Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Journal of the Southern African Institute of Mining and Metallurgy
versión On-line ISSN 2411-9717
versión impresa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.121 no.5 Johannesburg may. 2021
http://dx.doi.org/10.17159/2411-9717/16-478/2021
PROFESSIONAL TECHNICAL AND SCIENTIFIC PAPERS
A critical investigation into spontaneous combustion in coal storage bunkers
S. GovenderI; J.J.L. du PlessisII; R.C.W. Webber-YoungmanIII
IGrootegeluk Mine, Exxaro Resources, Pretoria, South Africa
IIDepartment of Mining Engineering, University of Pretoria, South Africa
IIIHead of Department, Department of Mining Engineering, University of Pretoria, Pretoria, South Africa
SYNOPSIS
Spontaneous combustion (SC) is a cold oxidation reaction that generates heat, causing a temperature rise of the reactant and leading, with limited heat dissipation, to self-ignition of the reactant, which occurs without an external heat source. Although not limited to coal, the most significant hazard of SC are the fires that occur in coal mining operations around the world. These fires pose a serious risk to the safety of workers in the mines as well as adverse effects on the environment, and can affect the quality of life for current and future generations. We investigated the occurrence of SC in raw coal storage bunkers with the purpose of compiling a decision analyser for engineers designing or working with coal storage bunkers. Specific experts were interviewed from different backgrounds and companies, followed by field research at a coal mine. The important factors affecting the possibility of SC occurring were the type of coal supplied to the bunker, the mining practice, and physical conditions around the bunker. The information presented here will assist in reducing the SC risk in raw coal storage bunkers. These findings, together with the systematic decision analyser developed, will assist design engineers and mine personnel to take early preventative steps in managing SC. The decision analyser was tested for many different scenarios and gave good guidance on how to minimize and prevent SC in bunkers.
Keywords: bunkers, coal storage, spontaneous combustion.
Introduction
In coal mining, spontaneous combustion (SC) can occur in many areas such as product or run-of-mine stockpiles, underground workings, waste dumps, coal faces, in-pit ramps, and backfill areas. Extensive research was done by Phillips, Uludag, and Chabedi (2011) on SC in coal stockpiles, dumps, and coal faces. SC is when an oxidation reaction occurs without an external heat source. Heat is generated spontaneously within an oxidized substance when dissipation of the heat generated is limited or cannot take place. This study investigated the occurrence of SC in coal storage bunkers, and established that there was no single research report available that addresses the problem adequately. The decision analyser steps that were developed in this investigation will assist towards a possible solution to problems associated with SC. The process described will assist personnel in understanding, preventing, and managing SC.
Background
In a surface mine, it is frequent practice to have haul trucks dumping material into a tipping bin in a continuous sequence, or from a stockpile of coal when it is positioned close to the tip. Storage bins or bunkers are frequently used to facilitate continuous feed to processing plants. At Grootegeluk Mine, two coal processing plants are fed from two raw coal storage bunkers in series (Figure 1) with storage capacities of 17.5 kt and 30.5 kt. This capacity allows for 8 hours of continuous plant operation.
During the commissioning of the bunkesr and plants in 2012, SC occurred in the storage bunkers. A risk assessment was done using a 5-by-5 risk matrix to assess the current SC problem as part of the normal course of operations. An initial risk rating of 22 was achieved, which is deemed to be extremely high. The implementation of additional known control measures reduced the risk rating to 15, which was still considered to be too high.
One of the proposed control solutions was that the bunker should be loaded from a shuttle car moving in sequence from right to left on one side and from left to right on the other side. The bunker should be kept at between 73 and 79% capacity, but should never exceed 85%. However, due to problems experienced with the shuttle car and the need to ensure that coal covers all draw-off points to prevent oxygen from entering the bunker, this sequence of loading could not be realized (Gerber, 2014). Figure 2 shows a side view of the storage bunker.
Due to the magnitude of the SC problem, further studies were necessary to find a more permanent solution to prevent and control SC. The proposed solution developed and presented in this paper provides design engineers with a decision analyser for evaluating the risk factors leading to potential SC in storage bunkers and assisting in designing preventative measures.
