Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.120 n.2 Johannesburg Feb. 2020
http://dx.doi.org/10.17159/2411-9717/894/2020
HEAVY MINERALS PAPERS
A literature review and interpretation of the properties of high-TiO2 slags
H. Kotzé
Consensi Consulting, Mtunzini, South Africa. https://orcid.org/0000-0002-8937-7511
SYNOPSIS
The properties of high-TlO2 slags are markedly different from those of many other metallurgical slags. The objective of this paper is twofold: firstly, to assemble in one document the TiO2 slag properties, including TÍ3+/TÍ4+ ratio, liquidus temperatures, heat capacity, density, viscosity, thermal conductivity, and electrical conductivity: all expressed in terms of temperature and composition dependency. Secondly, this paper attempts to correlate published experimentally measured data with theoretically based parameters, which will enable the application of the measured properties to wider slag compositions within the pseudobrookite-TiO2 slag system.
Keywords: high-TiO2 slag, properties.
Introduction
Slag properties are an essential tool in problem solving, both in equipment design and metallurgical process control. Experimental work on high-TiO2 slags is particularly difficult because of their chemically corrosive nature and compositional sensitivity to oxygen partial pressure. While ample publications are available and are still being produced for silicate-based slags, comparatively few results are available for high-TiO2 slags. The objective of this work is firstly to summarize the essential properties for liquid TiO2 slags. Secondly, an attempt is made to derive correlations from the published experimental work which are based on a theoretical concept. In this way, it is believed that the experimental data can be cautiously extrapolated to other high-TiO2 slags. To this end, a set of typical high-TiO2 slags based on a data-set published in earlier work (Kotze and Pistorius, 2010) is used throughout this paper. The chemical composition of this set is given in Table I. Although 94%TiO2 is not considered 'typical', it was included in the example slags because many producers migrate to higher TiO2 slags.
The experimental data cited in this work is by no means claimed to be all-inclusive. Glimmers of work done in Eastern European countries are present, though not easily interpreted by English-speaking researchers. On occasion experimental data was not used because of a discrepancy in the data or experimental method.
Slag properties
Ti3+/Ti4+ ratio
Titania slag compositions are typically expressed as TiO2 because analytical methods measuring the total titanium are fast and less cumbersome than the wet chemistry titration method required to measure Ti3+ (Tranell, Ostrovski, and Jahanshahi, 2002). When appreciable amounts of Ti2O3 are included in the TiO2 value, titania is expressed as the equivalent TiO2. In these instances, slag assays exceed 100 because the oxygen is overestimated. The assay total is therefore an indication of the level of reduction. Previous attempts by the author to correlate measured Ti3+ values with assay totals yielded unsatisfactory results.
The balance between Ti4+ and Ti3+ follows Reaction [1] and like other transition elements, it is governed by a redox reaction such as the O-type behavior of Reaction [2] (Tranell, Ostrovski, and Jahanshahi, 2002). The Ti3+/Ti4+ ratio is therefore expected to be dependent on the oxygen partial pressure and the oxygen activity in the slag bath. Tranell and co-workers (Tranell, Ostrovski, and Jahanshahi, 1997, 2002) investigated these relationships in TiO2-Ti2O3-SiO2-CaO slags and found the Ti3+/Ti4+ ratio to depend on the oxygen partial pressure raised to the power of 0.21 (Tranell, Ostrovski, and Jahanshahi, 1997) and 0.23 (Tranel, Ostrovski, and Jahanshahi, 2002). Using optical basicity to represent oxygen activity, they derived a correlation using temperature, oxygen partial pressure, and optical basicity to calculate the Ti3+/Ti4+ ratio. This correlation yielded good results with all the slags they investigated, but not with those of other researchers.
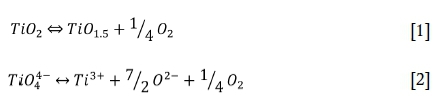
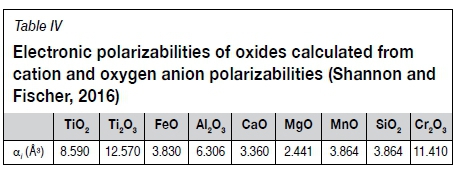
In this work, electronic polarizability was investigated as an alternative measure to predict the activity of oxygen. Electronic polarizability is described as the 'ability of oxygen to transfer electron density to surrounding cations' (Dimitrov and Komatsu, 2010). It is a measure of the propensity of compounds to donate charge from oxygen ions to the surrounding electron cloud (Duffy and Ingram, 1976). In this context, Ca2+ with a cation polarizability of 1.57Å3 is 'generous' while Si4+ at 0.284 Å3 is comparatively 'stingy.' A few mutually exclusive data-sets exist to calculate electronic polarizability. The data used in this work is based on the large data-set of dynamic polarizabilities published by Shannon and Fischer (2016) which includes a value for the cation polarizability of Ti3+. The Ti3+/Ti4+ ratios of the CaO-SiO2-TiOx slags from Tranell were found to correlate well with the ratio of the polarizabilities of Ca2+ and Si2+,
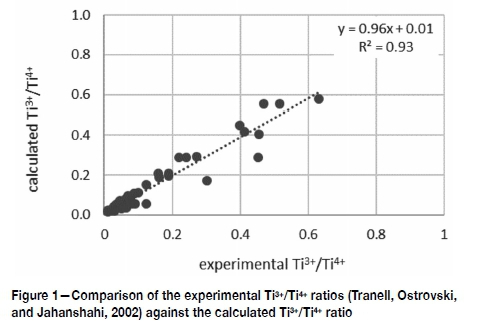
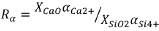 (polarizability Å3; mole fraction X) .This result corroborates the polarizability ratio of Ca2+ and Si4+ as a measure of the oxygen activity in the bath. The Ti3+/Ti4+ ratios of the slags Tranell studied were subsequently calculated using a correlation with two parameters: the experimental oxygen partial pressure and the polarizability ratio Ra. Figure 1 shows the results of this correlation to compare well with the experimental Ti3+/Ti4+ ratios.
