Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.119 n.11 Johannesburg Nov. 2019
http://dx.doi.org/10.17159/2411-9717/646/2019
GENERAL PAPERS
Improving the separation efficiency of Southern African haematite from slimes through selective flocculation coupled with magnetic separation
C. Da CorteI; C. BergmannI; L. WoollacottII
IMintek, South Africa
IIUniversity of the Witwatersrand, South Africa
SYNOPSIS
With depleting reserves of high-grade iron ores in South Africa, the local minerals processing industry is increasingly paying attention to fine tailings material as a potential resource for the future. A significant proportion of these tailings consists of slimes, i.e. material finer than 38 μηι. Upgrading of slimes by physical separation techniques is usually constrained by low efficiency at such fine sizes. Selective flocculation has the potential of overcoming these constraints to a degree. This paper reports on a study that investigated that potential by coupling selective flocculation with magnetic separation to improve separation efficiencies. The coupled process achieved an improvement in the grade of the magnetic concentrate from 52.3% to 59.2% Fe at much the same Fe recovery. This constitutes an improvement in separation efficiency from about 40% to 57%. These results were achieved under laboratory conditions, confirming the positive indications found in the literature, and give an indication that similar results may be possible at an industrial scale.
Keywords: selective flocculation, haematite, magnetic separation, SLon-100.
Introduction
In iron ore production in South Africa a significant proportion of the feed is lost as slimes. Dworzanowski (2014) reports that South Africa's major iron ore producer, Kumba Iron Ore's Sishen mine, discards approximately 3 Mt/a of -200 μηι material as tails. These tails contain about 60% -10 μm material (1.8 Mt/a) and constitute a valuable potential source of iron in the future.
Two approaches are currently being used for extracting iron ore from tailings, namely magnetizing roasting and direct reduction (Li et al., 2010). In the former case, roasting reduces paramagnetic haematite to ferromagnetic magnetite, enabling recovery via wet low-intensity magnetic separation. However, both options are energy intensive, with temperatures ranging from 700°C to 900°C required for magnetizing roasting and >1000°C for direct reduction. As the drive towards energy efficient processes becomes more critical, alternative approaches to tailings slimes treatment are desirable. However, the options are limited. Below 20 μι^ only enhanced gravity separation, high-intensity magnetic separation, and flotation are effective (Wills, 2016). Another option is to employ selective flocculation, which involves the selective aggregation of the desired mineral from a mixture of minerals using the bridging mechanism. Selective flocculation has been used to improve efficiencies, product grades, and recoveries when treating haematite slimes by selectively increasing the effective size of the targeted particles (Mathur, Singh, and Moudgil, 2000; Pascoe and Doherty, 1997; Roy, 2012; Song, Lu and Lopez-Valdivieso, 2002).
Selective flocculation of haematite from gangue can be achieved through various methods using microorganisms (Poorni and Natarajan, 2013; Pradhan et al., 2006); polymers (Abro, 2009; Abro et al., 2013; Ahmed and Mahran, 2013; Arol and Aydogan, 2004; Drzymala and Fuerstenau, 2014; Hanumantha Rao and Narasimhan, 1985; Kumar and Mandre, 2017; Ma, 2012; Weissenborn, 1996; Weissenborn, Warren, and Dunn, 1994), and collectors such as sodium oleate (Pascoe and Doherty, 1997; Roy, 2012; Song, Lopez-Valdivieso, and Ding, 1999; Song, Lu, and Lopez-Valdivieso, 2002; Yin et al., 2011).
Selective flocculation has been successfully applied at the industrial scale for haematite slimes at Tilden Mines in the USA (Haselhuhn, Carlson, and Kawatra 2012), copper ore from the Democratic Republic of Congo (Ansari, 1997), and potash ore (Forbes, 2011). However, certain factors have limited its widespread application. These include complex ore mineralogy, poor liberation, smearing of minerals, difficulties in controlling mineral surface properties and chemistry, water quality, and the entrainment and physical entrapment of gangue minerals in the voids in the flocs (Haselhuhn, Carlson, and Kawatra, 2012; Drzymala and Fuerstenau, 2014; Mathur, Singh, and Moudgil, 2000).
While flotation is the most commonly employed solid-solid separator in a selective flocculation process, magnetic separation can be used when the targeted minerals are paramagnetic (Arol and Aydogan, 2004; Roy, 2012; Song, Lu, and Lopez-Valdivieso, 2002). Selective flocculation coupled with magnetic separation has been termed the Floc Magnetic Separation (FMS) process by Song, Lu, and Lopez-Valdivieso (2002).
