Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.119 n.5 Johannesburg May. 2019
http://dx.doi.org/10.17159/2411-9717/17/484/2019
PAPERS OF GENERAL INTEREST
PGM recovery from a pregnant leach solution using solvent extraction and cloud point extraction: a preliminary comparison
L. MakuaI, II; K. LangaI, II; C. SaguruI, II; S. NdlovuI, II
ISchool of Chemical and Metallurgical Engineering, University of the Witwatersrand, South Africa
IIDST/NRF SARChI: Hydro-metallurgy and Sustainable Development; University of the Witwatersrand, South Africa
SYNOPSIS
Global demand for the three platinum group metals (PGMs) Pt, Pd, and Rh has been steadily growing due to their widespread use in emission control, industrial catalysis, and numerous other applications. The primary supply of PGMs is currently the mining industry. However, legislative, operational, and environmental challenges faced by many mining operations result in erratic supply patterns and deficits for these three metals. Naturally, recycling scrap material to recover Pt, Pd, and Rh has therefore aroused research interest in the scientific and engineering communities. Given that the majority of the global demand for these three metals is driven by emission control devices, autocatalytic converters are therefore a significant source of already processed and beneficiated PGMs for recycling.
Although pyrometallurgical processes dominate the recycling industry, hydrometallurgical processes may offer certain advantages, chief among these being lower emissions and reduced energy consumption. In this investigation, scrap converter material was leached in acidic chloride media and the leach liquor subjected to solvent extraction and cloud point extraction (CPE) to recover the PGMs. The recoveries for Pt and Pd using Alamine 308® dissolved in kerosene with decanol as a modifier were 95% and 81%, respectively. The maximum recoveries using CPE after complexation with 2-mercaptobenzothiazole (2-MBT) in the presence of Triton X-100 and tin (II) chloride dihydrate as a reductant were 97% for Pd, 96% for Pt, and 91% for Rh. This demonstrates that CPE, although relatively new, may have some potential as a breakthrough PGM recovery technology.
Keywords: ; platinum group metals, recycling, hydrometallurgy, chloride leaching, solvent extraction, cloud point extraction.
Introduction
Platinum group metals (PGMs) have found a wide variety of applications in different industries due to their chemical, catalytic, and physical properties. PGMs are essential in automobile emission abatement, petroleum refining, and the pharmaceutical and electrical industries (Carabias-Martinez et al., 2000). However, the abundance of PGMs in the Earth's crust is low and their primary sources are fast depleting (Crundwell et al., 2011).
The high demand for PGMs, together with social, economic, environmental, and political aspects related to their production, has led to concerns about their future supply. This has resulted in renewed interest in recycling end-of-life material. PGM supply from recycling has doubled over the last decade, and currently contributes 22% to global supply (Johnson Matthey, 2016). This is in part due to the environmental, social, and operational challenges associated with mining of PGMs. For example, labour unrest, energy cuts, declining commodity prices, and declining ore grades have collectively made the mining landscape economically challenging (KPMG, 2015). On the other hand, the high content of PGMs in secondary sources makes their recovery from the increasing quantities of waste products important from a resource conservation viewpoint. Moreover, PGMs obtained through recycling have been demonstrated to have a 1% environmental cost from a life-cycle perspective, whereas PGMs obtained from mining contribute the remaining 99% of environmental cost (Bossi and Gediga, 2017). Sustainable resource recovery and the economic incentive of recycling have therefore been major drivers to justify investment in PGM recycling. The autocatalytic converter industry accounts for about 70% of the global consumption of these metals (Johnson Matthey, 2016) and therefore recycling spent converters has become a major source of Pt, Pd, and Rh (Figure 1).
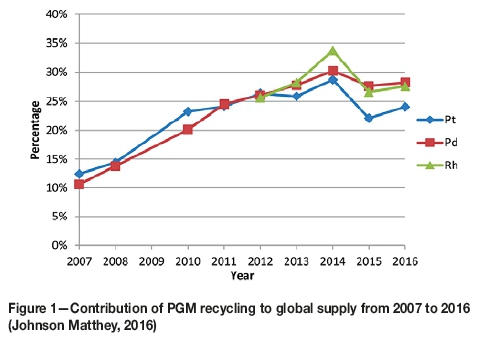
In the light of all these considerations, there have been concerted efforts to develop more efficient and more sustainable processes for the recovery of PGMs from secondary sources. Iodide complexation was investigated by Zanjani and Baghalha, (2009), and cyanide complexation by Chen and Huang, (2006) for leaching Pt, Pd, and Rh. Saguru and Ndlovu (2017) developed a leaching process for catalytic converters using acidic chloride media, which attained recoveries of 15%, 78%, and 86% for Rh, Pt, and Pd respectively.
