Kelly Ironmaking at BHP New Zealand Steel Limited, Glenbrook. New Zealand. Australasian Mining and Metallurgy: The Sir Maurice Mawby Memorial Volume (2nd edn). Monograph Series no. 19. Australasian Institute of Mining and Metallurgy, Melbourne.pp. 348-353. [ Links ]
Kelly, K. 2011. What Technology Wants. Penguin Books. [ Links ]
Killick, D. and Fenn, T. 2012. Archaeometallurgy: The study of preindustrial mining and metallurgy. Annual Review of Anthropology, vol. 41, no. 1. pp. 559-575. doi: 10.1146/annurev-anthro-092611-145719 [ Links ]
Killick, D. and Miller, D. 2014. Smelting of magnetite and magnetite-ilmenite iron ores in the northern Lowveld, South Africa, ca. 1000 CE to ca. 1880 CE. Journal of Archaeological Science, vol. 43, no. 1. pp. 239-255. doi: 10.1016/j.jas.2013.12.016 [ Links ]
Lepinski, J.A. 2000. Iron by direct reduction. Kirk-Othmer Encyclopedia of Chemical Technology. Wiley, Hoboken, NJ. pp. 1-15. doi: 10.1002/0471238961.091815 1412051609.a02 [ Links ]
Manamela, M.M. and Pistorius, P.C. 2005. Ore size does affect direct reduction of titaniferous magnetite. Journal of the South African Institute of Mining and Metallurgy, vol. 105, no. 3. pp. 183-185. [ Links ]
Matyas, A.G., Francki, R.C., Donaldson, K.M., and Wasmund, B. 1993. Application of new technology in the design of high-power electric smelting furnaces. CIM Bulletin, vol. 86, no. 972.. pp. 92-99. [ Links ]
Mcnulty, T. 1998. Developing innovative technology. Mining Engineering, vol. 50. pp. 50-55. [ Links ]
Mcnulty, T. 2014. Plant ramp-up profiles an update with emphasis on process development. COM 2014 - Proceedings of the Conference of Metallurgists. CIM, Montreal. [ Links ]
McNulty, T.P. 2004. Minimization of delays in plant startups. Improving and Optimizing Operations: Things That Actually Work - Proceedings of the Plant Operators' Forum 2004. Dowling, E.C. and Marsden, J.J. (eds). Society for Mining, Metallurgy, and Exploration, Inc., Littleton, CO. pp.113-120. [ Links ]
Miller, D., Killick, D., and van der Merwe, N.J. 2001. Metal working in the northern lowveld, South Africa a.d. 1000-1890. International Journal of Phytoremediation, vol. 21, no. 1. pp. 01-417. doi: 10.1179/jfa.2001.28.3-4.401 [ Links ]
New Zealand Steel. 2017. Ironmaking. http://www.nzsteel.co.nz/new-zealand-steel/the-story-of-steel/the-steel-making-process/iron-making/ [accessed 31 January 2017]. [ Links ]
New Zealand Steel. 2018. The History of Ironsand. Available: https://www.nzsteel.co.nz/new-zealand-steel/the-story-of-steel/the-history-of-ironsand/ [2018, December 02]. [ Links ]
Pang, K.N., Zhou, M.F., Qi, L., Shellnutt, G., Wang, C.Y., and Zhao, D. 2010. Flood basalt-related Fe-Ti oxide deposits in the Emeishan large igneous province, SW China. Lithos, vol. 119, no. 1-2. pp. 123-136. doi: 10.1016/j.lithos.2010.06.003 [ Links ]
Peck, D.C. and Huminicki, M.A.E. 2016. Value of mineral deposits associated with mafic and ultramafic magmatism: Implications for exploration strategies. Ore Geology Reviews, vol. 72. pp. 269-298. doi: 10.1016/j.oregeorev.2015.06.004 [ Links ]
Richards, S.R. and Davies, CE. 1980. Smelting alternatives for New Zealand titaniferous minerals. Proceedings of the Annual Conference, New Zealand. Australian Institute of Mining and Metallurgy, Melbourne.pp. 237-253. [ Links ]
Rohrmann, B. 1985. Vanadium in South Africa. Journal of the South African Institute of Mining and Metallurgy,.vol. 85, no. 5. pp. 141-150. [ Links ]
SAIMM. 1981. Minutes of the Annual General Meeting of the South African Institute of Mining and Metallurgy. http://www.saimm.co.za/Journal/v081n09p257.pdf%0A [ Links ]
Smirnov, L.A., Tret'yakov, M.A., and Gladyshev, v.I. 2000. Processing vanadium-bearing and titanomagnetite iron ores. Metallurgist, vol. 44. pp. 230-232. doi: 10.1007/BF02466943 [ Links ]
Steinberg, W., Geyser, W., and Nell, J. 2011. The history and development of the pyrometallurgical processes at Evraz Highveld Steel & Vanadium. Journal of the Southern African Institute of Mining and Metallurgy, vol. 111, no. 10. pp. 705-710. [ Links ]
Taylor, P.R., Shuey, S.A., vidal, E.E., Wang, W., and Gomez, J.C. 2005. Extractive metallurgy of vanadium containing titaniferous magnetite ores: A review. Proceedings of the 2005SME Annual Meeting. (May). Society for Mining, Metallurgy & Exploration, Englewood, CO. pp. 1-9. [ Links ]
US Geological Survey. 2021. Mineral Commodity Summaries 2021. Reston, VA. [ Links ]
 Correspondence:
Correspondence:
I.J. Geldenhuys
Email: isabel@pyro.co.za
Received: 13 Sep. 2021
Published: September 2021