The main factors that cause and/or determine the extent or severity of SC are the type of coal, the mining practice, and the physical conditions around the bunker. The grade, rank, sulphur content, and petrographic composition of the coal determine how the coal will react. Mining practice influences the average standing time of the coal in loose cubic metres (LCM) on the mining benches, the temperature of the coal when it is fed into the bunker, and the broken coal loading and cleaning practices. The physical conditions in and around the bunker include the ambient temperature, layout of the bunker with respect to the wind direction, and dead spots in the bunker structure.
Type of coal mined and supplied to a bunker
Coal analysis is based on the international classification of in-seam coals. In terms of this classification, four characteristics of coal were examined, namely the grade, the rank, the sulphur content, and petrographic composition.
Grade, rank, and sulphur content
The grade of the coal fed to the bunker ranges from very low to a carbonaceous rock grade coal. The ash content, which is important in determining the grade, ranges from 19.8-50% AD. Kaymakci and Didari (2002) posited that high-grade coals have a lower tendency to self-heat, while low-grade coals have a higher tendency to self-heat due to the higher volatile content and reactants, mostly vitrinite. A high ash content decreases the propensity of a coal to heat spontaneously. However, certain constituents of the ash (lime, soda, and iron compounds) may accelerate heating, whereas other constituents (alumina and silica) could have a retarding effect. Therefore, some components of ash promote combustion, while others inhibit it. The size distribution and permeability of the bunker feed also affect the risk of SC.
The rank of a coal refers to the degree of maturity of the coal. It is dependent on the degree of metamorphism, i.e. the properties of vitrinite, which is largely responsible for the coking properties of a coal. The greater the vitrinite content, the better the coking properties of the coal. Vitrinite reflectance is a direct measure of the rank of a coal, which is obtained through petrographic analysis.
Coal benches have different rank, grade, and sulphur ranges. For example, bench 2 could have a very low grade coal with a rank which is a high-volatile bituminous low rank C and a sulphur average of 1.4%. Bench 3 could have a carbonaceous rock grade coal with a rank corresponding to a high-volatile bituminous low rank C and a sulphur average of 1.26%. To establish the rank, grade, and sulphur range, a statistical analysis was done using information from the mine's geographic information system (GIS). This study could also have been conducted using the data from exploration boreholes. The data was separated into the different benches with their respective ash contents and calorific and sulphur values.
Phillips, Uludag, and Chabedi (2011) found that the rank of coal is one of the chemical factors that contribute to SC. Kaymakci and Didari (2002) state that it is widely recognized in the coal industry that lower-ranking coals are more susceptible to SC than higher-ranking coals. According to Adamski (2003), as the rank decreases towards lignite, the tendency for coal to self-heat increases, i.e. the lowest-ranked coal is more likely to self-heat under the same set of conditions.
Phillips, Uludag and Chabedi (2011) suggested that organic sulphur and the presence of pyrite contribute to SC. Kaymakci and Didari (2002) explain how the chemical factors such as pyrite content may accelerate SC. According to Panigrahi et al. (2005), at one mine coal with a sulphur content of 0.5% started to burn within two weeks of exposure. Guney (1968) concluded that the pyrite in coal may accelerate spontaneous heating due to the increased sulphur content. Guney further concluded that the pyrite must be present in a concentration greater than 2% before it can have any effect. However, SC can occur when sulphur or pyrite is present. The key factor is the size of the pyrite nodules and their exposure. The large nodules on their own, when exposed to oxidizing conditions or wetting after a dry period, can generate heat ten times faster than coal. This initiates 'hot spots' which then develop further into SC. Furthermore, pyrite oxidation is highly exothermic and could become autothermic/ self-sustaining depending on the balance of supply of reagents, reaction rate, rate of heat transfer, and accumulation of heat. A sufficient increase in temperature could trigger SC. A window of conditions is required for this to happen.
Petrographic composition
To better understand the variations in the physical and chemical properties of the coal at a mine a petrographic analysis is performed to confirm expectations of the coals and to assess their utilization potential (ALS Coal Technology, 2015). Petrographic analysis provides information that is used to establish how the coal will react under different conditions. ALS Coal Technology (2015) indicates that the greater the percentage of vitrinite and liptinite in the composition, the greater the reactivity, and the greater the percentage of inertinite, the lower the reactivity of the coal.
