Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.119 n.10 Johannesburg Oct. 2019
http://dx.doi.org/10.17159/2411-9717/693/2019
GENERAL PAPERS
Dissolution kinetics of tellurium from gold concentrate during alkaline sulphide leaching
W. YangI, II; H. CaoI, II; Y.P. WangIII; T. LongI, II; H. WanI, II; H. LiI, II; P. DongI, II
ISchool of Resources Engineering, Xi'an University of Architecture and Technology, China
IIKey Laboratory of Gold and Resources in Shaanxi Province, China
IIISchool of Foreign Languages, Baoji University of Arts and Sciences, China
SYNOPSIS
Alkaline sulphide leaching was adopted to extract tellurium from tellurium-bearing gold concentrate for the first time. The tellurium leaching kinetics were studied. The influence of average particle size, stirring speed, leaching temperature, Na2S concentration, and NaOH concentration on the tellurium extraction were investigated. It was found that tellurium extraction could reach 79.14% under the optimum conditions: 17.77 μηι average particle size, 500 r/min stirring speed, 80 g/L Na2S concentration, 30g/L NaOH concentration, 80°C leaching temperature, 4:1 liquid to solid ratio, and 180 minutes leaching time. The interface transfer and diffusion through the product layer or insoluble substance layer were the rate-controlling steps. The leaching kinetics equation was established, and the activation energy was 34.384 kJ/mol.
Keywords: Gold concentrate; tellurium leaching; alkaline suphide leaching; kinetics.
Introduction
Tellurium, a scarce and valuable element with relatively low abundance of 0.001-0.005 g/t in the Earth's crust (Kavlak and Graedel, 2013; Makuei and Senanayake, 2018), is widely used in the electronics industry, metallurgy, communications, aerospace, energy, medicine, and other fields. The bismuth or antimony compounds of tellurium are excellent refrigerating materials which can be employed in radars and underwater missiles (Siciliano et al., 2009). Lead and bismuth tellurides are often used to make photosensitive materials (Tsiulyanu et al., 2005). The cadmium telluride photovoltaic panel is one of the most common commercially available solar panels (Bhandari et al., 2015; Fadaam et aí., 2019). At present the market demand for tellurium continues to increase year by year (Candelise et al., 2012; Zhong et aí., 2018), but annual tellurium production is far behind the market demand (Yu et al., 2018).
Currently, about 90% of tellurium production is from copper anode slimes from the electrolytic refining of blister copper, and the remainder as a by-product of bismuth and lead processing (Makuei and Senanayake, 2018). Due to heavy losses of tellurium during mineral processing and smelting, the current routes for tellurium production as a metallurgical by-product cannot meet the demand. Therefore, the direct recovery of tellurium from tellurium-bearing ores has attracted increasing attention. Since primary tellurium deposits are very rare in nature, current research is focused mainly on the extraction of tellurium from daphyllite (Bi2Te2S) (Jiang, 2000; Lei and Xie, 2012). However, there are few studies on the extraction of tellurium from other tellurium-bearing ores, and even fewer on gold-telluride ores.
The gold-tellurium series minerals, second only to the gold-silver series, are important carriers of gold and silver. Among the 27 known types of gold-bearing minerals exploited, there are 11 goldtelluride minerals, including calaverite and petzite (Chen et al., 1999). The leaching rates of gold and silver in tellurium-bearing gold ore are low because telluride is difficult to dissolve in cyanide media and the TeO2 formed by the dissolution forms a layer on the gold surface, preventing the gold from contacting with the lixiviant. Tellurium leaching prior to cyanidation of gold and silver can not only effectively recover tellurium from this type of ore, but also improve the leaching rate of gold and silver (Ellis and Deschênes, 2016). Therefore, it is necessary to give more attention to the direct extraction of tellurium from tellurium-bearing gold ores.