Solvent extraction (Nguyen, Kumar, and Lee, 2016, Nguyen, Sonu, and Lee, 2016, Gandhi etal., 2015), ion exchange resin technology, classical precipitation (Crundwell et al., 2011) and more recently, cloud point extraction (CPE) (Suoranta et al., 2015) have been investigated for PGM recovery from chloride leach liquors. CPE has been used for the analytical determination of metals at low concentration, and due to the nature of the chemicals used, has been touted as a more environmentally friendly option. It was intended in this study to investigate the potential for recovering Pt, Pd, and Rh from a leach solution using CPE and then compare it to the more mature solvent extraction technology.
Solvent extraction (SX) refers to the distribution of a species between two liquid phases based on differences in solvation energies or relative affinities for the species. SX is a mature technology in the metal extraction industry, which nonetheless still has some operational demerits. The use of volatile solvents creates unsafe working conditions, and extractants are often expensive, translating to high operating expenditure. The technology has, however, been in the industry for over 50 years, and is well understood. Newer alternatives over the years have included ion exchange resins (Nikoloski and Ang, 2014), molecular recognition technologies (Izatt et al., 2015), and more recently CPE (Suoranta et al., 2015).
CPE is a technique used to preconcentrate and separate many different elements in biological and chemical systems. Much of the development and applications have dealt with the extraction and preconcentration of inorganic solutes. CPE has recently attracted a lot of attention, mainly because it is premised on 'green' sustainable chemistry.
CPE generally involves the following steps; forming neutral compounds of analytes with a chelating agent, extraction of these hydrophobic compounds to micelles formed by adding a surfactant, and separation of the surfactant-rich and aqueous phases by heating the system above its cloud point temperature (Hayes, 1993). The technique has been employed by analytical chemists as a preconcentration step in sample preparation for trace element analysis. Suoranta et al., (2015) used CPE to extract PGMs from a leach solution after microwave-assisted leaching. The underlying principles of CPE in the extraction of PGMs from leach solutions were reviewed by the authors. It was concluded that the efficiency of the CPE method depends on the pH of the solution, surfactant and complexing agent, hydrochloric acid concentration, and the presence of a reducing agent.
It was therefore the purpose of this research to conduct a preliminary comparison of using Alamine 308® in kerosene for SX with CPE using 2-mercaptobenzothiazole (2-MBT) in the presence of Triton X-100 and tin (II) chloride dihydrate. The underlying chemistry for both extraction schemes is presented in the following section.
Chemistry of PGM solvent extraction
Nguyen, Kumar, and Lee (2016) illustrated the potential for using tri-iso-octylamine (Alamine 308) to recover Pt (IV), Pd (II), and Rh (III) from a synthetic acidic chloride solution. We sought to investigate the performance of tri-iso-octylamine in a real leach solution. Tri-iso-octylamine is a tertiary amine with three alkyl (-C8H17) groups surrounding a single nitrogen atom. Metal extraction using tri-iso-octylamine occurs via protonation and anion exchange. Consequently, tri-iso-octylamine will only extract PGMs from acidic solutions. In the presence of an acid solution, for example in HCl, the basic N atom in the amine structure reacts with the excess protons and forms an amine salt according to Equation [1].
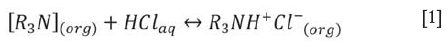
When this organic intermediary salt is in the vicinity of Pt-chloro anions, it exchanges its Cl- anion for the PtCl62- anion group:
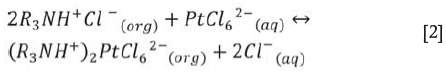
The required anion is thereby extracted into the organic phase.