In summary, if the ash values for the coal are high and it is a lower-grade coal then it is prone to SC. This is further dependent on the coal bench being mined and the amount of heat accelerators and retarders in the ash content of the coal. The lower the rank of coal the greater the tendency to SC. A high percentage of sulphur in the orebody could also give rise to SC.
Mining practice
The geological setting of the coal seam to be mined must be checked for faulting and faulted zones, which contribute to the dangers of SC by allowing air ingress into the coal mass (Kaymakci and Didari, 2002). The US Department of Energy (1994) claims that in opencast mines, the main factors responsible for mine bench fires due to SC are the presence of micro- and macro-cracks in the bench walls, which allow the entry of air, extended exposure of the bench walls to the open atmosphere, and accumulation of loose coal on the bench floor.
Standing time of loose cubic metres on the benches
The short-term production schedule for the case study coal mine indicated that broken coal fed to the plants had a standing time on the benches of about 6 days to 2 weeks on average, during which it is exposed to ambient environmental conditions. This allows for air ingress that can lead to oxidation and heating of the coal. If left unattended the coal will self-ignite once it reaches the critical SC point.
According to de Korte (2014), the time taken for heating to occur varies considerably. Product stockpiles and coal inventory in the mine should not be left longer than the incipient heating period. Both run-of-mine (ROM) and saleable product coal have been known to be susceptible to SC. A layer of coarse particles at the base and edges of the stockpile may result in increased ventilation. The situation is exacerbated by prevailing hot, moist winds and rain, which may increase the risk of SC in the summer months.
The US Department of Energy (1994), Adamski (2003), Phillips, Uludag and Chabedi (2011), and Humphreys (2004) reached similar conclusions, that freshly extracted coal absorbs oxygen more quickly than coal mined earlier. The oxygen absorption rate is a function of the coal's age, and fresh coal is more reactive than aged coal or coal that has been exposed to oxygen for longer periods. The reactivity decreases with exposure and oxidation over time. According to the US Department of Energy (1994), hot spots may not be present for the first one to two months. The heat transfer within the pile is controlled largely by the air flow through the coal, and will be less significant at very low flow rates. The surface temperature of the pile controls the convective heat loss at the pile's surface. These heat transfer processes are dependent on the temperature distribution and geometry of the reacting coal pile.
Phillips, Uludag, and Chabedi (2011) stated that when rainfall erodes the coal stockpiles, it exposes more coal to oxygen and, depending on the properties of the coal, heating of broken coal left in stockpiles in the pit or elsewhere on the mine can occur after a certain period of time.
Kaymakci and Didari (2002) concluded that the moisture content has an influence on the propensity of coal to self-heat, whereas Lyman and Volkmer (2001) established that the wetting and drying of coal provides an opportunity for heat transfer to occur in coal stockpiles. According to Lyman and Volkmer (2001), increases or decreases in heat affect the moisture content and oxidation rate, which explains most of the heat generated in the coal. The critical issue here is that when coal has been dried below its natural inherent moisture content, and then is exposed to moisture again, an exothermic process, termed 'heats of wetting', takes place, which generates sufficient heat to create a trigger point that in turn initiates hot spots. The coal dries out in winter and when exposed to rain or high humidity in spring, hot spots occur in places where just enough air passes through the coal bed to allow heats of wetting to occur, which then proceeds to oxidation and SC. Too much water or rain would swamp the process and too great an air flow would carry the heat away. So, the prime 'spot' for heating to start is where the conditions are optimal for heating to take place which is not dissipated. Therefore, porosity and size are key factors here.