The Floc Magnetic Separation (FMS) process
Song, Lu, and Lopez-Valdivieso (2002) applied the principle of selective flocculation to the treatment of haematite slimes with magnetic separation used as the solid-solid separator. Haematite fines were first selectively flocculated, then subjected to magnetic separation at a medium field intensity rather than high-intensity or high-gradient magnetic separations, which are energy intensive. As illustrated in Figure 1, the FMS process consists of four stages, namely dispersion, selective hydrophobization, hydrophobic flocculation, and magnetic separation. Dispersion is necessary to prevent unwanted particle aggregation. In selective hydrophobization a surfactant is added to selectively render the target mineral (haematite) hydrophobic so that in the flocculation stage only those targeted particles flocculate. Magnetic separation then removes the flocs, leaving behind the unflocculated gangue particles in the nonmagnetic product. Each of these stages is further described in more detail below.
> Dispersion is a critical first step in the FMS process as it minimizes aggregation of fines, clay particles, and slime coatings on the mineral particles by controlling the charge density at the solid-liquid interfaces or the electrical charges of ultrafine particles (Bulatovic, 2007). Sodium silicate, along with pH modifiers, is commonly used as a dispersant in the minerals industry. In the FMS process, typical dosage rates of sodium silicate dispersant range from 900 g/t to 1 000 g/t (Song, Lu, and Lopez-Valdivieso 2002; Roy, 2012).
> Selective hydrophobization, the second step in the FMS process, involves the addition of appropriate surfactants to render the magnetic particles, haematite, hydrophobic so that the next step, hydrophobic flocculation, will flocculate haematite particles selectively. Sodium oleate is typically used as the surfactant with dosages in the region of 0.5 kg/t to 8 kg/t at a pH range of 2.5 to 11 (Pascoe and Doherty, 1997; Roy, 2012; Song, Lu, and Lopez-Valdivieso, 2002; Yin etal. 2011). Yin et al. (2011) showed that pH affects the equilibrium between the molecules of oleic acid and oleate ions in solution, thereby affecting both hydrophobicity and the size of the flocs that are subsequently formed.
> Hydrophobicflocculation involves the addition of a flocculant that adsorbs onto hydrophobic surfaces under conditions that grow flocs of a desirable size and condition. Medium-density non-polar oils such as kerosene, diesel, and paraffin are used as the flocculating agents in this stage of the process. Song, Lu, and Lopez-Valdivieso, (2002) found that kerosene dosages in the range of 1 kg/t to 5 kg/t with 20 minutes conditioning time gave good results. The growth of flocs is facilitated by mechanical conditioning at low shear rates for a specific period. Conditioning imparts sufficient kinetic energy to the particles to overcome the energy barrier between them.
At a pH of 8, Pascoe and Doherty (1997) achieved the biggest floc size (50% of the sample by volume larger than 30 μιη) at a stirring speed and time of 1200 r/min and 20 minutes respectively. At an optimal stirring speed of 1400 r/min, Yin et al. (2011) determined that flocculation commenced after 10 minutes of stirring with increasing floc size observed after 20 minutes. Yin et al. (2011) also found that increasing the residence time to 25 minutes resulted in the rupture of flocs. To avoid froth formation during stirring due to the presence of oleate ions, Song, Lu, and Lopez-Valdivieso (2002) fixed the stirring speed to 1200 r/min and varied the stirring time from 5 to 30 minutes. They found that the iron grade and recovery increased with stirring time and reached a plateau at approximately 20 minutes.
> Magnetic separation separates the paramagnetic haematite flocs from the unflocculated nonmagnetic gangue such as quartz. Song, Lu, and Lopez-Valdivieso, (2002) and Roy (2012) both used the conventional Jones Wet High-Intensity Magnetic Separator (WHIMS) at intensities of 1 T to 1.52 T. Svoboda and Fujita (2003) showed that at a magnetic field intensity of 1.06 T, haematite particles smaller than 25 μΓη cannot be recovered as the drag forces overcome the magnetic forces. However, Roy (2012) found that increasing the intensity to 1.40 T and 1.52 T decreased the particle size limit threshold to 22 μm and 21 μm respectively.
The objective of the study
In the previous studies the conventional WHIMS unit was used as the solid-solid separator. The factor differentiating this study from previous work is the use of a laboratory-scale pulsating WHIMS (SLon-100). The pulsating action in the SLon-100 helps to free nonmagnetics trapped within the magnetic concentrate and increases Fe recoveries at lower magnetic field intensities. The pulsating action is also expected to reduce gangue entrainment in the floc structure. The objectives of the study were twofold:
> To investigate the impact of selective flocculation on the product Fe grade and recovery achieved with the SLon
> To ascertain the optimal range of operating parameters for selective flocculation for the particular ore studied, while simultaneously maximizing the three response variables, namely: separation efficiency, Fe grade, and Fe recovery.