(polarizability Å3; mole fraction X) .This result corroborates the polarizability ratio of Ca2+ and Si4+ as a measure of the oxygen activity in the bath. The Ti3+/Ti4+ ratios of the slags Tranell studied were subsequently calculated using a correlation with two parameters: the experimental oxygen partial pressure and the polarizability ratio Ra. Figure 1 shows the results of this correlation to compare well with the experimental Ti3+/Ti4+ ratios.
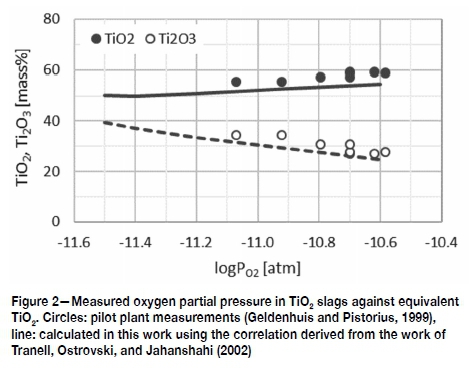
The validity of the correlation between oxygen partial pressure, electronic polarizability, and Ti valency was tested against industrial TiO2 slags using measurements reported by Geldenhuys and Pistorius (1999). These workers measured the oxygen partial pressure in pilot plant TiO2 slags and reported the
TiO2 and Ti2O3 values of the slags at the time of measurement. Their measured oxygen partial pressures in the slags are plotted as the circles in Figure 2. Since the full assays of the slags used in the Geldenhuys study were not given, their results are compared with the calculated TiO2 and Ti2O3 values of the example slags in Table I using the correlation derived from the work by Tranell. This is not ideal, but a reasonable replacement, as the impurity levels of the ilmenites used in the Geldenhuys study and those used in the Kotze (Kotze and Pistorius, 2010) study - on which the example slags were based were similar. The calculated results shown with the solid and broken lines in Figure 2 compare well with the experimental data. It is worthwhile to note that several studies found the same Ti3+/ Ti4+ ratios in industrial slags as were found in the Geldenhuys pilot plant study. Few of these results are published such as the industrial slag data points given in Figures 4 to 6, but many are unpublished due to intellectual property rights.
The dependency of the Ti3+/Ti4+ ratio on the oxygen partial pressure and CaO/SiO2 ratio (based on the work by Tranell) is summarized in Figure 3. Lower oxygen partial pressures lead to greater reduction and hence higher Ti3+/Ti4+ ratios. Higher CaO/ SiO2 ratios create higher oxygen activity, which in turn decreases the Ti3+/Ti4+ ratio. In a given smelting process the oxygen partial pressure is largely governed by the Fe FeO equilibrium in the slag (Pistorius, 2007), while the CaO/SiO2 ratio is determined by the raw material quality.
In an earlier paper Tranell also reports MgO to affect the Ti3+/Ti4+ ratio (Tranell, Ostrovski, and Jahanshahi, 1997). A correlation including the polarizability of Mg2+ did not fit the industrial data. The industrial data was a small set, though, and it does not exclude the potential of MgO (as possibly Al2O3) to -similarly to CaO and SiO2 influence the Ti3+/Ti4+ ratio. Future work will investigate this possibility.
Liquidus temperatures
Pesl and Erig (1999) conducted experimental work on slags of the TiO2-Ti2O3-FeO system, controlling the oxygen partial pressure with CO-H2 gas mixtures, and calibrating slag samples at 1500°C and 1600°C in platinum or molybdenum crucibles for 6 to 8 hours before rapidly quenching them to capture the high-temperature phases. Their predicted liquidus isotherms for 1500°C and 1600°C are reproduced for axes in mass% and given in Figure 4 and Figure 5 as dotted lines. No accuracy or error prediction was reported.
Seim melted slags in an induction furnace with a slag freeze-lining to protect the crucible walls (Seim, 2011; Seim and Kolbensen, 2010). Experiments were conducted in an Ar atmosphere, using titanium metal and haematite additions to control the level of reduction. Liquidus temperatures were determined from cooling curves captured by a spectropyrometer looking down onto the upper surface of the slag. The tolerances for individual measurements on the iron-saturated samples are given as 4.9°C to 33.3°C. Seim modelled a liquidus surface for the FeTiO3-Ti2O3-TiO2 system using the measured liquidus temperatures of the iron-saturated slags and expanding the thermodynamic data-set with two optimized ternary parameters. The model is considered to accurately predict the measured liquidus temperatures of the iron-saturated slags. The liquidus temperature measurements on the iron-unsaturated samples were unexpectedly and unexplainably lower and not used in the modelling exercise. Despite this, the measured liquidus temperatures of the iron-unsaturated slags plot on average 108°C below the modelled liquidus and 59°C above the modelled solidus temperatures. The model isotherms for 1500°C, 1600°C, and 1700°C were reproduced to have TiO2, Ti2O3, and FeO as corners of the ternary and are shown in Figure 4, Figure 5, and Figure 6 as broken lines.