Chemistry of PGM extraction using cloud point extraction
The mechanisms CPE are complex and not yet fully understood (Yu et al., 2010). However, in essence CPE involves the transfer of a surfactant from one liquid phase to another by heating the system above its cloud point temperature (CPT). The surfactant molecules form micelles at their critical micelle concentration (CMC). On increasing the temperature of the solution, the micelles are dehydrated and therefore accumulate (Yu et al., 2010). This results in the phase separation of the solution into a surfactant-micelle-rich phase and a surfactant-poor phase (aqueous phase). The surfactant concentration and temperature are manipulated to extract solutes into the micelle phase (Streat, 1999). Consequently, metal ions can be preconcentrated and/or separated from an aqueous solution using CPE.
Experimental methodology
The experimental work consisted of two parts, the solvent extraction experiments and the CPE experiments. A leach solution was obtained following the work conducted by Saguru and Ndlovu, (2017), in which an autocatalytic converter was crushed, ground, homogenized, and leached in an acidic chloride medium. The leach solution used for the extraction experiments assayed 22 ppm Pt, 408 ppm Pd, and 11 ppm Rh.
Solvent extraction experiments
Pt and Pd were co-extracted using Alamine 308 dissolved in kerosene with a 5% v/v n-decanol modifier following the method of Nguyen, Kumar, and Lee (2016). Based on preliminary experiments, it was determined that an acidification procedure was necessary prior to extraction, and as such HCl was used to acidify the pregnant leach solution. In these acidification experiments, 15 mL of the pregnant leach solution was added to 5 mL of hydrochloric acid at five different concentrations. The acidified solutions were then individually mixed with 20 mL of 0.1 M organic Alamine 308 in kerosene and shaken on a reciprocal shaker for 30 minutes at 300 r/min and room temperature. After shaking, the two layers were allowed to settle and separate into an organic and an aqueous phase. The aqueous phase was submitted for analysis and the percentage extraction calculated using mass balances.
Once the acidification concentration that produced a maximum extraction was determined, 750 mL of the pregnant leach liquor was mixed with 250 mL of acid at the chosen concentration to form a bulk solution for subsequent extraction experiments. In the second round of solvent extraction experiments, 20 mL of the acidified aqueous PGM solution was added to 20 mL of the organic solution at different concentrations, mixed for 30 minutes using a reciprocating shaker at 300 r/min, and allowed to settle. The two phases were separated by decanting and the aqueous phase sent for analysis, with percentage extraction calculated by mass balances. All experiments were duplicated.
Cloud point extraction experiments
Solutions of 10% m/v Triton X-100, 10% m/v tin (II) chloride in 6 M hydrochloric acid, 4 M NaOH, and 0.5 M ammonium hydroxide were prepared by dissolution in distilled water following the method of Suoranta et al. (2015). To the ammonium hydroxide solution, 1% m/v 2-mercaptobenzotiazole (2-MBT) was added to prepare the stock 2-MBT solution.
20 mL of pregnant leach solution was first diluted using water (the dilution ratio varied between 1 and 33) and then pipetted into 50 mL centrifuge tubes. 2 mL of the previously prepared Triton X-100 solution and 1 mL of 2-MBT solution were added to the centrifuge tubes and the solutions allowed to stand for 15 minutes, after which 1.5 mL of the prepared tin (II) chloride solution was added. The open centrifuge tubes were then placed in a thermostat-controlled water bath at 90°C. The solutions were heated in the water bath for 25 minutes and then held at 90°C for 120 minutes. A refrigerator set at 8°C was used to cool the solutions for 30 minutes, followed by further cooling in a freezer set to -10°C for 15 minutes. The aqueous phase was then separated from the surfactant-rich phase by decanting, and sent for analysis.
In this set of experiments, the dilution factor and starting pH were varied to obtain an optimum dilution ratio and pH. Once these were established, the additions of Triton X-100 (0.25 mL to 2 mL), 2-MBT (0.5 mL to 3 mL), equilibration temperature (70°C to 95°C) and incubation time (30 minutes to 130 minutes) were varied to identify their effects on PGM extraction efficiency. All experiments were duplicated.
Results and discussion
Solvent extraction results
Effect of HCl concentration on PGM recovery
The effect of HCl concentration on PGM extraction is presented in Figure 2.
