Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.119 n.5 Johannesburg May. 2019
http://dx.doi.org/10.17159/2411-9717/85-192-1/2019
PAPERS OF GENERAL INTEREST
Metal recovery from TiCl4 slurry by evaporation and acid leaching
X. XiangI; X. WangII; W. XiaI; J. YinI
ISchool of Metallurgical and Materials Engineering, Central South University, Changsha, China
IISchool of Metallurgy and Environment, Chongqing University of Science and Technology, Chongqing, China
SYNOPSIS
TiCl4 slurry containing valuable metals is an unavoidable by-product of the titanium ore chlorination process. The recovery of these valuable metals, which include titanium, niobium, tantalum, and aluminum, is an urgent issue to tackle in the titanium industry. The results of this investigation show that the valuable metallic elements can be recovered from the slurry by evaporation in a sealed container and leaching with dilute hydrochloric acid. After evaporation at 200°C for 60 minutes, nearly 99% of the titanium was recovered in the form of TiCl4, which was formed by the reaction of the TiO2 in the slurry with AlCl3. After evaporation, metals like niobium, aluminum, and tantalum remained in the residue. By leaching with 2.1 mol/L HCl at a L/S ratio of 6:1 mL/g at 80°C for 60 minutes, the soluble metals, such as aluminum, iron, and copper were all removed from the residue, and the niobium and tantalum were further enriched in the leach residue. A concentrate containing 53.40 wt% Nb and 5.57 wt% Ta was obtained by washing the leach residue with dilute aqueous ammonia under stirring. A potential waste water purifying agent containing 263.75 g/L AlCl3 was produced by purifying the leaching solution with Al(OH)3 and modified polyacrylamide.
Keywords: TiCl4 slurry, niobium, recovery, evaporation,leaching.
Introdution
Titanium tetrachloride (TiCl4) is an important intermediate in titanium metallurgy that is widely used in the production of titania and titanium sponge (Akhtar, Xiong, and Pratsinis, 1991). In conventional titanium metallurgical processes, high-titanium materials such as synthetic rutile and high-titanium slag are reacted with chlorine to produce TiCl4 in a fluidized-bed furnace. Accompanying elements, such as Al, Nb, and Fe also react with chlorine to form chlorides, which are mixed with gaseous TiCl4 (Anderson, 1917). In the condensation process, gaseous TiCl4 is refrigerated and high-boiling-point chlorides are precipitated in the liquid TiCl4 to form a slurry containing 50~60 wt% TiCl4 (Wang, Xiang, and Wang, 2012).
Various methods have been proposed to recover TiCl4 from the slurry, including microwave heating and spray drying (Wang, Xiang, and Wang, 2012, Wang et al., 2010a). To date, the only way to recover the TiCl4 is to return the slurry to the fluidized-bed furnace. However, this causes fluctuations in the temperature in the furnace, resulting in incomplete reaction between titanium-rich materials and chlorine. Therefore, the slurry is often rinsed with water and neutralized by adding lime, which caused serious environmental pollution and wastes a valuable resource (Roy, Bhatt, and Rajagopal, 2003).
The TiCl4 slurry contains considerable quantities of niobium (Wang et al., 2012), which is one of the accompanying elements in titanium ore. Niobium is a rare metal that is widely used in steel, electronics, and other high-tech industries (Miller, 1959a; He, Liu, and Zhang, 1998). In nature, niobium is often associated with titanium, tantalum, the rare earth elements, and tin (Gupta and Suri, 1994; Miller, 1959b), and is usually recovered by hydrometallurgical methods such as decomposing tantalum-niobium concentrate with hydrofluoric acid (El-Hussaini, 2001; Gupta and Suri, 1994; Wang et al., 2010) and leaching niobium from low-grade niobium materials with concentrated KOH solution (Zhou, Zheng, and Zhang, 2005; Wang et al., 2009a; Zhou et al., 2005b). These methods cannot be used to recover niobium from the slurry because of the high content of TiCl4. However, the niobium can be easily recovered from the slurry by leaching after the TiCl4 has been removed by evaporation (Fran, 1978).