Experimental
Ore sample
The haematite ore used in the study was selected for its simple mineralogy. The material originated from Angola and graded at 37.34 ±0.47% Fe with silica as the main diluent (41.78 ±0.21%). The material was ball milled to artificially create slimes (82.3% passing 38 μιη). Table I shows the material's mineralogical characteristics measured by the Mineral Liberation Analyser (MLA) and indicates the milled material was well liberated; i.e. 89.0-91.5% of the dominant minerals were found in particles where the mineral of interest constituted more than 80% of the particle area. It also confirms the simple mineralogy of the ore tested, with haematite the dominant Fe mineral and quartz the dominant gangue mineral.
Reagents for selective flocculation
Sodium silicate was used as the dispersant, sodium oleate as the collector, and paraffin was used as the non-polar oil. The required mass of paraffin was weighed out to ensure the target dosage was added. Sodium hydroxide and sulphuric acid were used for pH modification and were prepared to solution concentrations of 1% and 0.5% by mass respectively.
Sodium silicate and sodium oleate were prepared as solutions in concentrations of 1% by mass. The latter was prepared according to the method recommended by Pascoe and Doherty (1997). Water was heated to 60°C, the pH adjusted to 10.5, and the sodium oleate was added and stirred. Once the sodium oleate had dissolved the solution was allowed to cool to room temperature before use. Pascoe and Doherty (1997) also recommended that fresh stock solutions of sodium oleate be prepared weekly, and thus all reagents were prepared on a weekly basis in the study. All reagent solutions were prepared with tap water.
Optimizing the conditions for selective flocculation
The design of the experimental programme was complicated by the four-stage nature of the FMS process, the interdependence between stages, and the large number of variables (seven in total) in the process. Song, Lu, and Lopez-Valdivieso (2002) found that the most critical variables affecting flocculation were particle hydrophobicity, kinetic energy input, and non-polar oil addition, while Pascoe and Doherty (1997) found that sodium oleate concentration, pH, shear rate, and agitation time were the main factors that affected floc size. From the literature it was evident that the most critical variables for selective hydrophobization were pH and the sodium oleate dosage and, for the flocculation stage, the dosage of the non-polar oil (paraffin), conditioning time, and the shear rate during conditioning. To simplify the optimization programme and to reduce the number of variables that had to be optimized, the following experimental design was adopted.
1. Dispersion was optimized independently using rheological tests to determine the conditions that would achieve the lowest yield stress in the slurry of haematite fines. The yield stress indicates the force required to overcome the attractive forces between the solids, thereby allowing for adequate dispersion. The optimum conditions were found to be a sodium silicate dosage of 1 kg/t at a pulp density of 45% solids.
2. The shear rate during conditioning was fixed at 1200 r/min as per the work of Song, Lu, and Lopez-Valdivieso (2002).
3. The selective flocculation conditions were optimized in two stages. The pH, which affects both hydrophobization and floc growth, was optimized first by conducting tests at pH values in the range from 9.5 to 11, with typical values for the other relevant variables - i.e. 1 kg/t sodium silicate, 1 kg/t sodium oleate, 3 kg/t paraffin, and 10 minutes conditioning time (Song, Lu, and Lopez-Valdivieso, 2002).
For each pH test, a representative 200 g sample of solids was added to a 1 litre Denver flotation cell with 244.4 g of tap water added to the cell to create a pulp density of 45% solids by mass. The pulp was agitated at 1200 r/min to ensure a homogeneous pulp. The sodium silicate was added and the pH of the pulp was adjusted to the required level. The sodium oleate and paraffin were then added and the pH re-adjusted and the pulp conditioned for 10 minutes. Thereafter the pulp was washed from the Denver cell into a beaker and subjected to either sizing or magnetic separation. Floc size was measured by laser diffraction using the Malvern Mastersizer 2000E with a sample dispersion unit (Hydro-2000 MU). Selectivity of flocculation was measured by extracting the flocs from the flocculated slurry using the magnetic separator and determining their composition.
4. The second step in optimizing the conditions for selective flocculation focused on the remaining three critical variables: the dosages of sodium oleate and kerosene, and the conditioning time. To optimize these variables, tests were conducted in which the other variables were set at their already-determined optimum values. As in the optimizing of the pH level, selective flocculation was coupled with magnetic separation so that the effectiveness of the selective flocculation conditions could be evaluated in terms of overall metallurgical performance. A Box-Behnken experimental design (NIST, 2012) was employed for these optimization tests. Seventeen runs in triplicate were employed to study the effect of the three factors (sodium oleate dosage, paraffin dosage, and conditioning time, each at three levels) on the response variables, namely the concentrate Fe grade and recovery, and separation efficiency. Separation efficiency is defined in Equation [1].