Thermodynamic data for the binary systems TiO2-FeO and Ti2O3-TiO2 were published by Eriksson and Pelton (1993) and they illustrated the validity of using this data to calculate the solid phase relationships in the ternary system below 1400°C. They did not publish liquidus predictions (Eriksson and Pelton, 1996). The maximum inaccuracy of the data was given as ±20°C. In the present work the thermodynamic data from Eriksson were used in the Modified Quasichemical Approach (Pelton and Blander, 1986) to calculate the liquidus temperatures in the TiO2-Ti2O3-FeO system. A temperature, dependent activity coefficient was calculated for the pseudobrookite M3O5 phase from the experimental data by Pesl (Pesl and Eric, 1999). The calculated liquidus isotherms for 1550°C, 1600°C, and 1700°C are shown Figure 4, Figure 5, and Figure 6 as solid lines. The low-temperature trough these isotherms along approximately 55%TiO2 is in line with the experimentally measured low melting point measured in argon by Brauer and Littke (1960) for the TiO2 Ti2O3 binary: 1660°C between TiO170 and TiO175 (43% to 53% TiO2).
All three isotherm sets show a liquid slag field with (i) an upper phase boundary with the liquid slag + TiOx phase field, (ii) a lower phase boundary with the liquid slag + liquid iron phase field, and (iii) a boundary with the liquid slag + M3O5 + liquid metal phase field. The two upper phase boundaries at 1700°C (i.e. the calculated, and Seim prediction) are very similar, and so are the upper phase boundaries of these two sets for 1500°C and 1600°C up to 20%Ti2O3 - refer to Figure 7. Beyond 20% Ti2O3 the calculated isotherms predict the liquid slag phase field to extend towards TiO2.Ti2O3, similar to the dotted 1600°C and 1500°C isotherms by Pesl. The lower boundary separating the liquid slag from the liquid slag + liquid iron phase field, is predicted to lie closer to the TiO2 apex by the Seim model.
To place the liquidus isotherms in Figures 4 to 6 in context with industrial slags, compositions of typical as-tapped TiO2 slags from three different smelters (Pistorius, 2002) are shown as dark squares on the ternaries. For the high-FeO slags the dotted 1500°C and 1600°C upper boundary isotherms by Pesl imply a 100°C drop in liquidus with a ±1% change in FeO (approximately 19% to 18%). This seems an unlikely discontinuity in the known FeO-liquidus temperature relationship of the system. The more likely case is a more gradual temperature gradient as shown by the calculated 50°C spaced isotherms in Figure 7. These are also in agreement with the isotherms by Seim - at least up to 20%Ti2O3.
According to the calculated isotherms the industrial slag series have liquidus temperatures ranging from 1550°C for 20%FeOeq up to 1700°C for ±9%FeOeq, while the broken line isotherms (Seim model) indicate a liquidus range starting somewhere above 1600°C to just over 1700°C. Given the stated tolerances and inaccuracies, the different sets of isotherms are regarded to give similar results, especially in the area of concern for typical industrial TiO2 slags. It will be worthwhile, though, to clarify the discrepancy in the size of the liquid slag phase fields as predicted by the different sets of isotherms.
Figure 7 shows how the liquidus increases with decreasing %FeO: higher reduction yields a higher slag liquidus. However, for a given FeO content, e.g. 10mass%, higher reduction will increase the Ti3+/Ti4+ ratio, which will decrease the liquidus, until the Ti3+/Ti4+ ratio crosses the low liquidus trough between TiO2 and Ti2O3. On the lower TiO2 and higher Ti2O3 side of this trough, the liquidus will increase again with increasing Ti3+/Ti4+. In practice, where a process receives a stable ilmenite quality, higher reduction will lead to a simultaneous decrease in FeO and an increase in Ti2O3, and the net effect will be an increase in liquidus, though smaller in size than what would have resulted from a decrease in FeO only.
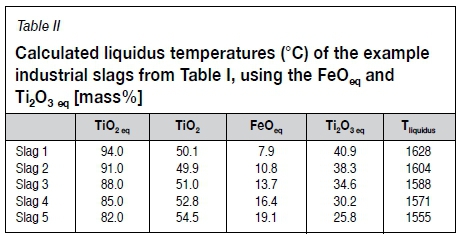
To apply phase temperature behaviour from the ternary FeO-TiOx (Figure 7) system to industrial slags, the gangue oxides are grouped into the so-called equivalent FeO and equivalent Ti2O3 values, calculated with Equations [3] and [4] (percentages as mass%, molecular weight M [g/mol]) (Pistorius, 2007). FeOeq and Ti2O3eq were shown to correlate, following the stoichiometric relationship of the pseudobrookite solid solution TiO2.(Ti3+Al3+Cr3+)2O3 (Fe2+Mg2+Mn2+)O.2TiO2, in short referred to as M3O5. Researchers have shown MgO, MnO, and Al2O3 to partition to the dominant M3O5 (pseudobrookite) phase thereby stabilizing this phase (Borowiec, 2009; Pistorius, 2002). There are indications that MnO does not fully partition to the M3O5 phase but that a potentially significant portion thereof separates to the silicate phase (Seim, 2011; Pesl and Eric, 2002). Without quantitative data on the behaviour of MnO though, the correlation showed by Pistorius is followed. The liquidus isotherms in Figure 7 can therefore applied to industrial slags when the FeO and Ti2O3 are expressed in their equivalent forms. The FeOeq and Ti2O3eq of the example slags (Table I), together with their calculated liquidus temperatures, are listed in Table II.