The objective of this study is to transform the slurry from a waste material into a valuable resource and reduce pollution. The methods employed include evaporation of the slurry in a sealed container and leaching of the residue with dilute hydrochloric acid.
Experimental
Materials and analysis
All the chemical reagents used in this study were analytical grade, and deionized water was used. The TiCl4 slurry was obtained from Zunyi Titanium Industry Co. Ltd. Its chemical composition is listed in Table I. It was found that about 80% of the titanium existed as TiCl4 in the slurry, which was confirmed by washing the slurry with CCl4.
The contents of titanium, aluminum, niobium etc. were measured by inductively coupled plasma atomic emission spectroscopy (ICP-AES) using a PS-6 Plasma Spectrovac, Baird (USA). Samples analysed for niobium were dissolved in hydrofluoric acid and hydrochloric acid (Lima and Conte, 2003; Lu and Makishima, 2007; Yang and Pin, 2002). The X-ray diffraction (XRD) patterns were recorded on a Rigaku Miniflex diffractometer with Cu K X-ray radiation at 35 kV and 20 mA.
Experimental procedure
Table I shows that the contents of titanium, aluminum, and niobium are much higher than those of other metallic elements in the slurry. Hence, the experimental work focused on the recovery of titanium, niobium, and aluminum. The experimental procedure was performed as shown in Figure 1. First, TiCl4 was recovered from the slurry by evaporation and condensation. The residue was then leached with dilute hydrochloric acid to remove the aluminium and enrich the niobium content. The leach residue was washed with ammonia solution and filtered to recover a niobium concentrate. The leaching solution was purified to produce an AlCl3 solution, which could be used as a purifying agent in waste water treatment.
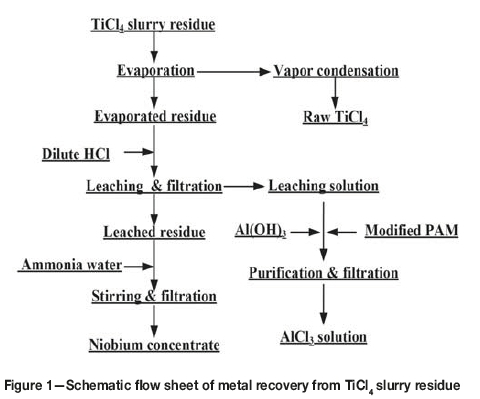
Figure 2 is a schematic of the experimental set-up for TiCl4 recovery from the slurry. During the operating process, the slurry (25 g each test) was added into a 50 ml stopper flask which was heated by an oil bath. TiCl4 in the slurry was volatilized from the flask and condensed in the forced-water condenser with a vortex tube. The liquid TiCl4 was collected in a calibrated measuring cylinder. After evaporation for the required time, the residue was removed and weighed.
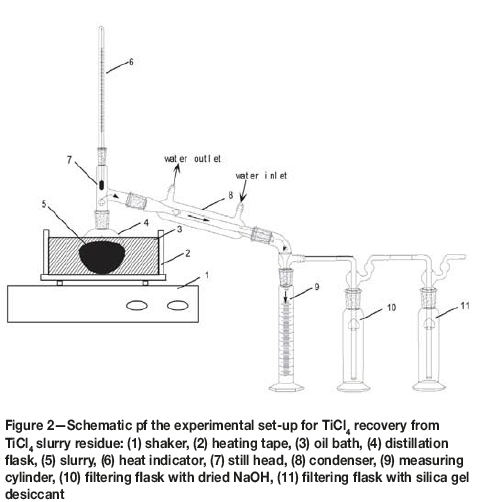
The leaching experiments were conducted in a 300 ml glass flask, which was heated by a water bath. After the required contact time, the suspension was vacuum-filtered. The leach residue was washed with ammonia solution while stirring to remove residual chlorine, then dried and analysed for niobium and other elements.