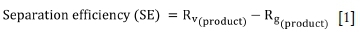
where Rv(product) is valuables (total Fe) recovery to the magnetic concentrate, and Rg(product) is gangue (SiO2) recovery to the magnetic concentrate.
5. The optimum conditions found in the selective flocculation optimization test work were retested three times to ensure statistical confidence in the results.
Table II summarizes the experimental design employed and shows which aspect was investigated, the associated operating variables, the criteria used for finding their optimum values, and the methodology used to determine those values.
As Table II indicates, magnetic separation was needed for different purposes at three points in the experimental programme.
1. In 'baseline tests' to establish the metallurgical performance of magnetic separation on samples before selective flocculation. For the beneficiation of iron ore slimes, Da Corte et al. (2015) determined the optima for most of the SLon-100 operating variables and only the background magnetic field intensity needed to be optimized for this study for the unflocculated slimes. The intensities tested ranged from 3 kG to 6 kG for the baseline magnetic separation tests. Although Da Corte et al. (2015) tested a magnetic field intensity of 8 kG, a lower range of intensities was selected as higher intensities require higher energy inputs and to contain costs a maximum of 6 kG was selected. The best intensity was used for determining the selectivity during the pH optimization stage and selective flocculation optimization stage.
2. To extract flocs from the flocculated slimes when finding the optimum pH for selective flocculation.
3. To establish the metallurgical performance of magnetic separation after selective flocculation when finding the optimum conditions for selective flocculation.
Results
Baseline tests: Magnetic separation without selective flocculation
Three tests were conducted at three background magnetic field intensity settings. The average results along with the associated error bars (two standard deviations indicating 95% confidence limits) are shown in Figure 2. The results follow the expected trend, with increased mass pull and Fe recovery of the magnetic product as the background magnetic field intensity increased. The Fe grade of the magnetic product remained relatively constant and within the 95% confidence limits across the intensities tested.
The separation efficiency increased with increasing background magnetic field intensity due to increased Fe recovery. Within the constrained intensity range tested for the SLon-100 in this study, the highest intensity of 6 kG was taken as the best setting for the magnetic separator. In addition, the metallurgical performance at this setting was taken as the baseline performance of magnetic beneficiation of the iron ore slimes without selective flocculation.
pH optimization
The average size distribution of flocs for each pH tested is presented in Figure 3. It indicates increasing floc size with increasing pH. For the feed pH 9, pH 10, pH 10.5, and pH 11, 50% of the sample by volume passes 13 , 15 , 19 , 21, and 33 μm respectively.
Figure 3 suggests that a pH of 11 will yield the coarsest flocs. However, as Figure 4 indicates, selective flocculation coupled with magnetic separation indicated that a pH of 10 yielded the best average Fe grades, recoveries, and separation efficiencies. Larger standard deviations were observed at a pH of 9.5 compared to the other tests. At 95% confidence, the individual response variables for the remaining pH values are within range of one-another and indicate no substantial variation with pH. A pH of 10 was therefore selected for further test work: this pH yielded the lowest standard deviation and, from a practical point of view, the associated reagent costs would be less than for higher pH values.
Comparison of the floc size distribution with the metallurgical performance data in Figure 4 indicates that the latter was the better indicator to use when optimizing conditions for selective flocculation. Therefore, the final stage of optimization involved tests in which selective flocculation was coupled with magnetic separation.
FMS optimization tests: Magnetic separation coupled with selective flocculation
The experimental data was analysed using the statistical software Design Expert (Stat-ease Inc., 2016). Design Expert incorporates ANalysis Of VAriance (ANOVA) to analyse the significance of each factor (i.e. of the relevant operating variables) and to develop empirical models that describe the response variable as a function of the factors. The models were used to generate response surface contours for each of the significant response variables. Thereafter the optimum operating range of each factor was identified and experiments conducted at these optimum conditions to validate the predicted optimal selective flocculation performance.
Response variable 1: Separation efficiency
Table III summarizes the results of the ANOVA with regard to separation efficiency and shows that it is most sensitive to the interaction between the sodium oleate dosage and conditioning time (F=11.32), followed by the paraffin dosage (F=7.90), and finally by sodium oleate dosage (F=6.04). The model developed for this response variable using these factors and interactions is given by Equation [2]. The model is significant, as suggested by the high F-value and low p-value.