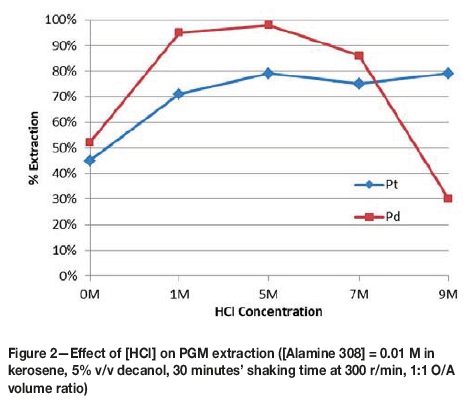
The extraction of Pt and Pd is directly correlated to the amount of excess H+ ions available to protonate the amine complex. As such, it was expected that increasing the HCl concentration would result in an increase in Pt and Pd co-extraction into the organic phase.
Increasing HCl concentration is also expected to increase the proportion of non-hydrated PGM chloro-complexes in the pregnant leach solution. Hydrated PGM chloro-complexes are chloro-complexes in which one or more of the chloride ions have been substituted by a hydroxide ion. The amount of hydrated PGM chloro-complexes increases with increasing pH of solution. Hydrated PGM chloro-complexes are hydrophilic - i.e. they attract more water molecules around each hydrated chloro-complex. This increases the solvation layer of the complex, which translates to fewer coulombic interactions between the protonated amine complex and the negative PGM chloro-complex. The decreased strength of attraction reduces the efficiency of extraction of the hydrated PGM chloro-complex into the organic phase. Decreasing the pH, by adding concentrated HCl, was therefore expected to reduce the proportion of hydrated PGM chloro-complexes, which increased PGM extraction as illustrated in Figure 2. An HCl concentration of 5 M, with an acidification volume ratio of PGM aqueous solution to acid of 3:1 was then chosen for the subsequent organic extractant concentration experiments.
Effect of Alamine 308 concentration on PGM recovery
An increase in the extractant concentration resulted in an increase in PGM extraction, peaking at 0.1 M concentration as shown in Figure 3. For all organic acid concentrations, it was also observed that Pt extraction was consistently higher than Pd extraction, which is in agreement with results obtained by Nguyen, Kumar, and Lee (2016). The PdCl42- ion has a higher charge density than PtCl62-. It is therefore highly polarizing and attracts water molecules. This increases its solvation layer and reduces the strength of attraction between the PdCl42- anion and the slightly positive end of the protonated amine, much like how hydration reduces attraction as explained in the previous section. This therefore reduces the degree of extraction of the Pd chloro-complex into the organic phase, compared to the Pt chloro- complex.

Cloud point extraction results
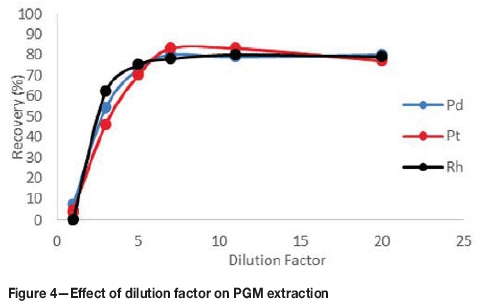
Effect of dilution ratio
CPE has previously been applied for analytical purposes in low concentration ranges. In order to apply the technique to PGM leach liquor, a dilution factor had to be established. From Figure 4, it can be observed that the recovery increases with an increase in dilution factor. At a dilution factor of unity, the recoveries were not quantitative. The concentrations of the analytes were too high and therefore most of the analytes could not successfully be extracted and entrapped in the micelles. PGM extraction peaked at a dilution factor of seven, as illustrated in Figure 4, and this dilution factor was used for the rest of the tests.
Effect of pH
The pH is a critical parameter for the complexation of metal ions and the coacervation of the micelles (Bezerra, Arruda, and Ferreira, 2005). Figure 5 shows the effect of pH on the recovery of PGMs.
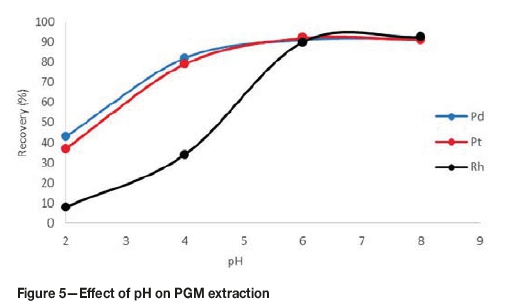
The effect of pH was investigated in the range 2-8. At a pH of 2, the recoveries for all the PGMs were below 45%. The recoveries increased with increasing pH value, with recoveries around 90% for all PGMs at pH 6. There was no significant change in recoveries with a further increase in pH .