Experimental fundamentals
From Table I, it can be seen that the slurry was mainly composed of chlorine, titanium, aluminum, and niobium. Most of the titanium in the slurry was in the forms of TiCl4 and TiO2
(Wang, Xiang, and Wang2012). During evaporation, TiCl4 was preferentially vaporized from the slurry as its boiling point is lower than that of other chlorides such as AlCl3, NbCl5, and FeCl3 (Dean, 1985; Lide, 1991). During evaporation, complex chemical reactions can take place in the slurry (Den, 2010), expressed as follows:
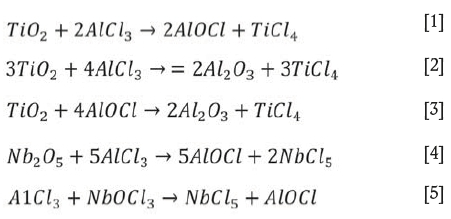
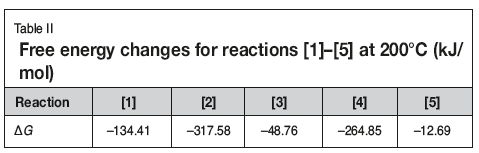
The free energy changes of reactions [1]-[5] at 200°C are listed in Table II. As seen, the reactions can occur when the slurry is heated to 200°C. Although the free energy changes are different, the reactions are not selective as they take place not in solution but in the slurry. Therefore the TiO2 in the slurry can transform into TiCl4, which then vaporizes. Hence, by controlling the evaporation temperature, titanium can be effectively separated and recovered from the slurry. Subsequently, aluminum, iron. and copper remaining in the residue can be easily leached with dilute hydrochloric acid, while the niobium, zirconium, and tantalum are insoluble in dilute acid (Dean, 1985; Zhu and Cheng, 2011; Gibalo, 1970). After washing with ammonia solution, chloride remaining in the leached residue is almost completely removed. Therefore, the niobium contained in the slurry can be effectively enriched by using the process shown in Figure 1.
Results and discussion
TiCl4 slurry evaporation
Effect of evaporation temperature
Figure 3 shows the effect of temperature on the evaporation of titanium, niobium, and aluminium from the slurry over 60 minutes of heating. It was found experimentally that only when the temperature was increased to 145°C could liquid TiCl4 be observed in the condenser, because the temperature difference between the oil bath and the still head outlet was about 25°C. In order to avoid the evaporation of high-boiling-point chlorides, the test temperature was changed from 150°C to 220°C. As can be seen, the evaporation of titanium increased considerably as the temperature increased from 150 to 164°C, and then increased very slowly in the range of 164-220°C. After evaporation at 164°C for 60 minutes, 3.5% of the titanium remained in the residue, decreasing to 1.5% when the temperature was raised to 200°C. This can be attributed to the reaction between TiO2 and gaseous AlCl3, because the sublimation temperature of AlCl3 is 178°C (Dean, 1985).
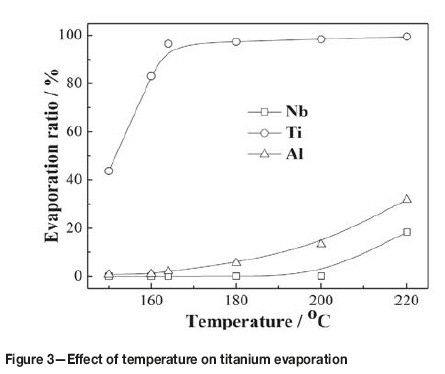
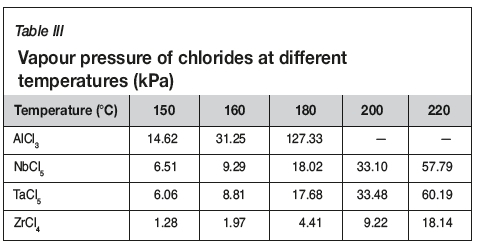
Figure 3 shows that the evaporation of titanium reached up to 98.5% at 200°C, whilc the evaporations of niobium and aluminum were 0.1% and 13.27% respectively. With further increases in temperature, the evaporation of titanium remained constant, while the evaporation of niobium and aluminum increased quickly, which might be due to the evaporation of AlCl3 and NbCl5. Table III gives the vapour pressures of chlorides at different temperatures (Dean, 1985). As can be seen, the vapour pressure of AlCl3 and NbCl5 increases with increasing temperature, and the vapour pressures of AlCl3 and NbCl5 are higher than those of TaCl5 and ZrCl4 at temperatures below 200°C. However, it is interesting to see that the evaporation of AlCl3 is lower than expected, which may be due to the inhibitory effect of TiO2 and Nb2O5. Therefore, to separate and recover TiCl4 from the slurry, the evaporation temperature should not exceed 200°C.