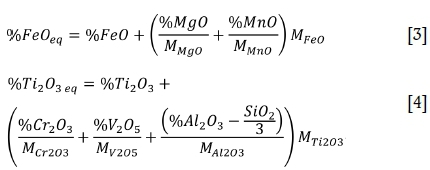
High-TiO2 slags have a narrow gap between liquidus and solidus temperatures. Seim measured the liquidus and solidus temperatures on synthetic slags using differential thermal analysis/thermal gravity analysis (Seim, 2011). For a TiO2-Ti2O3-5.6%FeO slag the liquidus and solidus were 1696°C and 1682°C respectively, and for a TiO2-Ti2O3-16.9%FeO slag, 1664°C and 1644°C respectively. These results imply a narrow temperature gap of 14°C to 20°C between liquidus and solidus. Borowiec (2009) measured solidus and liquidus temperatures on slags with 11-12%, 6-7%, and 2% gangue impurities (the publication gives the impurities for the first and third slag, while impurities for the second slags were deduced from other publications using the same slag). The temperature differences were 136°C, 83°C, and 66°C respectively (Borowiec, 2009). Provided the SiO2 content in the gangue impurities is low (i.e. <5% in >10% total gangue, and <3% in <7% total gangue), these differences are indications of the solidus temperatures of high-TiO2 slags.
Heat capacity
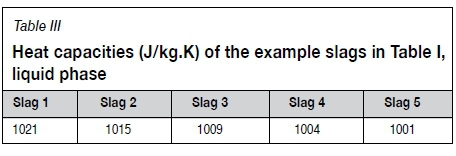
The heat capacities of liquid TiO2 slags were reported by Kotze and Pistorius (2010) and are repeated here, Equation [5] (Cpin J/ kg.K). The heat capacity values for the example slags in Table I are listed in Table III.
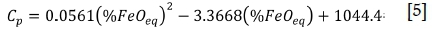
Density
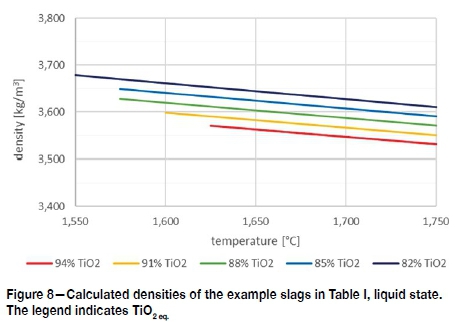
With most liquid slag systems, a density calculation needs to incorporate the enthalpy of mixing. For slags with only a small deviation from ideality, such as high-TiO2 slags (Eriksson and Pelton, 1993; Gaskell, 2008, p. 247), a weighted average of the densities of the pure liquids suffices. The density of liquid TiO2 was taken as 3.3 g/cm3 at 1600°C from the measurements by Dingwell (1991). The density of liquid Ti2O3 was measured by Ikemiya et al. under an Ar-H2 gas mixture (1993); extrapolated to 1600°C, Ti2O3 has a density of 4.0 g/cm3. Finally, the density of liquid FeO was taken as 4.4 g/cm3 at 1600°C based on the measurements done by Xin et al. (2019). The resultant calculated densities for the example slags (Table I) are given in Figure 8. It is concuded that typical high-TiO2 slags have a density range of 3.5 g/cm3 to 3.7 g/cm3. This is lower than the temperature-independent value of 3.8 g/cm3 often cited in literature (Kotze and Pistorius, 2010; Zietsman and Pistorius, 2006). Figure 8 shows that the slag densities decrease with increasing temperature, as well as with increasing total TiO2.
Electrical conductivity
The experimental measurements of the electrical conductivities of TiO2 slags by Desrosiers are reproduced in Figure 9 (Desrosiers, Ajersch, and Grau, August 1980). The electrical conductivity of TiO2 slags is exceptionally high compared to silicate slags, which have conductivities in the order of 0.1 up to less than 10 Ω1. cm1. High-FeO slags (>70%FeO) have similar and even higher electrical conductivities compared to the titania slags (Jiao and Themelis, 1988; Frederikse and Hosler, 1973). The Desrosiers slags form two distinct groups: those with low gangue (oxides excluding Fe and Ti oxides: 5-6%, 66% to 79%TiO2eq), and high gangue (15-17% gangue, 80% to 88%TiO2eq).
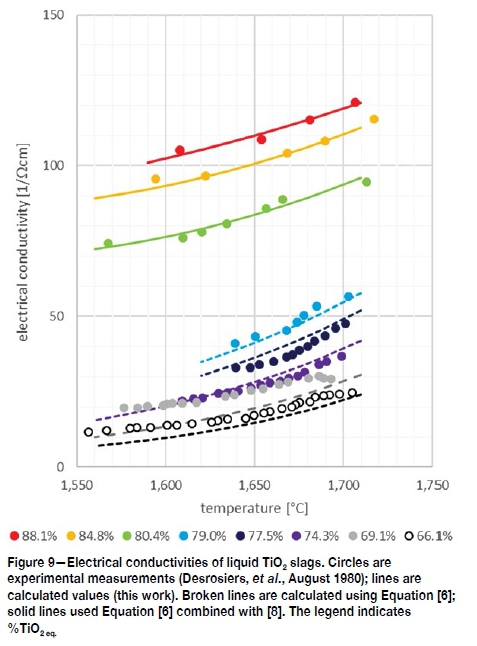
Since electrical conductivity is the movement of electron charges, the concept of electronic polarizability was tested against the experimental electrical conductivity measurements. By using the polarizability of the oxide components (Table IV), it was possible to reproduce the electrical conductivities of the high-gangue TiO2 slags using a correlation of the form given in Equation [6] (electrical conductivity σ [Ω-1cm-1]; electronic polarizability of the slag aslag [Å3] calculated with Equation [7] and the oxide polarizabilities given in Table IV; a a constant; and n a temperature-dependent constant). The calculated electrical conductivities for the high-gangue group (lower TiO2eq) are given by the broken lines in Figure 9: these calculated values correspond remarkably well with the measured data from Desrosiers.