A range of techniques, including sulphation roasting (Xu, Li, and Guo, 2014), soda roasting (Chen and Li, 2008), pressure sulphate leaching (Zhang, Wang, and Peng, 2007), pressure alkaline leaching (Fan et al., 2013), and bioleaching (Rajwade and Paknikar, 2003) have been reported for recovering tellurium from metallurgical by-products. However, these techniques have many disadvantages, such as harsh leaching conditions, excessive consumption of reagents, and serious corrosion of equipment. The development of a reasonable and efficient method to extract tellurium is therefore required. Guo et al., (2017) used alkaline sulphide leaching to recover tellurium from high-tellurium materials obtained from the basic refining of crude bismuth, and tellurium was enriched from 11.60% to 91.24%. Additionally, the alkaline sulphide leaching method can recover arsenic and antimony (Awe et al., 2013; Curreli et al., 2009; Li et al., 2011). However, little attention has been paid to using the method to recover tellurium from tellurium-bearing gold ores.
In this work, tellurium was extracted directly from tellurium-bearing gold flotation concentrate by alkaline sulphide leaching for the first time. The effects of different leaching conditions on the leaching efficiency of tellurium were investigated, and the kinetics of tellurium leaching are discussed on this basis. This study will provide a technological and theoretical basis for the efficient extraction of tellurium from tellurium-bearing gold ores.
Experimental
Materials
The tellurium-bearing gold flotation concentrate was obtained from a tellurium-type gold mine in Qinling Mountains, Shannxi Province, China. Table I shows the chemical composition of the samples and Table II shows the main mineral content. The distribution of major elements in the minerals is shown in Table III.
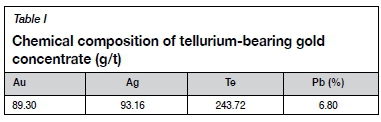
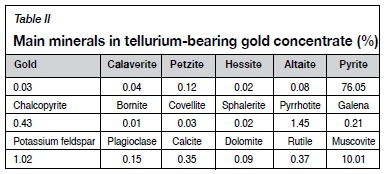
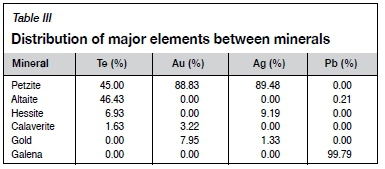
Table II shows that the main metal sulphide minerals are pyrite, pyrrhotite, chalcopyrite, and galena whereas the main nonmetallic minerals are muscovite, quartz, and potassium feldspar. Table III shows that most of the tellurium is contained in the petzite and altaite, and a small amount in the hessite and calaverite.
Experimental procedure
The tellurium samples were ground in a laboratory planetary ball mill. Particle size distribution analysis of the ground samples was performed using a Malvern laser particle-size analyser (Mastersize 2000). Five size fractions with volumetric average particle sizes of 56.58, 44.50, 28.67, 17.77 , and 10.23 μm were obtained to study the effect of particle size on tellurium recovery. The PSDs of these fractions are shown in Figure 1 (a, b, c, d, and e respectively).
The leaching experiments were carried out in a 500 mL four-necked round-bottomed glass reactor fitted with a digitally controlled mechanical stirrer, condenser tubes, and mercury thermometers. Na2S-9H2O and NaOH of analytical grade and deionized water were used throughout the experiments. The leaching solution was prepared by dissolving sodium sulphide and sodium hydroxide in deionized water. The lixiviant (200 mL) was first added to the reactor, and the reactor was heated to the desired temperature in a superthermostatic water bath. Ground sample (50 g) was added when the desired stirring rate was reached. Samples of the leaching solution (2 mL) were taken at different time intervals, filtered, and submitted for tellurium analysis. The sample loss was compensated by adding 2 mL of fresh leaching solution. After leaching, the slurry was filtered and the residue was washed, dried, and weighed. The contents of main minerals and distribution of major elements in the tellurium samples were analysed by mineral liberation analyser (MLA650, FEI, USA). Tellurium in the solution was determined by ICP-AES (PS-6, Baird Corp, USA).