Effect of evaporation time
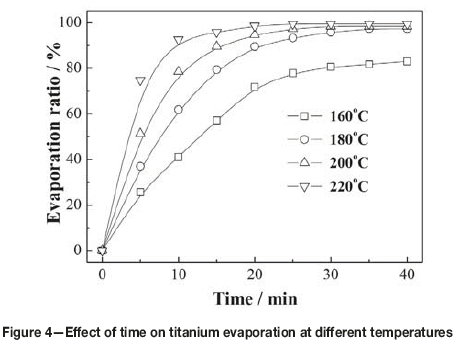
Figure 4 shows the effect of varying evaporation time from 5 to 40 minutes on the evaporation of titanium at different temperatures. It can be seen that the evaporation of titanium was fast initially, and then gradually slowed as evaporation neared completion. The times needed for evaporation at temperatures 220°C, 200°C, 180°C, and 160°C were 25, 30, 35, and 40 minutes respectively, which indicates that the lower the evaporation temperature and the less the amount of TiCl4 remaining in the slurry, the more difficult the evaporation of titanium from the residue. From Figures 3 and 4, it can be determined that the recovery of titanium from the slurry should be carried out in the temperature range of 180-200°C for more than 35 minutes.
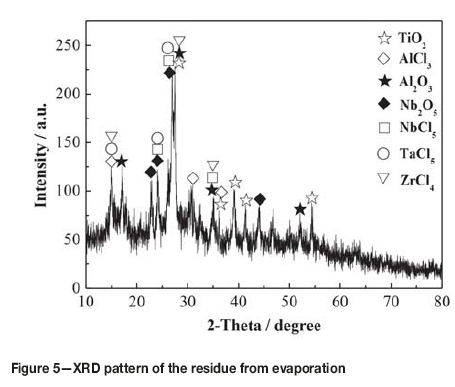
The residue obtained by evaporating the slurry at 200°C for 60 minutes (see Table I) was used for niobium recovery experiments. As can be seen from Table I, after evaporation, the content of titanium decreased from 17.41 wt% to 0.79 wt% while the contents of aluminum, niobium, zirconium, tantalum, copper, and iron all increased proportionally. Compared with a titanium content of 29.6 wt% in the residue obtained by spray drying with argon at about 250°C (Wang etal., 2012), it can be seen that evaporation in a closed container is an effective method of recovering titanium from the slurry. During the evaporation process, 66.7 wt% of the slurry evaporated to form a yellowish distillate with a TiCl4 content of 97.5%, together with some impurities such as AlCl3, NbCl5, and FeCl3. Therefore, the distillate represents a crude titanium tetrachloride and could be used to produce pigment or titanium sponge after purification (Den, 2010). Figure 5 shows the XRD pattern of the residue from evaporation. As seen from Figure 5 and Table I, the high-boiling-point chlorides and oxides all remained in the residue, indicating that the most appropriate method of separating and recovering titanium from TiCl4 slurry is to evaporate the slurry in a closed container at 200°C for 60 minutes.
Leaching of the evaporation residue
It can be seen from Table I that the metallic elements contained in the evaporation residue can be divided into two types - those that are soluble in dilute hydrochloric acid, which include aluminum, copper, and iron, and those that are insoluble, including niobium, zirconium, and tantalum (Dean, 1985). The content of aluminum in the residue is much higher than that of copper and iron. Therefore, this paper focuses on the separation of aluminum and niobium.