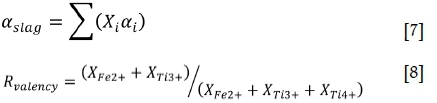
Applying Equation [6] to the low-gangue group (high-TiO2eq) yielded fair, but not, good results. The addition of a temperature-dependent valency ratio RYden(y, Equation [8], improved the calculation significantly: the results are shown by the solid lines in Figure 9.
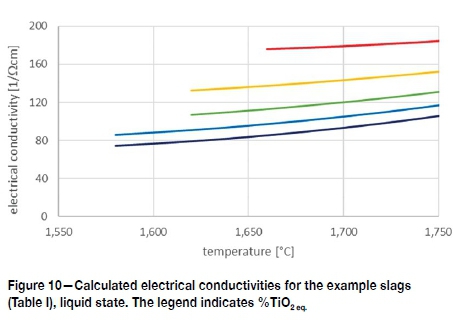
The electrical conductivities calculated for the example slags (Table I) are shown in Figure 10. The results of the 82%, 85%, and 88%TiO2eq slags correspond with the measured values of similar slags shown in Figure 9. The predicted electrical conductivities for the 91% and 95% TiO2 slags cannot be verified against experimental data, but since the calculation is based on parameters which supports the theory of electrical conductivity in slags, the prediction carries some level of confidence. Judging from the intervals between the lines of the five example slags, the increase in electrical conductivity accelerates with increasing TiO2eq. A similar phenomenon is shown for high-FeO slags up to 100% FeO (Jiao and Themelis, 1988).
Viscosity
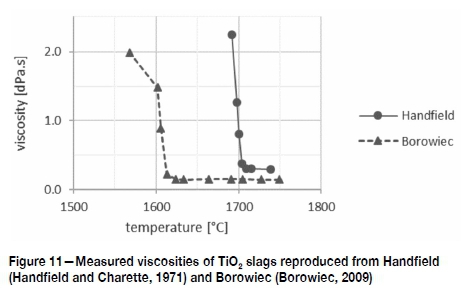
The viscosity of slags is known to be structure-dependent and high-TiO2 slags are no different: above their liquidus temperatures TiO2 slags dissociate into Ti4+, Ti3+, and Fe2+; these have no structure such as SiO2 networks provide for silicate slags. Consequently, fully liquid TiO2 slags have very low viscosities. The measured data for 'low-FeO' Sorel slag (Handfield and Charette, 1971) and 95%QMM slag (Borowiec, 2009) are reproduced in Figure 11: above their liquidus temperatures these slags measured 0.3 dPa.s and 0.15 dPa.s. A recent study reports 0.6 dPa.s for fully liquid slags (Hu etat., 2018). Though these numbers are different, they are all very low and not sensitive to composition or temperature. A second point of importance is that the critical temperature where viscosity increases sharply is only slightly below the slags' liquidus temperatures (Borowiec, 2009; Hu et at., 2018).
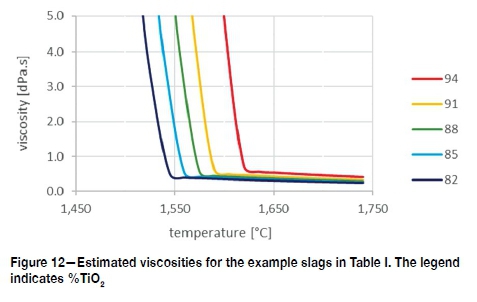
Considering these results, the viscosities of the example slags (Table I) are estimated as shown in Figure 12. The dominant feature in these viscosity curves is the sharp upwards turn, the estimated positioning of which, was based on the calculated liquidus temperatures of the example slags given in Table II.
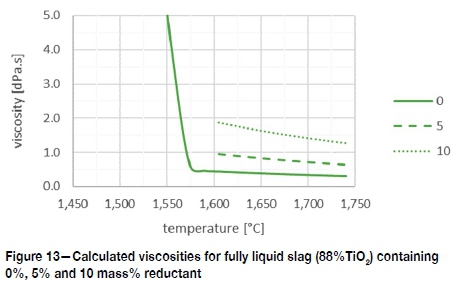
In industrial smelting the slag bath is likely to contain some unreacted reagent. The Einstein-Roscoe equation was used to quantify the impact thereof on the viscosity of the liquid bath (Equation [9]: viscosity of the liquid with solids, η [Pa.s]; viscosity of fully liquid phase η0 [Pa.s];ƒ volume fraction of the solids; assumed density for reductant 0.9 g/cm3). This is an approximation because the Einstein-Roscoe equation was derived for solid spheres and reductants are often angular. The calculated
viscosities for 0%, 5%, and 10mass% reductant in the 88%TiO2 fully liquid slag are shown in Figure 13. At 10mass% reductant the viscosity of the slag bath increases from around 0.4 dPa.s to 1.5 dPa.s. This is similar to a change from olive oil at 35°C to maple syrup at 25°C (Nierat, Musameh, and Abdel-Raziq, 2014; Ngadi and Yu, 2004): not unmanageable - as Handfield points out: blast furnace slags are still tappable at 5 dPa.s, but also not insignificant considering the foaming tendency of TiO2 slag baths.