The pH of the solution affects the overall charges of the PGM-chloro complexes and therefore affects the formation of metal complexes. The optimal pH range matches the range most favourable for complex formation, but should still be lower than the pH range for precipitation of the metals. The low recoveries at acidic conditions are due to the fact that complexation is weak under acidic conditions. Under acidic conditions, the metal ions compete with hydrogen ions for binding with the complexing agent, therefore resulting in lower recoveries. At lower pH values the micelles are also easily destroyed, reducing the system's entrapping capability. The pH was set to 6 for the rest of the tests.
Effect of amount of surfactant
Triton X-100 was chosen as the surfactant because of its commercial availability, stability, low toxicity, low cost, and low cloud point temperature. Figure 6 shows the effect of Triton X-100 dosage on the PGM recoveries. At a dosage of 0.25 mL, recoveries were below 50%. An increase in surfactant dosage resulted in an increase in recoveries until a dosage of 1 mL, after which the recoveries remained constant despite increasing surfactant dosage. High recoveries above 80% were achieved at a dosage range of 1-2 mL. The low recoveries at a low dosage of 0.25 mL were due to the surfactant concentration being too low to form an adequate number of micelles with an adequate aggregation number to entrap the hydrophobic metal complexes quantitatively. With increasing surfactant concentration, the aggregation number of the formed micelles increased, which resulted in an increase in recoveries until a dosage of 1 mL, where the micelles were able to successfully entrap most of the complexes. A further increase in surfactant dosage resulted in no significant change in the recoveries. The for surfactant dosage was therefore set to 1 mL.

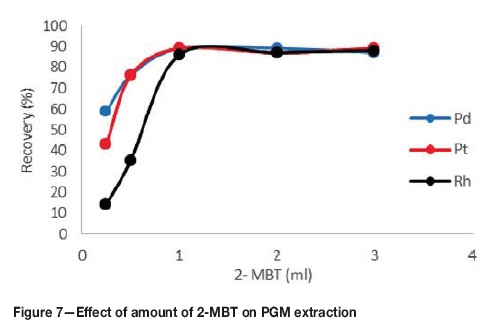
Effect ofamount of2-MBTcomplexing agent on PGM extraction
2-MBT was chosen as the complexing agent because it produces sufficiently hydrophobic complexes with PGM-chloro complexes and it is selective for the PGMs. The complexing agent dosage was investigated in the range 0.25-3 mL. From Figure 7, it can be observed that the recovery of the complexes increases with an increase in complexing agent dosage. Above a dosage of 1 mL the recoveries remained constant. The low recoveries at a low dosage of 0.25 mL were due to insufficient complexing agent for reaction with all the PGMs. Above a dosage of 1 mL, the recoveries remained constant because the complexing agent was now in excess. Initially, the recoveries of rhodium were very low compared to the other PGMs. This is probably due to the fact that rhodium is very inert and the probability of it reacting with a very small amount of the complexing agent is low. The amount of complexing agent was therefore set to 1 mL.
Effect of equilibration temperature and incubation time
It is desirable to employ the lowest possible equilibration temperature and shortest incubation time from a process economics and productivity viewpoint. From Figure 8 it can be observed that the recoveries increase with an increase in equilibration temperature up to 90°C, and decrease above 90°C. Below 90°C, the system has not reached its cloud point temperature, which is why recoveries are lower. Temperature is the driving force for phase separation. When the system is below its cloud point temperature the phase separation between the bulk aqueous phase and the surfactant-rich phase is incomplete, and therefore some of the complexes are still in the aqueous phase. At 90°C the system is above its cloud point temperature, which explains the maximum recoveries achieved. Above 90°C the recoveries begin to decrease due to the decomposition of the metal complexes at temperatures higher than the cloud point temperature. The optimum equilibration temperature was therefore set to 90°C.
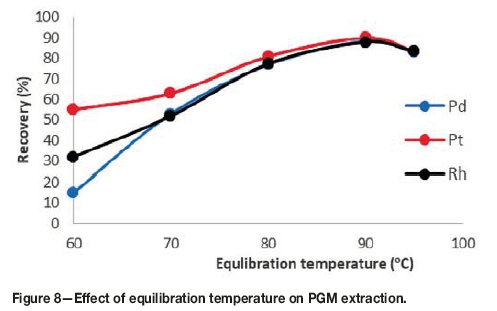
Figure 9 show that an increase in incubation time resulted in an increase in recoveries. At an incubation time of 30 minutes the recoveries were very low. The maximum recoveries were achieved after 120 minutes. The gravitational settling of the micelles with the attainment of cloud point temperature to form a surfactant-rich phase depends on the time for complete phase separation. The optimum incubation time was chosen as 120 minutes.