Adamski (2003) and Grossman, Davidi, and Cohen (1995) drew similar conclusions, namely that where oxygen is in contact with the coal and oxidation starts to produce heat, and if this heat cannot dissipate, SC is initiated. Extra heat due to the condensation of moisture can lead to runaway self-heating if a coal pile is at a critical temperature point and oxygen is available. If the stockpile temperature increases above 30°C, then the moisture content of the coal vaporizes and carbon dioxide and hydrocarbons of lower molecular weight are emitted. The vaporization and emission rates of gases decrease with temperature, as vaporization is an endothermic process. However, the moisture is transferred by diffusion to drier parts of the stockpile, causing condensation and an increase in temperature, since condensation is an exothermic process. These processes can decrease or increase the ignition point of the coal in different parts of the stockpile. The autogenous heating of stockpiled coal is restricted to small distinct areas, referred to as hot spots, which are characterized by good oxygen diffusion and insufficient heat dissipation. The oxidation-induced autogenous heating of coal causes a loss in calorific value and can also lead to safety and handling problems.
Evidence suggests that coal stockpiles should not be left on the mining benches for longer than is necessary for loading, and this time period should be determined through a risk assessment process for each bench. The mixing of freshly mined coal and aged coal should be avoided to minimize spontaneous heating, since. when old oxidized, weathered and dry coal is in contact with to wet fresh coal, self-heating occurs through heats of wetting once again. This is a dangerous situation as hot spots form all along the interface of the two lots of coal. This can occur in many situations, including when old coal is mixed with fresh coal when being loaded on board ships as well as on stockpiles.
Physical conditions at the storage bunker
Detailed information on the physical conditions around the bunker operation that may contribute to SC was obtained, including the fines ratio, temperatures in and around the bunker, and wind velocity. Monthly average wind speeds, wind direction, temperatures, pressure, and humidity data were obtained from a local weather station. Figure 3 shows the various physical measurements taken around the bunker.
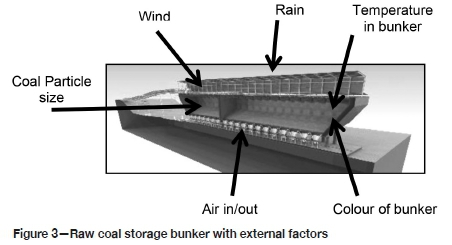
To establish the impact of particle size on SC, samples were taken before the coal was fed into the ROM bunker and at the exit from the bunker. This was done to establish the change in coarse to fines ratio as the particles flow through the bunker. To obtain the best results it was critical to ensure that the bunker was empty and at its lowest level when the samples were taken. The coal height will drop a maximum of about 22 m during operations. The samples were screened at apertures from 0 to 150 mm.
The results indicated that 14.8% of the material fed into the bunker is -4 mm, increasing to 20% -4 mm at the discharge end. The reason for this test was to ascertain whether the ratio of coarse to fine material can affect the flow of air in the bunker. According to Itay, Hill, and Glasser (1989) the most important factor contributing to SC is particle size. Very fine coal does not burn due to a shortage of oxygen, whereas coarse does not burn easily as the rapid and easy passage of air through the body of the coal dissipates any heat generated. There is a critical middle size range where the air flow is just enough for oxidation and therefore heat production, but not fast enough to dissipate the heat. The most dangerous combination is a mixture of fine and coarse material, which increase the permeability of the mixture. However, the air velocity measurements taken at the discharge point of the bunker indicated that a reasonable proportion of fine and coarse coal particles minimizes the flow of air entering into the bunker.
The ambient conditions around a coal mine, in particular the daily temperature and the weather patterns, influence the temperature of the coal entering the bunker. If the wind direction is in line with the bunker, air could be forced into the bunker thereby allowing the entry of oxygen, resulting in SC. It therefore becomes important to position the bunker loading and exit chutes out of the direct line of the prevailing wind direction.
The presence of 'dead spots' in the bunker could result in coal standing and oxidizing at these points, thereby creating hot spots (Figure 4), which could damage the structure of the bumker.

Results
The coal type, mining practice, and physical conditions around the bunker have an impact on the occurrence of SC. A diagrammatic plan view of an opencast mine is depicted in Figure 5, showing the pit layout, the loading area, the crushers, and the storage bunker.
The geological setting is an important contributor, especially if the coal seam is prone to faulting. SC is influenced by the type of coal being mined in terms grade and heat accelerators in the ash content. The high vitrinite and liptinite in the coal and low rank result in an increased oxidation rate and heating, which leads to a possibility of SC. A high content of organic and pyritic sulphur in the coal also increases the possibility of SC.