Thermal conductivity
The thermal conductivity of solid TiO2 slags was reported as 0.00175T + 0.3 W/m°C, which gives a thermal conductivity range of 1.0 to 2.9 W/m°C from 400°C to 1500°C (Kotze and Pistorius, 2010). Thermal conductivity measurements on solid TiO2 slags from room temperature to 1050°C varied from just above 1 W/mK to approximately 2.5 W/mK with mostly, but not always, a positive relationship with temperature (Heimo, 2018). A single measurement in the same study gave 5 W/m°C at 1400°C. In the absence of data for liquid slag, 1 W/m°C is sometimes used in modelling (Zietsman and Pistorius, 2006). In the following paragraphs an attempt is made to approximate the thermal conductivity of high-TiO2 slags based on the structure of these slags and their optical properties.
The thermal conductivity of slags is a combination of lattice kbtt, radiation kmd, and electronic conduction ke(Mills and Susa, 1992). Lattice conduction is structure-dependent because it relies on the velocity of phonons v through the slag, and the mean free path of phonons L through the slag, (Equation [10]; heat capacity at constant pressure Cp). In solids the crystal lattice provides the structure along which phonons travel; in silicate slags, the SiO2 network provides the means. Thermal conductivity values measured with the transient hot wire method, a technique which isolates lattice conductivity, range from 0.03 to 1 W/mK for silicate slags (Glaser and Sichen, 2013; Kang etat., 2012). Lattice thermal conductivities decrease with increasing temperature, reflecting the diminishing slag structure with increasing temperature. In TiO2 slags - judging from the low viscosities above liquidus temperatures - no significant structure exists. Lattice thermal conductivities in high-TiO2 slags are therefore expected to be small. Nevertheless, the lattice conductivity where estimated from a correlation between reported transient hot wire thermal conductivities and calculated viscosities of silicate slags. The model used for viscosity calculations is based on the work by Zang (Zhang et at., 2012, Zhang and Chou, 2012), a little-known model for SiO2-Al2O3-CaO-MgO-FeO-MnO-K2O slags, but with good reproducibility of experimental data, and with potential to be expanded to more oxide components (Kotze, 2017).

The radiative component of thermal conductivity kradis calculated with Equation [11] (Mills and Susa, 1992) (Stefan Boltzmann constant σ = 5.67x10-8 W/m2K4; refractive index of the slag n [dimensionless]; temperature T [K]; absorption coefficient β [cm-1] usually denoted as a in the literature but changed here to avoid confusion with the earlier mentioned electronic polarizability). The refractive indexes of the transition metal oxides are generally higher: 2 and more, compared to 1.535 for SiO2, 1.805 for CaO, 1.715 for MgO, and 1.79 for Al2O3. Based on this the radiative component in the TiO2 slags could be expected to be higher than for silicate slags. However, the absorption coefficient for FeO is two orders of a magnitude higher (Susa, Nagata, and Mills, 1993), and that of Ti2O3 three orders of a magnitude higher (Li, 2016) than those of SiO2, CaO, MgO and Al2O3. Consequently, the radiative component of high-TiO2 slags is low compared to low FeO silicate slags.

The electronic thermal conductivity kewas calculated with the Wiedemann-Franz formula (Ok et al., 2018) (Equation [12], electrical conductivity σ [Q-1m-1]; Lorenz number L = 2.44x10-8 WQ/K2; temperature T [K]). Due to the high electrical conductivity of TiO2 slags, electronic conductivity, which is negligible in silicate slags, can be significant for TiO2 slags.

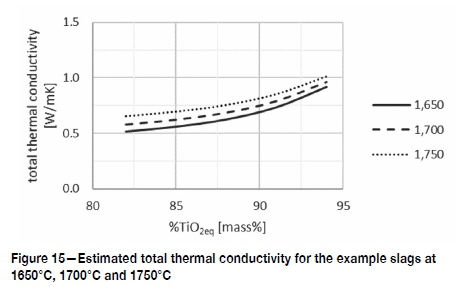
The etimated lattice, and calculated radiative and electronic conductivities of example slags 1, 3, and 5 (94%, 88%, and 82% TiO2eq) are shown in Figure 14. Total thermal conductivities are shown with the thicker solid lines. The total thermal conductivity for liquid high-TiO2 slags is estimated to range from 0.4 W/mK to close to 1 W/mK over the liquid temperature range. The thermal conductivity is predicted to increase non-linearily with increasing TiO2eq (Figure 15) which is attributed to the increase in electronic conduction with increasing TiO2eq.
Summary
Experimentally measured properties of high-TiO2 slags were investigated and correlated with theoretically based parameters. Based on these correlations, the properties for five typical high-TiO2 slags were calculated.
➤The Ί13+/Ί14+ ratio of the liquid slags depends on the oxygen partial pressure and the oxygen activity in the bath. The polarizability ratio between Ca2+ and Si4+ cations was found to be a good parameter to quantify the oxygen activity. Future work will endeavor to investigate this concept against more TiO2 slag data and other slag systems.
➤Liquidus temperatures increase with decreasing FeO, but can increase or decrease with increasing Ti2O3 depending on the ratio between Ti3+ and Ti4+. Following on from the Ti3+/Ti4+ correlations, liquidus temperatures depend therefore on FeO, oxygen partial pressure, and the CaO and SiO2 impurities. Other impurities such as MgO, MnO, Al2O3, Cr2O3, and V2O5 also affect liquidus values when they stabilize the M3O5 pseudobrookite phase. The partitioning of MnO between the M3O5 and silicate phases needs to be reviewed.