Effect of acid concentration
The effect of acid concentration on the separation of aluminum and niobium was tested at 30°C at a liquid-to-solid ratio of 6:1 mL/g and leaching time of 120 minutes. Figure 6 shows that the acid concentration was an important factor in the separation. The leaching ratio of aluminum increased with increasing acid concentration, with more than 97% of the aluminum being leached when the acid concentration increased to 2.1 mol/L. A further increase in acid concentration had little effect. The remaining aluminum might be occluded by the leach residue. The leaching ratio of niobium was nearly zero at an acid concentration below 1.7 mol/L, and increased quickly at acid concentrations over 2.1 mol/L. This could be attributed to the dissolution of niobium oxides at high acid concentrations (El-Hussaini and Mahdy, 2002), and the possible reaction is given in Equation [6]. Therefore, to separate aluminum from niobium, the preferred hydrochloric acid concentration was determined to be
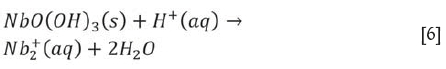
Effect of temperature
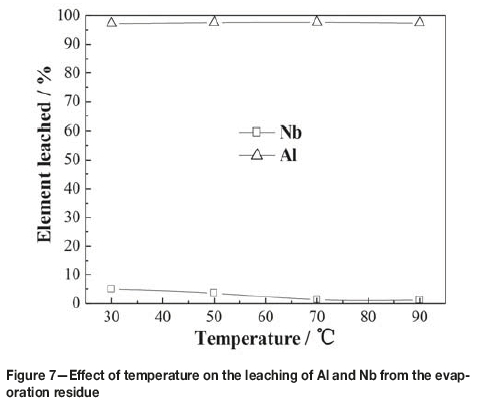
The effect of temperature on the leaching of the residue from evaporation is illustrated in Figure 7. The experiments were performed with liquid-to-solid ratio of 6:1 mL/g, and a leaching time of 120 minutes in 2.1 mol/L HCl. As can be seen from Figure 7, the effect of temperature on aluminum leaching was slight, while the effect on niobium leaching was more pronounced. As the temperature was increased from 30°C to 70°C, leaching of niobium decreased slowly, which might be attributed to hydrolysis of niobium in the leaching solution. Only 1.28% of the niobium was leached at 70°C, and further increase of temperature had little effect. Therefore, keeping the leaching temperature over 70°C is propitious for the enrichment of niobium.
Effect of time
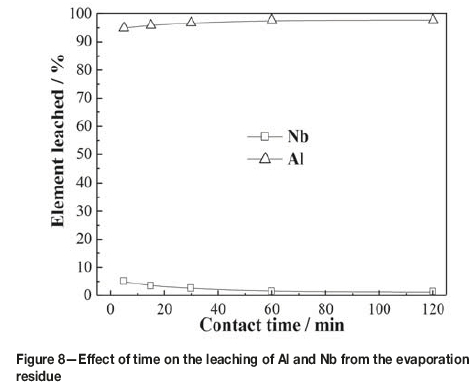
Figure 8 shows the results obtained by varying the leaching time from 5 to 120 minutes at 80°C with a liquid-to-solid ratio of 6:1 mL/g in 2.1 mol/L HCl. As can be seen, 95% of the aluminum was leached within 5 minutes, while only 5% of the niobium was leached. During the experiment, it was found that once the residue was added into the hydrochloric acid solution under stirring, the solid became suspended in the solution and the amount decreased appreciably. This indicated that aluminum contained in the evaporation residue dissolved easily in dilute hydrochloric acid. As the leaching time increased, the leaching ratio of aluminum increased slowly, while the leaching ratio of niobium decreased markedly. With a leaching time of 60 minutes, the leaching ratio of aluminum increased to 97.67%, while that of niobium decreased to 1.39%. The leaching ratio of niobium remained almost constant with further increases in leaching time. As aluminum was leached, the acidity of the leaching solution decreased rapidly, which caused the hydrolysis precipitate of niobium to dissolve in the solution. Therefore, a contact time of 60 minutes was selected for the following experiments.