The standing time of the coal LCM on the mining benches and the possibility of it undergoing SC is influenced by many factors such as loading and cleaning cycles, ambient temperature, and exposure to moisture.
High temperatures in and around the bunker increase the likelihood of SC. If coal is found to be hot at the crusher it should be rerouted to the hot coal stockpile for cooling and disposal, as shown in Figure 5. This coal should not be fed to the bunker.
The loading and exit chutes of the bunker should be out of the direct line of the prevailing wind direction to avoid air being forced into the bunker, resulting in SC. An appropriate ratio of fine to coarse material should be maintained to minimize air ingress and decrease the possibility of SC. The presence of 'dead spots' in the bunker could result in coal standing and oxidizing at these points, thereby creating hot spots and resulting in SC.
The assessment of the factors influencing SC was utilized to develop a decision analyser that can be used to assist mine personnel in understanding the potential causes of SC. The decision analyser was split into six simple steps that address the possibility of SC from the orebody to the bunker. These steps involve determining the impact of faulting and fault zones, the grade, reactivity, rank, and of the coal, confirming the sulphur content of the coal, the coal standing time on the benches, and the physical conditions at the bunker.
Step 1: Impact of faulting and fault zones
The decision process starts once the coal has been blasted (Figure 6). It is important to establish whether the blasted material is lying on a fault or in a fault zone that could allow air ingress into the stockpile, leading to oxidation and heating. If this is not the case, normal mining operations can continue using the mine's standards and procedures. The standing time of the coal on the mine bench must be minimized, and the coal should be moved or processed as soon as possible.
Step 2 Confirm grade of coal
This step deals with understanding the impact of coal properties on SC. It starts with establishing the grade of coal present (Figure 7). This is determined by the ash content - the higher the ash content the lower the grade. Once the grade has been established, the presence of heat accelerators must be established. If the ash contains a high amount of lime, soda, or iron, then SC is possible, whereas high amounts of alumina or silica reduce the possibility of SC. Once it has been established that SC is possible, measures must be put in place to manage the risk and monitor the coal temperature in the operations. These measures could include reducing the standing time of coal on the benches, by ensuring that heat is dissipated from the stockpile, or taking steps to block air ingress into the stockpile.
Step 3: Confirm reactivity and rank of coal
The next step is to establish the reactivity of the coal, since the reactivity is a direct measure of the rank. A high content of vitrinite and liptinite results in higher reactivity, which increases the oxidation rate and results in heating of the coal (Figure 8). A low-rank coal is more prone to SC than a high-rank coal. Once it has been established that SC is probable, measures must be put in place to manage the risk and monitor the coal temperature.
Step 4: Impact of sulphur content of the coal
In this step the chemical properties must be confirmed, especially the sulphur content. The amount of organic and pyritic sulphur in the coal has an influence on SC (Figure 9). High amounts of organic and pyritic sulphur indicate a greater possibility of SC. If there is a possibility of SC, measures must be put in place to manage the risk and monitor the coal temperature.
Step 5: Determine coal standing time on the benches
To determine the coal standing time with low risk of SC on the benches it is important to consider the outcomes of steps 1-4.
These outcomes, together with other influencing factors such as inadequate loading, poor cleaning cycles, coarse coal at the base of the stockpile, exposure of coal after rain, the wetting of coal and a high ambient temperature (Figure 10) will indicate whether SC is likely. Thereafter measures must be put in place to manage the risk at the source and to monitor the coal temperature regularly.
Step 6: Impact of physical conditions at the bunker
The last step in this decision process relates to where the coal has been loaded and is being transported to the bunker. If the coal has been loaded into the crusher and the monitoring equipment detects a high temperature, the coal must be redirected to a hot coal area or lay-down area for cooling and disposal (Figure 5). Such coal should not be transported to the bunker. It is advisable that temperature monitoring devices be installed in the transporting section to assist decision-making as to whether to load or redirect hot coal. This should be the first line of defence in order to prevent hot coal from entering the bunker. At the bunker (Figure 11), continuous monitoring is necessary to detect high-temperature areas. The following parameters need to be monitored: air ingress into the bunker, the fines content, and dead spots that could evolve into hot spots. If SC is detected in the bunker, the only option is to stop loading and remove coal from the affected area immediately. A gas monitoring device could be installed at the bunker to measure CO levels. If the CO level is found to be above 4%, the coal is self-heating in areas not often visible to the naked eye, devolatilizing gases are being emitted, and an explosion is possible, as the CO level has reached the lower explosive limit (LEL).