➤The heat capacity of liquid high-TiO2 slags cover a narrow range from 1000 to 1020 J/kgK.
➤Liquid slag densities range from 3.5 to 3.7 g/cm3, with the lower range applying at higher temperatures and to higher TiO2 eq slags.
➤ The electrical conductivities of liquid high-TiO2 slags are one to two order of magnitudes higher than those of silicate slags and increase with increasing temperature. This is an important point for both AC and DC furnace design. The electrical conductivity of high-gangue TiO2 slags correlates with the slags' electronic polarizability, while for low-gangue TiO2 slags (high-TiO2eq), the electrical conductivity was reproduced by using the slags' electronic polarizability and the valency ratio between Fe and Ti cations.
➤The viscosities of high-TiO2 slags above liquidus temperatures are very low but increase sharply just below liquidus temperatures. Though the viscosities of fully liquid slags are low, the presence of unreacted reductant in the bath will increase its viscosity.
➤The thermal conductivities of liquid high-TiO2 slags were derived from their viscosities and optical properties. For the example slags the thermal conductivities are estimated to range from 0.5 to 1 W/mK. Electronic thermal conduction is dominant and contributes to most to the total thermal conductivity.
Though the intention of this paper is to create a basis for extrapolation to TiO2 slags not covered in the specific set of experimental work, the correlations derived here are still only applicable to the typical TiO2, pseudobrookite M3O5 slags.
Slag properties are evasive: the need to estimate the thermal conductivity of the TiO2 slags from theoretical considerations is a point in case. It is a complex field but our understanding of slag properties has expanded over the last approximate two decades. It is the author's hope that many a publication on the properties of TiO2 slags is still to see the light.
Acknowledgements
I would like to express sincere gratitude to all the researchers who contribute to the field of slag properties. My gratitude also goes to the referees who fulfilled their duty to challenge my thoughts and assumptions. And to all the designers and producers who apply this understanding of slag properties to optimize their processes: thank you for your support.
References
BoRowEc, K. 2009. Partition of impurities within titania slag. Santiago, Chile, s.n., pp. 23-31. [ Links ]
Braüer, G. and Littke, W. 1960. On the melting point and thermal dissociation of titanium dioxide. Journal of Inorganic and Nuclear Chemistry, vol. 16. pp. 67-76. [ Links ]
Desrosiers, R., Ajersch, F., and Grau, A. August 1980. Electrical conductivity of industrial slags of high titania content. Halifax, Nova Scotia, s.n. [ Links ]
Dimitrov, V. and Komatsu, T. 2010. An interpretation of optical properties of oxides and oxide glasses in terms of the electronic ion polarizability and average single bond strength. Journal of the University of Chemical Technology and Metallurgy, vol. 45, no. 3. pp. 219-250. [ Links ]
Dingwell, D.B. 1991. The density of titanium(IV) oxide liquid. Journal of the American Ceramic Society, vol. 74, no. 10. pp. 2718-2719. [ Links ]
Duffy, J. and Ingram, M. 1976. An interpretation of glass chemistry in terms of the optical basicity concept. Journal of Non-Crystalline Solids, vol. 21, pp. 373-410. [ Links ]
Eriksson, G. and Pelton, A.D. 1993. Critical evaluation and optimization of the thermodynamic properties and phase diagrams of the MnO-TiO2, MgO-TiO2, FeO-TiO2, Ti2O3-TiO2, Na2O-TiO2, and K2O-TiO2 systems. Metallurgical TransactionsB, October, Volume 24B, pp. 795-805. [ Links ]
Eriksson, G. and Pelton, A.D. 1996. Measurement and thermodyamic evaluation of phase equilibria in the Fe-Ti-O system. Ber. Bunsenges Phys Chem, vol. 100, no. 11. pp. 1839-1849. [ Links ]
Frederikse, H. and Hosler, W. 1973. Electrical conductivity of coal slag. Journal of the American Ceramic Society, August, pp. 481-419. [ Links ]
Gaskell, D.R. 2008. Introduction to the Thermodynamics of Materials. Fifth edition ed. New York, London: Taylor & Francis. [ Links ]
Geldenhuis, J. and Pistorius, P. 1999. The use of commercial oxygen probes during the production of high titania slags. Journal of the South African Institute of Mining and Metallurgy, January/February, pp. 41-48. [ Links ]
Glaser, B. and Sichen, D. 2013. Thermal conductivity measurements of ladle slag using transient hot wire method. Metallurgical and Materials Transactions B, February, vol. 44B. pp. 1-4. [ Links ]
Handfield, G. and Charette, G. 1971. Viscosity and structure of industrial high-TiO2 slags. Canadian Metallurgical Quarterly, vol. 10, no. 3. pp. 235-243. [ Links ]
Heimo, J. 2018. Thermal conductivity of titanium slags, Aalto: Aalto University. [ Links ]
Hu, K. et al., 2018. Viscosity of TiO2-FeO-Ti2O3-SiO2-MgO-CaO-Al2O3 for high-titania slag smelting process. Metallurgical and Materials Transactions B, August, vol. 49B. pp. 1963-1973. [ Links ]