Results and discussion
Alkaline sulphide leaching of tellurium
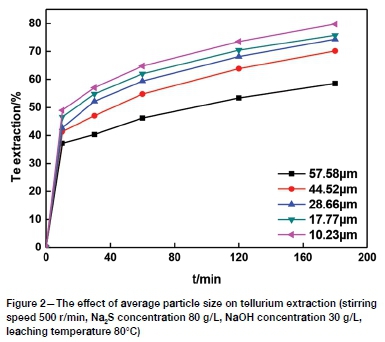
The effect of average particle size
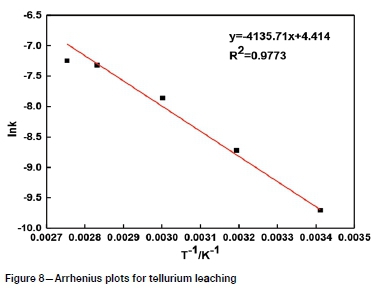
Samples with average particle sizes of 57.58, 44.52, 28.66, 17.77, and 10.23 μηι were leached in a solution containing 80 g/L Na2S plus 30 g/L NaOH at 80°C using a stirring speed of 500 r/min. The results (Figure 2) show that the leaching rate increases with decreasing particle size. A tellurium extraction of 58.56% was obtained for the 57.58 μm size fraction after leaching for 180 minutes, while 75.77% was obtained for the 17.77 μm size fraction. This could be caused by the increase in specific surface area with the decrease in particle size (Xiao et al., 2019; Zheng and Chen, 2014a), so that the mass transfer process is enhanced. In addition, solid particles are also activated during grinding and the occluded tellurium is exposed, enhancing contact with the lixiviant. Considering the grinding energy consumption and subsequent solid-liquid separation process, 17.77 μπ is recommended for the average mineral particle size.
The effect of stirring speed
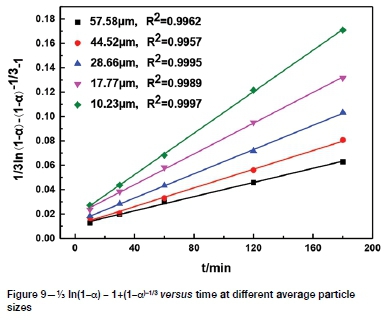
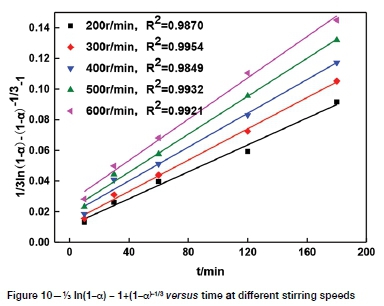
The effect of stirring speed on tellurium leaching was studied using speeds from 200 to 600 r/min. Other conditions were the same as those in the previous section. As shown in Figure 3, the reaction rate was very fast in the first 30 minutes at any stirring speed. After leaching for 30 minutes, tellurium extraction continued to increase, but the rate of extraction gradually slowed. When the stirring speed was increased from 200 r/min to 600 r/ min, the increase of tellurium extraction was only 7.45-11.89%. In the solid-liquid reaction process, when liquid film diffusion is the controlling step, the stirring speed has a great influence on the reaction rate (Tavakoli and Dreisinger, 2014).Therefore, the leaching of tellurium is probably not controlled by the external diffusion. A stirring speed of 500 r/min was chosen for the following experiments.

The effect of leaching temperature
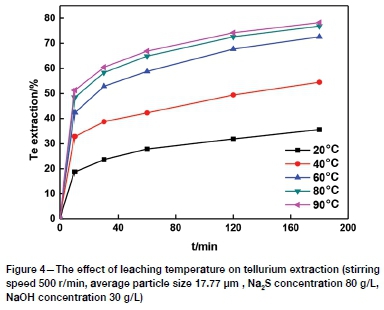
The effect of leaching temperature on tellurium extraction was investigated in the range of 20-90°C. Figure 4 shows the significant effect of temperature on the leaching kinetics. The tellurium extraction increased from 35.60% to 78.23% as the temperature was increased from 20°C to 90°C. This is expected from the exponential dependence of the rate constant in the Arrhenius equation (Zheng and Chen, 2014b). Furthermore, the energy available for atomic and molecular collisions increases with increasing temperature (Rao et al., 2015). Only a minimal increase in extraction was observed at temperatures higher than 80°C, therefore 80°C was selected as the optimum leaching temperature.