Effect of liquid-to-solid ratio
The effect of liquid-to-solid (L/S) ratio was examined in 2.1 mol/L HCl at 80°C for 60 minutes. The results are presented in Figure 9. As can be seen, the leaching ratio of aluminum increased from 80.16% to 97.73% when the L/S ratio increased from 2:1 to 6:1 mL/g, and then remained constant. However, the leaching ratio of niobium remained almost constant as the L/S ratio increase from 2:1 to 6:1 mL/g. At a L/S ratio over 6:1 mL/g, the leaching ratio of niobium increased markedly. Therefore, a L/S ratio of 6:1 mL/g was considered appropriate in this work.
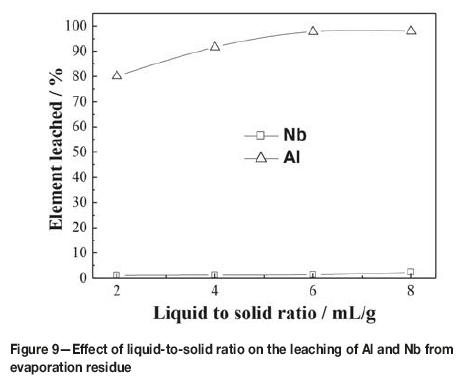
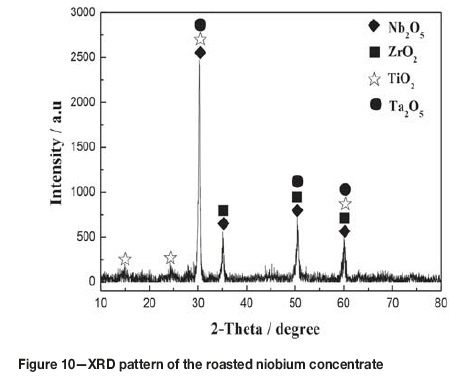
The compositions of the leached residue and leaching solution are listed in Table I and Table IV respectively, obtained by treating the evaporation esidue with a L/S ratio of 6:1 mL/g in 2.1 mol/L HCl at 80°C for 60 minutes. As can be seen, aluminum, iron, and copper were almost completely leached from the evaporation residue, while the leaching of chlorine was only about 85%. This indicated that the leached residue might contain oxychlorides (Rodrigues and Silva, 2010; Guo and Wang, 2009). In order to remove the residual chlorine, the leached residue was washed with 2 mol/L aqueous ammonia at a L/S ratio of 4:1 mL/g under stirring at room temperature for 30 minutes and then filtered. After drying at 115°C for 24 hours, a niobium concentrate with the composition listed in Table I was obtained. Table I shows that after evaporation of the TiCl4 slurry in a closed container, leaching of the residue with dilute HCl, and washing with dilute ammonia, the niobium, tantalum, and zirconium contents in the slurry were enriched dramatically. Although the content of Nb was 53.40 wt% in the concentrate, which is equivalent to 76.39 wt% of Nb2O5, it was found that the niobium in the concentrate was amorphous. Figure 10 shows the XRD pattern of the niobium concentrate roasted at 600°C for 4 hours. As seen, the Nb2O5 changed from amorphous to crystalline after roasting. It can also be seen from Table I that the Nb2O5 plus Ta2O5 content can reach up to 83.19wt% in the Nb concentrate. Therefore, this is a high-quality raw material for extracting niobium and tantalum by hydrofluoric acid decomposition and solvent extraction (El-Hussaini, 2001; Gupta and Suri, 1994; Guo and Wang, 2009).
Purification ofleaching solution
Table IV shows that the leaching solution contains aluminum, iron, copper, titanium, niobium, zirconium, and tantalum. To recover aluminum, the impurities should be removed from the solution. The pH of the solution was adjusted to 3.5 by adding 36 g of Al(OH)3 to 1000 mL of leaching solution, which caused Fe, Ti, Zr, Nb, and Ta to precipitate. Subsequently, modified polyacrylamide (PAM) was added to remove Cu and other trace heavy metals before filtration (Li etal., 2010). The composition of the purified solution after filtration is listed in Table IV. The purified solution contained 263.75 g/L AlCl3, which can be used as a purifying agent for waste water (Zhao et al., 2009).