Ikemiya, N., Umemoto, J., Hara, S., and ogino, K. 1993. Surface tension and densities of molten Al2O3, Ti2O3, V2O5 and Nb2O5. ISIJ International, vol. 33, no. 1. pp. 156-165. [ Links ]
Jiao, Q. and Themelis, N. 1988. Correlations of electrical conductivity to slag composition and temperature. Metallurgical Transactions B, February, vol. 19B. pp. 133-140. [ Links ]
Kang, Y. etal., 2012. Thermal conductivity of the molten CaO-SiO2-FeOx system. Metallurgical and Materials Transactions B, December, vol. 43B. pp. 1420-1426. [ Links ]
Kotze, H. 2017. Comparison between viscosity models, Consensi internal study. [ Links ]
Kotze, H. and Pistorius, P. 2010. A heat transfer model for high titania slag blocks. Journal of the South African Institute of Mining and Metallurgy, February, vol. 110. pp. 57-66. [ Links ]
Li, Y. 2016. Investigation of titanium sesquioxide Ti2O3: synthesis and physical properties, Thuwal, Kingdom of Saudi Arabia. [ Links ]
Mills, K. and Susa, M. 1992. Thermal Conductivities of Slags, Teddington, United Kingdom: National Physical Laboratory. [ Links ]
Ngadi, M. and Yu, L. 2004. Rgeological properties of Canadian maple syrup. Canadian Biosystems Engineering, vol. 46. pp. 3.15-3.18. [ Links ]
Nierat, T., Musameh, S., and Abdel-Raziq, I. 2014. Temperture-dependence of olive oil viscosity. Materials Science An Indian Journal, vol. 11, no. 7. pp. 233-238. [ Links ]
Ok, K.M. et al., 2018. Effect of point and planar defects on thermal conductivity of TiO2-x. Journal of the American Ceramic Society, vol. 101. pp. 334-346. [ Links ]
Pelton, A.D. and Blander, M. 1986. Thermodynamic analysis of ordered liquid solutions by a modified quiasochemical approach - application to silicate slags. Metallurgical Transactions B, December, vol. 17B. pp. 805-815. [ Links ]
Pesl, J. and Eric, R. 2002. High temperature carbothermic reduction of Fe2O3-TiO2-MxOy oxide mixtures. Minerals Engineering, vol. 15. pp. 971-984. [ Links ]
Pesl, J. and Eric, R.H. 1999. High-temperature phase relations and thermodynamics in the iorn-titanium-oxygen system. Metallurgical and Materials Transactions B, August, vol. 30B. pp. 695-705. [ Links ]
Pistorius, P. 2007. Ilmenite smelting: the basics. s.l. The Southern African Institute of Mining and Metallurgy, pp. 75-84. [ Links ]
Pistorius, P. C. 2002. The relationship between FeO and Ti2O3 in ilmenite smelter slags. Scandinavian Journal of Metallurgy, vol. 31. pp. 130-125. [ Links ]
Seim, S. 2011. Experimental investigations and phase relations in the liquid FeTiO3-Ti2O3-TiO2 slag system, Trondheim. [ Links ]
Seim, S. and Kolbensen, L. 2010. Update on the equilibrium between liquid Fe-Ti-O slags and metallic iron. Steel Research International, vol. 81, no. 12. pp. 1051-1055. [ Links ]
Shannon, R.D. and Fischer, R.X. 2016. Empirical electronic polarizabilities of ions for the prediction and interpretation of rerfactive indices: oxides and oxysalts. American Mineralogist, vol. 101, pp. 2288-2300. [ Links ]
Susa, M., Nagata, K., and Mills, K. 1993. Absorption coefficients and refractive indices of synthetic glassy slags containing transition metal oxides. Ironmaking and Steelmaking, vol. 20, no. 5. pp. 372-378. [ Links ]
Tranell, G., ostrovski, o., and Jahanshahi, S. 1997. The thermodynamics of titanium in CaO-MgO-Al2O3-SiO2-TOx slags. Sydney, pp. 501-506. [ Links ]
Tranell, G., ostrovski, o., and Jahanshahi, S. 2002. The equilibrium partitioning of titanium between Ti3+ and Ti4+ valency states in CaO-SiO2-TiOx slags. Metallurgical and Materials Transactions B, February, vol. 33B. pp. 61-67. [ Links ]
Xin, J., Gan, L., Wang, N., and Chen, M. 2019. Accurate density calculation for molten slags in SiO2-Al2O3-CaO-MgO-FeO-Fe2O3 systems. Metallurgical and Materials Transactions B, 50B(December). pp. 2828-2842. [ Links ]
Zhang, G.-H. and Chou, K.-C. 2012. Measuring and modeling viscosity of CaO-Al2O3-SiO2(-K2O) melt. Metallurgical and Materials Transactions B, August, vol. 43B. pp. 841-848. [ Links ]
Zhang, G.-H., Chou, K.-C., and Mills, K. 2012. Modelling viscosities of CaO-MgO-Al2O3-SiO2 molten slags. ISIJ International, vol. 52, no. 3. pp. 355-362. [ Links ]
Zhang, G.-H., Chou, K.-C., Xue, Q.-G., and Mills, K. C. 2012. Modeling viscosities of CaO-MgO-FeO-MnO-SiO2 molten slags. Metallurgical and Materials Transactions B, February, vol. 43B. pp. 64-72. [ Links ]
Zietsman, J. H. and Pistorius, P. 2006. Modelling of an ilmenite-smelting DC arc furnace process. Minerals Engineering, vol. 19. pp. 262-279. [ Links ] ♦
 Correspondence:
Correspondence:
H. Kotze
Email: hanlie.k@consensi.co.za
Received: 23 Aug. 2019
Revised: 24 Jan. 2020
Accepted: 13 Feb. 2020
Published: February 2020
This paper was first presented at The Eleventh International Heavy Minerals Conference, 5-6 August 2019, The Vineyard, Cape Town, South Africa.