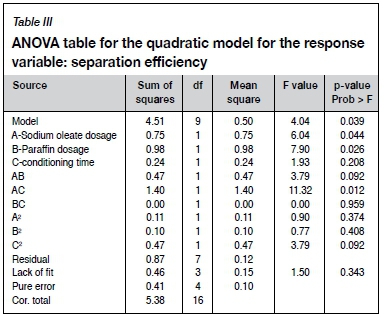

The interaction between sodium oleate dosage and conditioning time is presented graphically by the response surface shown in Figure 5. The interaction yields a relatively flat response curve with maximum separation efficiency at the two extremes of the range tested, namely:
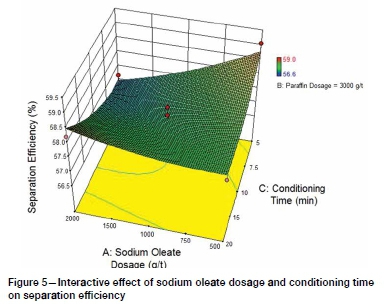
> 2000 g/t sodium oleate and 20 minutes conditioning time
> 500 g/t sodium oleate and 5 minutes conditioning time.
Response variable 2: Concentrate Fe grade
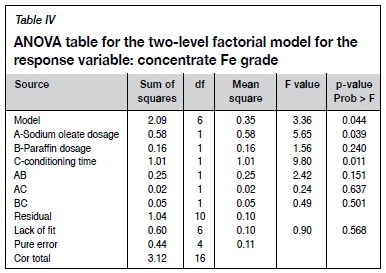
Unlike separation efficiency, the concentrate Fe grade is most sensitive to conditioning time, followed by sodium oleate dosage, as indicated by the high F-value and low p-value (Table IV). As can be seen by the p-value, the two level factorial model for the concentrate Fe grade is significant and is given by Equation [3].
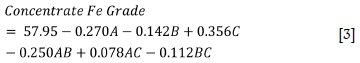
Increasing the conditioning time increases the concentrate Fe grade. This is shown in Figure 6, along with related data from the literature. In order to compare the results of the current study to previous work it was important to compare similar conditions for both studies. Due to the Box-Behnken design, only one data-point from the study could be compared with the data from the literature that is presented in Figure 6. The same applies to Figures 8 and Figure 9. The red solid lines in Figures 6, Figure 8, and Figure 9 represent the predicted values of the response variables derived from the respective models generated from the Box-Behnken experimental design.
The trend in Figure 6 is similar to that found by Song, Lu, and Lopez-Valdivieso (2002), although in the current study the concentrate Fe grade reaches a plateau after 5 minutes rather than 10 minutes. The plateau indicates that increasing the conditioning time above 5 minutes provides no significant benefit.
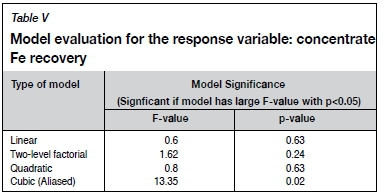
Response variable 3: Concentrate recovery
No significant relationship was found between the recovery of iron to the concentrate and the three operating variables: sodium oleate dosage, paraffin dosage, and conditioning time. This can be seen from Table V, which summarizes the statistical data for four alternative empirical models of how the operating variables influenced concentrate Fe recovery. The first three models were not significant; their p-values were greater than 0.05. In the fourth model, the cubic model, aliasing of variables occurred so the model was omitted from further comparisons. (Aliases occur when two or more variables vary together, and the effect of one variable cannot be separated from the effect of the others.)
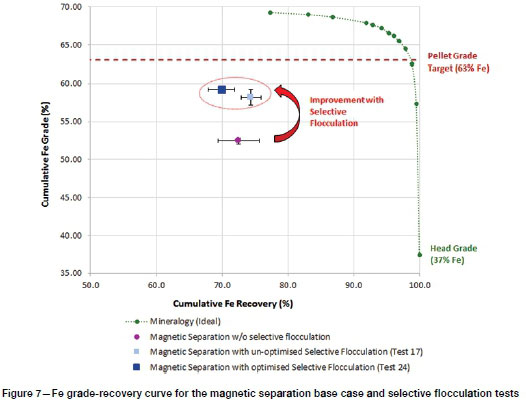
Comparison of magnetic separation with and without selective flocculation
Figure 7 presents grade-recovery data from the study that is relevant to an evaluation of the metallurgical performance of selective flocculation coupled with magnetic separation. It shows the ideal grade-recovery curve derived from the mineralogical analysis, and the Fe grade and recovery with and without selective flocculation. Two data-points are shown to indicate the performance after selective flocculation; one indicates the best performance achieved among the 17 optimization tests before the optimum conditions had been established (test 17); and the other the test conducted at the optimized conditions (test 24). Test 17, the best result for unoptimized conditions, showed that selective flocculation had improved both the Fe grade and recovery at an intensity of 6 kG at a sodium oleate dosage of 500 g/t, paraffin dosage of 3 000 g/t, and conditioning time of 5 minutes. Test 24, under optimized conditions (i.e. the same sodium oleate dosage but half the paraffin dosage and slightly lower conditioning time of 4.6 minutes) achieved better Fe grades at a slight drop in recovery. Although the process separation efficiency for slimes treatment is low compared to ideal separation as suggested by mineralogy, selective flocculation under optimized conditions improved the product Fe grade from 52.28% to 59.20% Fe with a slight decrease in recovery from 72.1% to 69.9%.