The effect of Na2S concentration
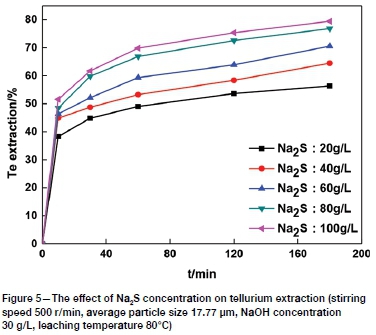
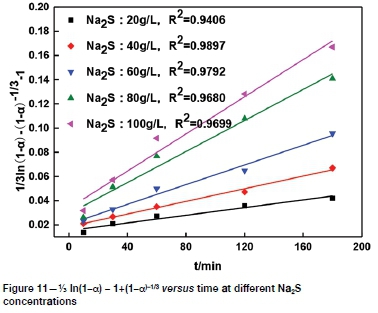
The effect of Na2S concentration on tellurium leaching was determined using Na2S concentrations of 20, 40, 60, 80, and 100 g/L. Figure 5 shows that the tellurium extraction increased rapidly from approximately 56.36% to 79.43% with an increase in Na2S concentration from 20 g/L to 100 g/L, indicating that Na2S concentration has a great influence on tellurium leaching. Tellurium is sulphurophilic. An increase in Na2S concentration increases the activity of sulphur ions, and then tellurium dissolves in the Na2S solution and is transformed to soluble thiotellurate (TeS42-) (Guo et al., 2017), contributing to the enhanced reaction between tellurium and sodium sulphide and thus improving the leaching rate. Based on these results, 80 g/L was considered to be the optimum Na2S concentration.
The effect of NaOH concentration
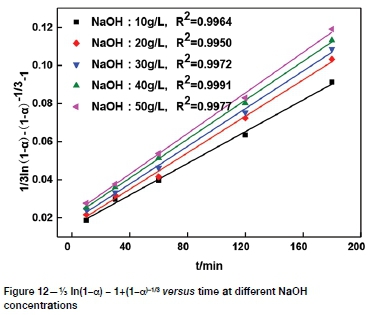
The effect of NaOH concentration on the leaching rate of tellurium was studied at NaOH concentrations of 10, 20, 30, 40, and 50 g/L (Figure 6). It can be seen that tellurium extraction increased as NaOH concentration increased, but only slightly, by 4.40-6.33% as the NaOH concentration increased from 10 g/L to 50 g/L. NaOH concentration has no marked influence on tellurium leaching, and 30 g/L was therefore selected as the optimum NaOH concentration.

Based on these results, the optimum leaching conditions were determined as 500 r/min stirring speed, 17.77 μηι average particle size, 80 g/L Na2S, 30 g/L NaOH, 80°C leaching temperature, a L/S ratio of 4:1, and 180 minutes leaching time. Under these optimum conditions, 79.14% of the tellurium was extracted into solution. The result indicates that alkaline sulphide is an effective lixiviant for tellurium dissolution from tellurium-bearing gold ores. In order to further enhance the leaching process, it is necessary to do some kinetic analysis to identify the rate-controlling steps in the leaching process.