As the coal proceeds through the different steps the risk of SC increases. The first priority is therefore to put measures in place to prevent SC.
Control measures
The control measures can be broken up into two main areas: the bunker design and management control
Bunker design
The bunker must be so designed so as to prevent dead spots or areas where hotspots can form, as per Figure 4. A particle flow analysis can be done by using simple software or a discrete element method to simulate the flow of material in the bunker or silo. The simulation will provide insight into the mass flow/final flow, residence time/product degradation, rat-holing, bridging, blockage, dust formation, bunker/silo shape, cone shape, output dimension, and wall thickness calculations.
The designer must consider the static and dynamic operation of the mine. During normal production, breakdowns, planned maintenance, and scheduled routine inspections occur. At times, these could last for hours, during which time the coal is standing inside the bunker and most likely to combust spontaneously. The temperature of the coal should be monitored continuously during any such abnormal periods. If the coal temperature starts to increase above 50°C it is best to remove the coal from the bunker. Therefore, one can assume that a safe duration for the coal standing in the bunker is dependent on the temperature of the coal.
Thermal cameras or temperature probes should be installed to monitor the coal entering the crusher and bunker or silo. This will inform the operator if hot coal is being fed from the mine or source.
Air flow measurements must be taken in order to establish whether air is entering the bunker or silo. This will influence the oxidation rate of the coal. These measurements must be taken regularly by the mine hygiene specialist using a well-calibrated velocity meter.
If the bunker has a number of coal-extraction points, it becomes important to ensure that the sequence of loading coal into the bunker is designed in such a way as to minimize the quantity of air being drawn into the bunker. This will help to prevent oxidation and can be done by ensuring that there is always coal at all the extraction points or that they are sealed off. The process engineer needs to build in a standard operating procedure which will ensure that coal is not drawn off completely from the extraction point. This operating procedure can be applied by using simple instrumentation and programmable software, which will then trigger a warning to the operator when the extraction point is reaching a dangerously low level.
Depending on the amount of dust generated when the bunker or silo is loaded, it will be advisable to install explosion-proof equipment in that area or within that control zone. This dust is carbonaceous dust and if present the area is referred to as a Class II location. The ignition temperature, electrical conductivity, and thermal blanketing effect of the dust are all critical when dealing with heat-producing equipment such as lighting fixtures and motors.
Management and control
To address the issue of the standing time of coal on the benches, a risk assessment must be done and a standard operating procedure established as to how to manage the raw coal standing time on the benches. Training in this procedure must be provided to all mine personnel.
Mining best practices must be established to control and treat hot coal. If hot coal is detected, it should be moved to a safe location and allowed to burn out in a controlled environment. After it has been burnt out, it should be disposed of safely. The cause of the SC must be established and proactive measures put in place to prevent its reccurrence.
There should be a standard operating procedure for emptying the bunker in a controlled manner in the event of SC. This should follow from a risk assessment and adequate training of operating personnel.
Conclusions
This study examined the factors that could influence SC of coal at various stages from the mine (pit) to the storage bunker. It is evident that the type of coal mined and the mining practice play a major role in the management of the SC risk. The physical factors pertaining to the bunker, although less important, also influence the likelihood of SC occurring in the bunker. These findings, together with the systematic decision analyser presented, will assist mine personnel to take early preventative steps to manage and control the risk of SC in the bunker. This decision tool was tested for many different scenarios and gave good guidance on how to minimize and prevent SC in a bunker. The SC problem has been reduced to a bare minimum. The outcomes of this study can be applied to similar situations in other mines.