Discussion: Comparison with the literature
Several researchers have conducted investigations similar to those reported in this paper, so a comparison of findings is of considerable interest and can provide useful insights into the factors influencing selective flocculation of haematite fines.
Comparisons with respect to the optimum pH for selective flocculation
Zhou (2008), as cited by Yin et al. (2011), investigated shear flocculation of ultrafine haematite and the solution chemistry of sodium oleate. Zhou (2008) found that at a pH of 8.6 the oleic acid molecules and oleate ions in solution are in equilibrium, resulting in maximum hydrophobicity and floc size. The work of Yin et al. (2011) indicated that strong alkaline conditions (pH >10) resulted in decreased floc size due to the increased concentration of OH- ions that compete with RCOO- for adsorption onto the surfaces of haematite particles, thereby reducing the hydrophobicity of the particle surfaces. However, the current study showed the opposite - floc size increased with increasing pH. The discrepancy between the two studies could be attributed to different ore characteristics and different experimental conditions; namely the water employed (deionized vs tap water), stirring time (20 minutes vs 10 minutes), and sodium oleate dosage (3.94 χ 10-4 M vs 2.69 χ 10-2 M).
In addition, this study found that floc size was not necessarily the best criterion for determining optimum conditions for selective flocculation; the metallurgical performance achieved when selective flocculation was coupled with magnetic separation was found to be a better criterion. Accordingly, in the Box-Behnken design of the optimization test work it was assumed that the optimum pH would be relatively independent of the conditioning time and sodium oleate and paraffin dosages.
Comparisons with respect to separation efficiency
The study found that separation efficiency was lowest at the highest sodium oleate dosage and lowest conditioning time. With regard to conditioning time, Williams and Arbiter (1968) as cited by Quast (2015) found that several micro-processes may occur during the conditioning of haematite when fatty acids such as oleic acid and tall oil are used as collectors in flotation. These are
> Removal of alteration films from mineral surfaces
> Dispersion of undissolved collector and insoluble hydrocarbon oil
> Mineral/collector interaction
> Mineral/hydrocarbon oil interaction
> Desorption of collector from gangue minerals
> Flocculation of floatable mineral in micrometre and submicrometre sizes
The work of Laskowski and Nayamekye (1994), however, suggests that at alkaline conditions the oleate is in soluble form and equilibrium is established fairly quickly so that conditioning time has little impact on the transportation of the collector species to the mineral surfaces. Since the current study was conducted under alkaline conditions (pH 10), the observation of decreasing separation efficiency with decreasing conditioning time at sodium oleate dosages exceeding 1000 g/t can be attributed to:
> Lower probability of collision between the collector and mineral surfaces
> Lower shear stresses on the initially formed aggregates and expulsion of mechanically entrained gangue
> Insufficient time for the desorption of the collector from gangue minerals.
It is envisaged that increased dosages of collector and non-polar oil strengthen the adsorption of oleate ions onto the haematite surfaces and lead to the creation of oil bridges, both of which contribute to an increase in hydrophobicity. The stronger adsorption may have resulted in a slight increase in the entrainment of gangue, thereby reducing the separation efficiency. The results obtained in this study correlate well with those obtained by Roy (2012) as presented in Figure 8, with the separation efficiency relatively constant at sodium oleate dosages below 2 kg/t and decreasing separation efficiency as the collector dosage increases. The results indicate that the range selected for the collector dosage in this study was appropriate.
Comparisons with respect to concentrate Fe grade
The study found that, as with separation efficiency, the concentrate Fe grade decreased slightly with increasing sodium oleate dosages. Figure 9 shows the effect along with findings from two other sources. Interestingly, the work conducted by Song, Lu, and Lopez Valdivieso (2002) at a similar Fe head grade indicates that the concentrate Fe grade plateaus in the same region as that for the current study. A comparison with the work of Roy (2012) indicates that higher Fe concentrate grades can be achieved at higher Fe head grades, neglecting the influence mineralogy has on the separation performance.
With separation efficiency and concentrate Fe grade being closely related to one another, the reasons for the effects of sodium oleate dosage and conditioning time on Fe concentrate grade are similar to those given for separation efficiency.