Kinetic analysis
Leaching kinetics
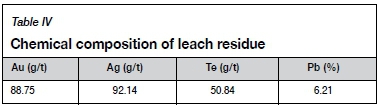
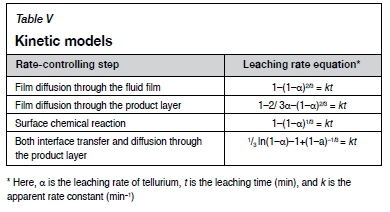
Table IV shows the chemical composition of the leach residue. As can be seen by comparing with Table I, the grade changes for gold and silver are very small, indicating that during the direct leaching of tellurium from gold concentrate very little dissolution of gold occurs, and there will therefore be no effect on the subsequent recovery of Au and Ag. Tellurium in gold flotation concentrate occurs mainly in petzite and altaite. During leaching, tellurium is dissolved mainly from petzite and altaite, and gold remains undissolved and locked in the mineral particles in some form. Therefore, the leaching of tellurium from the concentrate is a multiphase liquid-solid reaction. The rate-controlling processes include surface chemical reaction, film diffusion through a fluid film, and film diffusion through the product layer with the shrinking core model (Hua, 2004). A new model, proposed by Dickinson and Heal (1999), suggests that both the interface transfer and diffusion through the product layer or insoluble substance layer can affect the reaction rate. It is also used to explain the rate-controlling steps in the leaching process (Bingöl, Canbazoglu, and Aydogan, 2005; Rao et al., 2015). The leaching rate equations for different rate-controlling processes are shown in Table V.
Rate-controlling processes
Based on the experimental data at different leaching temperatures, the leaching kinetics of tellurium samples are fitted by the kinetic models in Table V. The kinetic model that gives the best-fitting results for the experimental data is defined as the rate-limiting step (Figure 7).
Table VI shows the correlation coefficients (R2) of the fitted data from Figure 7. It can be found that the R2 value of V3 ln(1-a)-1+(1-a)-1/3vs. t is closest to 1.0, indicating that V3 ln (1-α)-1+(1-α)-1/3 is the best model to simulate the leaching kinetics of tellurium. This observation confirms that the kinetics of leaching tellurium with alkaline sulphide solution is controlled by both interface transfer and diffusion across the product layer.
Additionally, the leaching rate is closely related to the activation energy, which can be calculated by the Arrhenius equation (ln k = -E/ RT+A). Based on the Arrhenius equation, lnk is plotted versus 1/T using the values of lnk and 1/T in Figure 7d, as shown in Figure 8. The data in Figure 8 is in an approximate straight line. The values of E and A are calculated to be 34.384 kJ/mol and 4.414, respectively. The activation energy of a diffusion-controlled process is typically from 4 to 12 kJ/mol, while that of a chemically controlled process is usually larger than 40 kJ/mol (Abdel-Aa, 2000; Habashi, 1969). Therefore, an activation energy of 34.384 kJ/mol demonstrates the tellurium leaching in alkaline sulphide solution is controlled by both interface transfer and diffusion through the product layer.
Leaching kinetics equation
The dependence of the rate constant on the stirring speed, average particle size, Na2S concentration, NaOH concentration, and leaching temperature may be expressed as
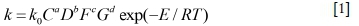
where k0is the frequency factor, E is the apparent activation energy, R is the gas constant, T is the leaching temperature, C is the stirring speed, D is the average particle size, F is the Na2S concentration, G is the NaOH concentration, and a, b, c, and d, are the constants (Demirkiran and Künkül, 2007).
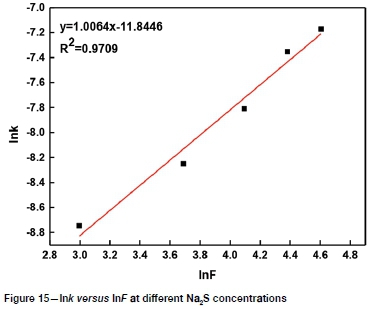
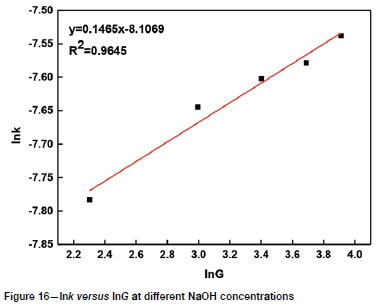
It can be seen from Figures 8-11 that 73 ln(1-a)-1+(1-a)-1/3 is related to average particle size, stirring speed, Na2S concentration, and NaOH concentration according to the experimental data in Figures 2, 3, 5, and 6.