Conclusions
Experiments confirmed that the valuable metals in TiCl4 slurry can be recovered by evaporation and acid leaching. In this process, TiCl4, niobium concentrate, and AlCl3 solution were obtained separately. More than 98% of the titanium was recovered from the slurry by evaporation at 200°C for 60 minutes. The residue was leached with 2.1 mol/L HCl at a L/S ratio of 6:1 at 80°C for 60 minutes, then washed with 2 mol/L aqueous ammonia at a L/S ratio of 4:1 mL/g under stirring at room temperature for 30 minutes. After filtration, a niobium concentrate containing 53.40 wt% Nb and 5.57 wt% Ta was obtained. The leaching solution was purified by adding Al(OH)3 and modified polyacrylamide, and a solution containing 263.75 g/L AlCl3 was produced. Future work will focus on testing the performance of the purified leaching solution during wastewater treatment.
Acknowledgements
The authors would like to thank the National Natural Science Foundation of China (No. 51604055 and No. 51674057) and the Chongqing Research Program of Basic Research and Frontier Technology (cstc2016jcyjA0645) for their financial support of this work.
References
Akhtar, M.K., Xiong, Y., and Pratsinis, S.E. 1991. Vapor synthesis of titania powder by titanium tetrachloride oxidation. Journal of Aerosol Science, vol. 22, suppl. 1. pp. S35-S38. [ Links ]
Anderson, R.J. 1917. The metallurgy of titanium. Journal of the Franklin Institute, vol. 184, no. 4. pp. 469-508. [ Links ]
Dean, J.A. 1985. Lange's Chemistry Handbook. 13th edn. McGraw-Hill, New York. [ Links ]
Den, G.Z. 2010. The Metallurgy of Titanium. Metallurgical Industry Press, Beijing [in Chinese]. [ Links ]
El-Hussaini, O.M. 2001. Extraction of niobium and tantalum from nitrate and sulfate media by using MIBK. Mineral Processing and Extractive Metallurgy Review, vol. 22. pp. 633-650. [ Links ]
El-Hussaini, O.M., and Mahdy, M.A. 2002. Sulfuric acid leaching of Kab Amiri niobium-tantalum bearing minerals, Central Eastern Desert, Egypt. Hydrometallurgy, vol. 64. pp. 219-229. [ Links ]
Frank, P. 1978. Metal extraction process. US patent, 4100252. [ Links ]
Gíbalo, I.M. 1970. Analytical Chemistry of Niobium and Tantalum. Ann Arbor Humphery Science Publishers, Ann Arbor, Michigan. [ Links ]
Guo, Q.W. and Wang Z.X. The Metallurgy of Niobium and Tantalum. Metallurgical Industry Press, Beijing [in Chinese]. [ Links ]
Gupta, C.K. and Suri, A.K. Extractive Metallurgy of Niobium. CRC Press, London. [ Links ]
He, C., Liu, Z., and Zhang, H. 1998 Treatment of fluorine-containing waste gas from hydrometallurgy of tantalum and niobium ore. Nonferrous Metal, vol. 4. pp. 141-142 [in Chinese]. [ Links ]
Li, F.T., Jiang, J.Q., Wu, S.J., and Zhang, B.R. 2010. Preparation and performance of a high purity poly-aluminum chloride. Chemical Engineering Journal, vol. 156. pp. 64-69. [ Links ]
Lide, D.R. 1991. Handbook of Chemistry and Physics. CRC Press, Boca Raton, Florida. [ Links ]
Lima, B.B. and Conte, R.A. 2003. Analysis of nickel-niobium alloys by inductively coupled plasma optical emission spectrometry. Talanta, vol. 59. pp. 89-93. [ Links ]
Lu, Y.H. and Makishima, A. 2007. Coprecipitation of Ti, Mo, Sn and Sb with fluorides and application to determination of B, Ti, Zr, Nb, Mo, Sn, Sb, Hf and Ta by ICP- MS. Chemical Geology, vol. 236. pp. 13-26. [ Links ]