References
Adamski, S.A. 2003. Prevention of spontaneous combustion in back-filled waste material at Grootegeluk Coal Mine. PhD thesis, University of the Witwatersrand. [ Links ]
ALS Coal Technology. 2015. Petrography and imaging centre. http://www.alsglobal.com/~/media/Files/Divisions/Energy/Coal/Coal%20Resources/Capabilities-and-Services-Fact-Sheets/ALS-Coal-Petrography-and-Imaging-Centre-Fact-Sheet.pdf [accessed: 27 September 2015]. [ Links ]
De Korte, J. 2014a. Managing spontaneous combustion of coal. Part 1: Causes, controls and case studies. Proceedings of the Fossil Fuel Foundation Conference on Spontaneous Combustion, Johannesburg, South Africa, 13-14 February 2014. [ Links ]
De Korte, J. 2014b. Managing spontaneous combustion of coal. Part 2: Tests to determine the spontaneous combustion propensity of coal. Proceedings of the Fossil Fuel Foundation Conference on Spontaneous Combustion, Johannesburg, South Africa, 13-14 February 2014. [ Links ]
Gerber, J. 2012. Operating philosophy - Grootegeluk Medupi Expansion Project. Grootegeluk Coal Mine, Operating Standards document No. MCG-B78-0000-0000-G-PHI-001. Lephalale, South Africa. [ Links ]
Gerber, J. 2014. Personal communication, 7 January. Grootegeluk Coal Mine, Lephalale, South Africa. [ Links ]
Grossman, S.L., Davidi, T.S., and Cohen, H. 1995. Explosion risks during the confined storage of bituminous coals. Fuel, vol. 74, no. 12. pp. 1772-1775. [ Links ]
Guney, M. 1968. Oxidation and spontaneous combustion of coal - Review of individual factors. Colliery Guardian, vol. 216. pp. 105-110 and 137-143. [ Links ]
Humphreys, D. 2004. The application of numerical modelling to the assessment of the potential for, and the detection of, spontaneous combustion in underground coal mines. PhD thesis, University of Queensland, Australia. [ Links ]
Itay, M., Hill, CR., and Glasser, D. 1989. A study of the low temperature oxidation of coal. Fuel Processing Technology, vol. 21, no. 2. pp. 81-97. [ Links ]
Kaymakci, E. and Didari, V. 2002. Relations between coal properties and spontaneous combustion parameters. Turkish Journal of Engineering & Environmental Sciences, vol. 26. pp. 59-64. [ Links ]
Lyman, R.M. and Volkmer, j.E. 2001. Pyrophoricity (spontaneous combustion) of Powder River Basin coals: Considerations for coal bed methane development. Coal Report CR 01-1. Wyoming State Geological Survey, Laramie, WY. [ Links ]
Moolman, J. 2010. Grootegeluk Medupi Expansion Project - Detailed design - 3D design showcase. Grootgeluk Coal Mine, Lephalale, South Africa. [ Links ]
Panigrahi, D.C., Udaybhanu, G., Yadav, M.D., and Singh, R.S. 2005. Development of inhibitors to reduce the spontaneous heating susceptibility of Indian coals. Proceedings of the Eighth International Mine Ventilation Congress, Brisbane, Queensland, Australia, July 2005. Australasian Institute of Mining and Metallurgy, Melbourne. [ Links ]
Phillips, H., Uludag, S., and Chabedi, K. 2011. Prevention and control of spontaneous combustion. Best Practice Guidelines for Surface Coal Mines in South Africa. Coaltech Research Association, Johannesburg. [ Links ]
Department of Minerals and Energy. 2002. Guideline for the compilation of a mandatory code of practice for the prevention of flammable gas and coal dust explosions in collieries. DME 16/3/2/1-A1, 1 August 2002. Government Printer, Pretoria. [ Links ]
US Department of Energy. 1994. Primer on spontaneous heating and pyrophoricity. DOE Handbook FSC-6910, DOE-HDBK-1081-94, Washington, DC.http://energy.gov/sites/prod/files/2015/01/f19/DOE-HDBK-1081-2014.pdf accessed: 15 November 2014]. [ Links ] ♦
 Correspondence:
Correspondence:
S. Govender
Email: Mervin.Govender@exxaro.com
Received: 14 Jul. 2016
Revised: 1 Oct. 2019
Accepted: 23 Feb. 2021
Published: May 2021
ORCID R.C.W Webber-Youngman https://orchid.org/0000-0002-0101-7125