To conclude this discussion, it should be noted that the conditions for the laboratory test work were somewhat idealized and therefore the optimum conditions in industrial contexts may be somewhat different from those found in the study. Examples of obvious differences include simple vs complex mineralogy; potable water supplied by Rand Water vs mine water (which may contain several impurities or ions that may adversely affect the selectivity of the flocculation process); artificial slimes with clean surfaces vs aged slimes with surface alteration; and laboratory control vs plant control.
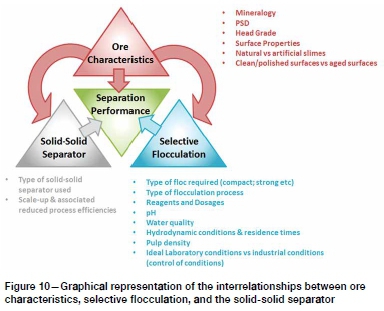
This study has shown that value can be derived from selective flocculation coupled with magnetic separation. However, as Figure 10 shows, the process is complex and numerous factors affect separation performance in interrelated ways to the extent that a careful laboratory and pilot programme is required before contemplating implementation in industry.
Conclusions
The study reported in this paper found that selective flocculation coupled with a SLon magnetic separator could recover 70% of the Fe in haematite slimes at a grade of 59% Fe. The sample originated from Angola and its head grade was 37% Fe. Without selective flocculation, about the same recovery of Fe was achieved using the SLon separator but at a much lower concentrate grade, i.e. 52% Fe. This shows that selective flocculation improved the separation efficiency from 40.0% to 56.8%. Although these are laboratory results obtained under somewhat idealized conditions, they do indicate the potential of the process quite clearly. They also confirm the positive findings from the literature regarding the potential of the FMS process as a viable means for recovering values from haematite slimes that have been or are currently being lost to tailings.
However, selective flocculation is a complex process with the ore characteristics, flocculation conditions, and solid-solid separator interacting in complex ways that affect separation performance. The application of the FMS process would need to be preceded by a careful experimental programme that deals appropriately with these complications. In addition to its findings, this study provides a useful guideline for how such a programme might be implemented.
Acknowledgements
The authors would like to acknowledge Mintek for funding the research project and for providing the necessary facilities and resources to successfully complete the study.
References
Abro, M.I. 2009. Upgradation of Dilband iron ore. PhD thesis, Mehran University of Engineering & Technology, Pakistan. [ Links ]
Abro, M.I., Pathan, A.C., Memon, A.R., and üddin, S. 2013. Dual polymer flocculation approach to overcome activation of gangue minerals during beneficiation of complex iron ore. Powder Technology, vol. 245. pp. 281-291. [ Links ]
Ahmed, H.A.M. and Mahran, G.M.A. 2013. Processing of iron ore fines from Alswaween, Kingdom of Saudi Arabia. Physiochemical Problems of Mineral Processing, vol. 49, no. 2. pp. 419-430. [ Links ]
Ansari, M. 1997. Fine particle processing - A difficult problem for mineral engineers. Proceedings of National Seminar on Processing of Fines, Jamshedpur, India, 9-10 January 1997. NML, Jamshedpur. pp.93-102. [ Links ]
Arol, A.I. and Aydogan, A. 2004. Recovery enhancement of magnetite fines in magnetic separation. Colloids and Surfaces A: Physiochemical Engineering Aspects, vol. 232, no. 2-3. pp. 151-154. [ Links ]
Da Corte, C., Sindane, Z., Sehlotho, Ν., Thiele, Η., and Bryson, M. 2015. Fine Fe ore processing by gravity, magnetic and flotation techniques. Internal Report 42023. Mintek, Randburg, South Africa. [ Links ]
Drzymala, J. and Fuerstenau, D.W. 2014. Selective flocculation of hematite in quartz-hematite-ferric-ion-polyacrylic acid system. Part 2. Effect of grinding and a hydrofluoric treatment on selectivity of flocculation. International Journal of Mineral Processing, vol. 129. pp. 1-5. [ Links ]
Dworzanowski, M. 2014. Maximizing haematite recovery within a fine and wide particle size distribution using wet high intensity magnetic separation. Journal of the Southern African Institute of Mining and Metallurgy, vol. 114. pp. 559-567. [ Links ]
Forbes, E. 2011. Shear, selective and temperature responsive flocculation: A comparison of fine particle flotation techniques. International Journal of Minerals Processing, vol. 99, no. 1-4. pp. 1-10. [ Links ]