Miller, G.L. 1959a . Metallurgy of the Rarer Metals-6, Tantalum and Niobium. Butterworths Scientific Publications, London. Chapter 4. [ Links ]
Miller, G.L. 1959b. Tantalum and Niobium. Butterworths Scientific Publications, London. [ Links ]
Rodrigues, L.A. and Silva, M.L.C.P. 2010. Thermodynamic and kinetic investigations of phosphate adsorption onto hydrous niobium oxide prepared by homogeneous solution method. Desalination, vol. 263. pp. 29-35. [ Links ]
Roy, P.K., Bhatt, a., and Rajagopal, C. 2003. Quantitative risk assessment for accidental release of titanium tetrachloride in a titanium sponge production plant. Journal of Hazardous Materials A, vol. 102. pp. 167-186. [ Links ]
Wang, W.H., Zheng, S.L., Xu, H.B., and Zhang, Y. 2009a. Leaching of niobium and tantalum from a low-grade ore using a KOH roast-water leach system, Hydrometallurgy, vol. 98. pp. 219-223. [ Links ]
Wang, X.W., Zhang, L.P., Shang, G.H., Zhang, G.Q., Yuan, J.W., and Gong, S.C. 2009b. Processing copper-vanadium precipitate formed from crude TiCl4 in titania and titanium sponge production. Hydrometallurgy, vol. 99, no. 3-4. pp. 259-262. [ Links ]
Wang, X.W., Tian, J.Q., Wang, M.Y., Guo, R.L., Peng, J., and Luo, L. 2010a. The method used to dry the slurry formed in raw titanium tetrachloride. Chinese patent, CN102092783A. [ Links ]
Wang, N., Gu, H.N., Jiang, Y., Fu, Y.H., and Tian, Y.J. 2010b. Study on niobium-tantalum resources in waste from the process of refining titanium tetrachloride by copper wires. Science Technology and Engineering, vol. 10, no. 31. pp. 7728-7730 [in Chinese]. [ Links ]
Wang, X.W., Xiang, X.Y., and Wang, M.Y. 2012. The method used to separate and recover titanium and niobium from the slurry formed in raw titanium tetrachloride. Chinese patent 201210259878.8. [ Links ]
Wang, X.W., Wang M.Y., Xiang X.Y., Gong S C., and Zhao, Y.R. 2012. Recovery of TiCl4 from precipitation slime by spray drying. Titanium Industry Progress, vol. 29, no. 5. pp. 36-38. [ Links ]
Yang, X.J. and Pin, C. 2002. Determination of niobium, tantalum, zirconium and hafnium in geological materials by extraction chromatography and inductively coupled plasma mass spectrometry. Analytica Chimica Acta, vol. 458. pp. 375-386. [ Links ]
Zhao, H.Z., Liu, C., Xu, Y., and Ni, J.R. 2009. High-concentration poly-aluminum chloride: Preparation and effects of the Al concentration on the distribution and transformation of Al species. Chemical Engineering Journal, vol. 155. pp. 528-533. [ Links ]
Zhou, H.M., Zheng, S.L., and Zhang, Y. 2005. Leaching of a low-grade niobium-tantalum ore by highly concentrated caustic potash solution. Hydrometallurgy, vol. 80. pp. 83-89. [ Links ]
Zhou, H.M., Zheng, S L., Zhang, Y., and Yi, D.Q. 2005. A kinetic study of the leaching of a low-grade niobium-tantalum ore by concentrated KOH solution. Hydrometallurgy, vol. 80. pp. 170-178. [ Links ]
Zhu, Z.W. and Cheng, C.Y. 2011. Solvent extraction technology for the separation andpurification of niobium and tantalum: A review. Hydrometallurgy, vol. 107.pp. 1-12. [ Links ] ♦
 Correspondence:
Correspondence:
X. Xiang
Email: xxycsu@163.com
Received: 5 Apr. 2018
Revised12 Jul. 2018
Accepted: 29 Oct. 2018
Published: April 2019
ORCiD ID: X. Xiang. https://orchid.org/0000-0002-7080-7980