The optimum parameters found in each test conducted were combined and tested again in a duplicate experiment which attained recoveries of 97% for Pd, 96% for Pt, and 91%, for Rh.
Comparison between solvent extraction and cloud point extraction, and recommendations for further test work
CPE has been used substantively in analytical test work. Recently, due to its environmental competitiveness, it has been shown to be a viable contender for PGM extraction, at least, at the bench scale. In this work, high recoveries were obtained using both CPE and solvent extraction. However, with CPE all the PGMs were extracted in a single step - which may or may not be an advantage. The advantage is that for operations that are in the middle of the value chain, CPE could be used to extract all PGMs into one phase before collective precipitation. The crude precipitate would then be sold to operations that are at the end of the value chain for final metal separation. However, metal stripping tests have not yet been conducted; therefore the selective separation of the metals is not well understood. In contrast, solvent extraction technologies are well understood. For example, it is known that Pt and Pd could be selectively stripped from the Alamine 308 organic solution using HCl and thiourea (Nguyen, Kumar, and Lee, 2016). In order for more comprehensive comparisons to be conducted, not only between CPE and solvent extraction, but also between CPE and any other recovery technology, there will be a need for comprehensive studies into the science and engineering of CPE.
For this preliminary study, the methodology of changing one factor at a time was employed, which is rather limited in terms of statistical robustness of the results. We therefore suggest the use of response surface methodology for statistical design in further test work. This research was aimed at providing an insight on the performance of this relatively new technology in a real leach solution for PGM extraction.
It was also established that CPE works best at low PGM concentrations, and therefore concentrated solutions may require dilution. Although this study was limited to the recovery of PGMs from an autocatalytic converter leach solution, CPE could be investigated for application on other low-concentration solutions, such as tailings solution streams. It is also imperative to investigate the extent of entrainment of other metal solutions before CPE can be seriously considered in the PGM extraction industry.
It is therefore recommended that further test work be conducted on CPE for PGM extraction, with the ultimate goal of comparing it against mature technologies like solvent extraction through piloting, cost-benefit analyses, and process costing. This work has illustrated that CPE could be a potential technology for recovery of PGMs.
Conclusions
Cloud point extraction using Triton X-100 as a surfactant and 2-mercaptobenzothiazole as the complexing agent was successfully used to extract PGMs from leach solutions. Although the results indicate that the CPE technique is feasible, much more work is required to compare CPE with existing extraction methods. CPE offers an attractive alternative to conventional extraction methods by reducing the consumption of, and exposure to, the solvents, disposal cost, and increasing environmental sustainability. CPE offers several advantages over conventional solvent extraction, ion exchange, and precipitation processes, including simplicity, safety, low cost, high concentration factors, and high recovery and selectivity.
Acknowledgements
The authors wish to acknowledge the National Research Fund and Department of Science and Technology, South Africa for funding this research work.