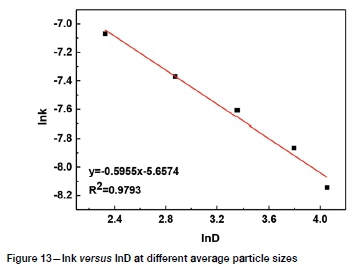
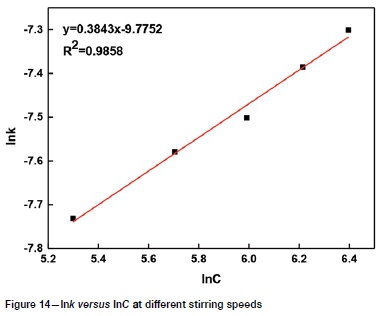
The slopes of the lines in Figures 9-12 represent the rate constant. The value of lnk can be calculated from these k values. The values of a, b, c, and d in Equation [1] are determined by linear regression in Figures 13-16: a = 0.3843, b = -0.5955, c = 1.0064, d = 0.1465. By combining the Arrhenius equation and Equation [1], the following expression is obtained:

Based on Equation [2], the value of k0 is calculated as 1.1854. The final leaching kinetics equation is
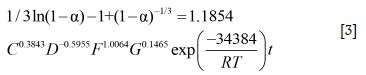
Recommendations
According to the above kinetic analysis, the leaching of tellurium from gold concentrate is controlled by interface transfer and diffusion across the product layer. However, through the condition test, it can be seen that optimizing the leaching conditions has little further effect on the tellurium leaching rate, or it is necessary to increase the tellurium leaching rate by increasing the energy input. Therefore, further studies should be undertaken to improve the tellurium leaching rate.
Future work will include investigation of tellurium minerals from leach residues to find out why they are difficult to leach, and then testing different measures to enhance the leaching process. It is suggested that different oxidants could be employed. The leaching kinetics of tellurium under different conditions is studied by researching the effect of changes in grain parameters, apparent activation energy, and the reaction order of tellurium under alkaline sulphide leaching and oxidation leaching. Tellurium leaching may also be enhanced by roasting pretreatment, especially oxidation roasting.
Conclusion
In the range of parameters studied, the most suitable conditions for the leaching process of tellurium from gold concentrate were an average particle size of 17.77 μιη, stirring speed 500 r/min, Na2S concentration 80 g/L, NaOH concentration 30 g/L, and leaching temperature 80°C. Under these conditions, a tellurium extraction of 79.14% was obtained. The leaching rate increased with increasing Na2S and NaOH concentration, stirring speed, and leaching temperature, and decreasing average particle size. The leaching temperature had the most significant effect on tellurium leaching, followed by Na2S concentration, average particle size, stirring speed, and NaOH concentration. The leaching kinetics of tellurium by alkaline sulphide is controlled by interface transfer and diffusion across the product layer. According to a new model and the Arrhenius equation, the leaching kinetics equation was represented as V3 ln(l-a)-1+( l-a)-1/3 =  exp
exp 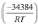 activation energy was 34.384 kj/mol.
activation energy was 34.384 kj/mol.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 51474169), Key Projects of Industrial Science and Technology in Shaanxi Province (Grant No. 2016GY-154), and Key Laboratory Research Project of Education Department in Shaanxi Province (Grant No.18JS061).