Hanumantha Rao, K. and Narasimhan, K.S. 1985. Selective flocculation applied to Barsuan Iron Ore tailings. International Journal of Mineral Processing, vol. 14. pp. 67-75. [ Links ]
Haselhuhn, H.J., Carlson, J.J., and Kawatra, S.K. 2012. Water chemistry analysis of an industrial selective flocculation dispersion hematite ore concentrator plant. International Journal of Mineral Processing, vol. 102-103. pp. 99-106. [ Links ]
Kumar, R. and Mandre, N.R. 2017. Recovery of iron from iron ore slimes by selective flocculation. Journal of the Southern African Institute of Mining and Metallurgy, vol. 117. pp. 397-400. [ Links ]
Li, C., Sun, Η., Bai, J., and Li, L. 2010. Innovative methodology for comprehensive utilization of iron ore tailings. Part 1. The recovery of iron from iron ore tailings using magnetic separation after magnetizing roasting. Journal of Hazardous Materials, vol. 174, no. 1-3. pp. 71-77. [ Links ]
Laskowski, J.S. and Nyamekye, G.A. 1994. Colloid chemistry of weak electrolyte collectors: the effect of conditioning on flotation with fatty acids. International Journal of Mineral Processing, vol. 40. pp. 245-256. [ Links ]
Ma, M. 2012. The significance of dosing sequence in the flocculation of hematite. Chemical Engineering Science, vol. 73. pp. 51-54. [ Links ]
Mathur, S., Singh, p., and Moudgil, B.M. 2000. Advances in selective flocculation technology for solid-solid separations. International Journal of Minerals Processing, vol. 58, no. 1-4. pp. 201-222. [ Links ]
NIST, 2012. e-Handbook of Statistical Methods. National Institute of Standards and Technology, US Department of Commerce, Gaithersburg, MD. http://www.itl.nist.gov/div898/handbook [accessed July 2015]. [ Links ]
Pascoe, R.D. and Doherty, E. 1997. Shear flocculation and flotation of hematite using sodium oleate. International Journal of Mineral Processing, vol. 51. pp. 269-282. [ Links ]
Poorni, S. and Natarajan, K.A. 2013. Microbially induced selective flocculation of hematite from kaolinite. International Journal of Mineral Processing, vol. 125. pp. 92-100. [ Links ]
Pradhan, Ν., Das, Β., Gahan, C.S., Kar, R.N., and Sukla, L.B. 2006. Beneficiation of iron ore slime using bacteria. Bioresource Technology, vol. 97, no. 15. pp. 1876-1879. [ Links ]
Quast, K. 2015. Use of conditioning time to investigate the mechanisms of interactions of selected fatty acids on hematite. Part 1: Literature review. Minerals Engineering, vol. 79. pp. 295-300. [ Links ]
Roy, S. 2012. Recovery improvement of fine magnetic particles by floc magnetic separation. Mineral Processing & Extractive Metallurgy, vol. 33. pp.170-179. [ Links ]
Song, S., Lopez-valdivieso, Α., and Ding, Y. 1999. Effects of nonpolar oil on hydrophobic flocculation of hematite and rhodochrosite fines. Powder Technology, vol. 101. pp. 73-80. [ Links ]
Song, S., Lu, S., and Lopez-Valdivieso, Α. 2002. Magnetic separation of hematite and limonite fines as hydrophobic flocs from iron ores. Minerals Engineering, vol. 15, no. 6. pp. 415-422. [ Links ]
Stat-ease Inc. 2016. Design-Expert Trial Version 10. Minneapolis, MN. [ Links ]
Svoboda, J. and Fujita, T. 2003. Recent developments in magnetic methods of material separation. Minerals Engineering, vol. 16, no.9. pp. 785-792. [ Links ]
Weissenborn, P.K. 1996. Behaviour of amylopectin and amylose components of starch in the selective flocculation of ultrafine iron ore. International Journal of Mineral Processing, vol. 47. pp. 197-211. [ Links ]
Weissenborn, P.K., Warren, L.J., and Dunn, J.G. 1994. Optimisation of selective flocculation of ultrafine iron ore. International Journal of Minerals Processing, vol. 42. pp. 191-213. [ Links ]
Wills, Β.Α. and Finch, J.A. 2016. Wills' Mineral Processing Technology: An Introduction to the Practical Aspects of Ore Treatment and Mineral Recovery. 8th edn. Butterworth-Heinemann, Amsterdam. p. 8. [ Links ]
Yin, W., Yang, X., Zhou, D., Li, Y., and Lu, Z. 2011. Shear hydrophobic flocculation and flotation of ultrafine Anshan hematite using sodium oleate. Transaction of Nonferrous Metals Society of China, vol. 21, no. 3. pp. 652-644. [ Links ]
 Correspondence:
Correspondence:
C. Da Corte
Email: carladc452@gmail.com
Received: 19 Feb. 2019
Revised: 14 Jun. 2019
Accepted: 10 Oct. 2019
Published: November 2019