References
Bezerra, M. de A., Arruda, M.A.Z., and Ferreira, S.L.C. 2005. Cloud point extraction as a procedure of separation and pre concentration for metal determination using spectroanalytical techniques: A review. Applied Spectroscopy Reviews, vol. 40. pp. 269-299. https://doi.org/10.1080/05704920500230880 [ Links ]
Bossi, T. and Gediga, J. 2017. The environmental profile of platinum group metals. Johnson Matthey Technology Review, vol. 61. p. 111. https://doi.org/doi:10.1595/205651317x694713 [ Links ]
Carabias-Martinez, R., Rodríguez-Gonzalo, E., Moreno-Cordero, Β., Perez-Pavon, J., García-Pinto, C., and Fernandez Laespada, E. 2000. Surfactant cloud point extraction and preconcentration of organic compounds prior to chromatography and capillary electrophoresis. Journal of Chromatography, vol. A 902. pp. 251-265. [ Links ]
Chen, J. and Huang, K. 2006. A new technique for extraction of platinum group metals by pressure cyanidation. Hydrometallurgy, vol. 82. pp. 164-171. https://doi.org/10.1016/j.hydromet.2006.03.041 [ Links ]
Crundwell, FX, Moats, M.S., Ramachandran, V., Robinson, T.G., and Davenport, W.G. 2011. Extractive Metallurgy of Nickel, Cobalt and Platinum-group Materials. Elsevier, Amsterdam, Boston. [ Links ]
Hayes, P.C. 1993. Process Principles in Minerals and Materials Production. Hayes Publishing, Brisbane, Australia. [ Links ]
Izatt, R.M., Izatt, S.R., Izatt, N.E., Krakowiak, K.E., Bruening, R.L., and Navarro, L. 2015. Industrial applications of molecular recognition technology to separations of platinum group metals and selective removal of metal impurities from process streams. Green Chemistry, vol. 17. pp. 2236-2245. https://doi.org/10.1039/C4GC02188F [ Links ]
Johnson M. 2016. PGM market report May 2016, Supply and demand in 2015. KPMG. 2015. Commodity insights bulletin (Insight). KPMG. [ Links ]
Nguyen, T.H., Kumar, Β.Ν., Lee, and M.S. 2016. Selective recovery of Fe(III), Pd(II), Pt(IV), Rh(III) and Ce(III) from simulated leach liquors of spent automobile catalyst by solvent extraction and cementation. Korean Journal of Chemical Engineering, vol. 33. pp. 2684-2690. https://doi.org/10.1007/s11814-016-0123-5 [ Links ]
Nguyen, T.H., Sonu, C.H., and Lee, M.S. 2016. Separation of Pt(IV), Pd(II), Rh(III) and Ir(IV) from concentrated hydrochloric acid solutions by solvent extraction. Hydrometallurgy, vol. 164. pp. 71-77. https://doi.org/10.1016/j.hydromet.2016.05.014 [ Links ]
Nikoloski, A.N. and Ang, K.-L. 2014. Review of the application of ion exchange resins for the recovery of platinum-group metals from hydrochloric acid solutions. Mineral Processing and Extractive Metallurgy Review, vol. 35. pp. 369-389. https://doi.org/10.1080/08827508.2013.764875 [ Links ]
Rajiv Gandhi, M., Yamada, M., Kondo, Y., Shibayama, A., and Hamada, F. 2015. Selective extraction of Pd(II) ions from automotive catalyst residue in Cl- media by O-thiocarbamoyl-functionalized thiacalix[n]arenes. Hydrometallurgy, vol. 151. pp. 133-140. https://doi.org/10.1016/j.hydromet.2014.11.017 [ Links ]
Saguru, C. and Ndlovu, S. 2017. Preliminary study on PGM leaching from used autocatalytic convertors using AlCl3 and HOCl. Proceedings of the European Metallurgical Conference 2017. Production and Recycling of 'Non-Ferrous Metals: Saving Resources for a Sustainable Future, GDMB, Leipzig, Germany. pp. 55-70. [ Links ]
Suoranta, T., Zugazua, O., Niemela, M., and Peramaki, P. 2015. Recovery of palladium, platinum, rhodium and ruthenium from catalyst materials using microwave-assisted leaching and cloud point extraction. Hydrometallurgy, vol. 154. pp. 56-62. https://doi.org/10.1016/j.hydromet.2015.03.014 [ Links ]
Yu, F., Xi, C., He, Z., and Chen, L. 2010. Development of cloud point extraction for simultaneous extraction and determination of platinum and palladium using inductively coupled plasma optical emission spectrometry in platinum-palladium spent catalysts. Analytical Letters, vol. 43. pp. 972-982. https://doi.org/10.1080/00032710903491112 [ Links ]
Zanjani, A. and Baghalha, M. 2009. Factors affecting platinum extraction from used reforming catalysts in iodine solutions at temperatures up to 95°C. Hydrometallurgy, vol. 97. pp. 119-125. https://doi.org/10.1016/j.hydromet.2009.02.001 ♦ [ Links ]
 Correspondence:
Correspondence:
C. Saguru
Email: tatendasaguruc@gmail.com
Received: 24 Jun. 2018
Accepted: 3 Oct. 2018
Published: May 2019
ORCiD ID: C. Saguru https://orchid.org/0000-0002-6014-2495