References
Abdel-Aal, E.A. 2000. Kinetics of sulfuric acid leaching of low-grade zinc silicate ore. Hydrometallurgy, vol. 55, no. 3. pp. 247-254. [ Links ]
Awe, S.A., Sundkvist, J.-E., Bolin, Ν.-]., and Sandström, Â. 2013. Process flowsheet development for recovering antimony from Sb-bearing copper concentrates. Minerals Engineering, vol. 49. pp. 45-53. [ Links ]
Bhandari, K.P., Koirala, P.K., and Ellingson, R.J. 2015. Iron pyrite nanocrystal film serves as a copper-free back contact for polycrystalline CdTe thin film solar cells. Solar Energy Materials and Solar Cells, vol. 140. pp.108-114. [ Links ]
Bingöl, D., canbazoGlu, Μ., and AydoGan, S. 2005. Dissolution kinetics of malachite in ammonia/ammonium carbonate leaching. Hydrometallurgy, vol. 76, no. 1-2. pp. 55-62. [ Links ]
candelise, c., Winskel, Μ., and Gross, R. 2012. Implications for CdTe and CIGS technologies production costs of indium and tellurium scarcity. Progress in Photovoltaics: Research and Applications, vol. 20, no. 6. pp. 816-831. [ Links ]
Chen, C., Cao, Z., Hou, X., Shuai, D., and Luo, Y. 1999. The distributive law and main minerogenic conditions of gold-telluride deposits in the world. Journal of Chengdu University of Technology, no. 3. pp. 34-41 [in Chinese]. [ Links ]
Chen, L. and Li, A. 2008. Progress in research on extracting and refining tellurium. Chinese Journal of Rare Metals, vol. 32, no. 1. pp.115-120 [in Chinese]. [ Links ]
Curreli, L., Garbarino, C., Ghiani, Μ., and Orrü, G. 2009. Arsenic leaching from a gold bearing enargite flotation concentrate. Hydrometallurgy, vol. 96, no. 3. pp. 258-263. [ Links ]
Demirkiran, Ν. and Künkül, A. 2007. Dissolution kinetics of ulexite in perchloric acid solutions. International Journal of Mineral Processing, vol. 83, no. 1-2. pp.76-80. [ Links ]
Dickinson, CF. and Heal, G.R. 1999. Solid-liquid diffusion controlled rate equations. Thermochimica Acta, vol. 340-341. pp. 89-103. [ Links ]
Ellis, S. and Deschênes, G. 2016. Treatment of gold-telluride ores. Gold Ore Processing. Adams, M.D. (ed.). Elsevier. pp. 919-926. [ Links ]
Fadaam, S.A., Mustafa, M.H., Abd AlRazaK, A.H., and Shihab, A.A. 2019. Enhanced efficiency of CdTe photovoltaic by thermal evaporation. Vacuum. Energy Procedia, vol. 157. pp. 635-643. [ Links ]
Fan, Y., Yang, Y., Xiao, Y., Zhao, Z., and Lei, Y. 2013. Recovery of tellurium from high tellurium-bearing materials by alkaline pressure leaching process: Thermodynamic evaluation and experimental study. Hydrometallurgy, vol. 139. pp. 95-99. [ Links ]
Guo, Χ., Xu, Z., Li, D., Tian, Q., Xu, R., and Zhang, Z. 2017. Recovery of tellurium from high tellurium-bearing materials by alkaline sulfide leaching followed by sodium sulfite precipitation. Hydrometallurgy, vol. 171. pp. 355-361. [ Links ]
Habashi, F. 1969. Principles of Extractive Metallurgy, Gordon & Breach, New York. pp. 153-163. [ Links ]
Hua, Y. 2004. Kinetic of metallurgical process. Metallurgical Industry Press, Beijing, pp.148-157 [in Chinese]. [ Links ]
Jiang, Χ. 2000. Separation of Te from Bi-Te Ore. Rare Metals and Cemented Carbides, no. 2. pp.8-11 [in Chinese]. [ Links ]
Kavlak, G. and Graedel, T.E. 2013. Global anthropogenic tellurium cycles for 1940-2010. Resources, Conservation and Recycling, vol. 76. pp. 21-26. [ Links ]
Lei, Ν. and Xie, H. 2012. Bioleaching of low grade tellurium sulfide mineral. Energy Procedia, vol. 16. pp. 946-951. [ Links ]
Li, Y., Liu, Z., Li, Q., Zhao, Z., Liu, Z., and Zeng, L.Y. 2011. Removal of arsenic from Waelz zinc oxide using a mixed NaOH-Na2S leach. Hydrometallurgy, vol. 108, no. 3-4. pp.165-170. [ Links ]
Makuei, F.M. and Senanayuíe, G. 2018. Extraction of tellurium from lead and copper bearing feed materials and interim metallurgical products - A short review. Minerals Engineering, vol. 115. pp. 79-87. [ Links ]
Rajwade, J.M. and Paknikar, K.M. 2003. Bioreduction of tellurite to elemental tellurium by Pseudomonas mendocina MCM B-180 and its practical application. Hydrometallurgy, vol. 71, no. 1-2. pp. 243-248. [ Links ]
Rao, S., Yang, Τ., Zhang, D., Liu, W., Chen, L., Hao, Z., Xiao, Q., and Wen, J. Leaching of low grade zinc oxide ores in NH4Cl-NH3 solutions with nitrilotriacetic acid as complexing agents. Hydrometallurgy, vol. 158. pp.101-106. [ Links ]
Siciliano, Τ., Di Giulio, Μ., Tepore, Μ., Filippo, E., Micocci, G., and Tepore, A. 2009. Ammonia sensitivity of rf sputtered tellurium oxide thin films. Sensors and Actuators B: Chemical, vol. 138, no. 2. pp. 550-555. [ Links ]
Tavakoli, M.R. and Dreisinger, D.B. 2014. The kinetics of oxidative leaching of vanadium trioxide. Hydrometallurgy, vol. 147-148. pp. 83-89. [ Links ]
Tsiulyanu, D., Tsiulyanu, Α., Liess, H.D., and Eisele, I. 2005. Characterization of tellurium-based films for NO2 detection. Thin Solid Films, vol. 485, no. 1-2. pp. 252-256. [ Links ]
Xiao, W., Zhao, Y., Yang, J., Ren, Y., Yang, W., Huang, Χ., and Zhang, L. 2019. Effect of sodium oleate on the adsorption morphology and mechanism of nanobubbles on the mica surface. Langmuir, vol. 35, no. 28. pp. 9239-9245. [ Links ]
Xu, Z., Li, D., and Guo, Χ. 2014. Research progress of tellurium extraction processes. Metal Materials and Metallurgy Engineering, vol. 42, no. 2. pp. 3-7+30 [in Chinese]. [ Links ]
Yu, Η., Chu, Y., Zhang, Τ., Yu, L., Yang, D., Qiu, F., and Yuan, D. 2018. Recovery of tellurium from aqueous solutions by adsorption with magnetic nanoscale zero-valent iron (NZVFe). Hydrometallurgy, vol. 177. pp. 1-8. [ Links ]
Zhang, Β., Wang, J., and Peng, J. 2007. Research on removing tellurium from copper anode slime by process of acid leaching under pressure. Nonferrous Metals (Extractive Metallurgy), no. 4. pp. 27-29 [in Chinese]. [ Links ]
Zheng, Y.-J. and Chen, K.-K. 2014a. Leaching kinetics of selenium from selenium-tellurium-rich materials in sodium sulfite solutions. Transactions of Nonferrous Metals Society of China, vol. 24, no. 2. pp. 536-543. [ Links ]
Zheng, Y. and Chen, K. 2014b. Leaching kinetics of selenium from selenium-tellurium-rich materials in sodium sulfite solutions. Transactions of Nonferrous Metals Society of China, vol. 24, no. 2. pp. 536-543. [ Links ]
Zhong, J., Wang, G., Fan, J., Ki, Q., Kiani, Μ., Zhang, J., Yang, Η., Chen, J., and Qang, R. 2018. Optimization of process on electrodeposition of 4N tellurium from alkaline leaching solutions. Hydrometallurgy, vol. 176. pp. 17-25. [ Links ] ♦
 Correspondence:
Correspondence:
W. Yang
yangweixauat@126.com
Received: 29 Mar. 2019
Revised: 5 Jul. 2019
Accepted: 30 Jul. 2019
Published: October 2019














