Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.118 n.11 Johannesburg Nov. 2018
http://dx.doi.org/10.17159/2411-9717/2018//v118n11a9
COPPER COBALT AFRICA
Techno-economic evaluation of BASF's new high-temperature solvent extraction reagent
J.J. TauteI; S.J. ArcherII
IBASF Corporation, Tucson, Arizona
IISENET (Pty) Ltd, Johannesburg, South Africa
SYNOPSIS
BASF Mining Solutions has developed a new-generation copper solvent extraction reagent that offers significant benefits over standard extractants currently available to the industry. As the name suggests, the new high-temperature (HT) extractant can be used at a higher operating temperature, but also exhibits significantly lower degradation rates, greater copper-to-iron selectivity, and is totally nitration-proof compared with the standard extractants. Pilot-plant trials have also shown that lower aqueous-inorganic entrainment can be achieved. This paper investigates the techno-economic benefit of using the HT extractant as an alternative to the standard extractant in a high-temperature application. The results of the technical evaluation showed that the use of the HT extractant reduces the extractant addition requirements and bleed volume from copper electrowinning; this smaller bleed requirement further reduces acid and limestone consumption, leading to a reduction in valuable metal losses. The results of the economic evaluation showed that the use of the HT extractant represents significant cost savings over that of the standard extractant.
Keywords: copper solvent extraction, reagents, high temperature, selectivity, entrainment
Introduction
Current solvent extraction (SX) reagents employed for the extraction of copper from typical pregnant leach solutions (PLS) at ambient conditions function well with minimal long-term effects on the extractant's performance (Taute et al., 2015). This performance, however, deteriorates at elevated temperatures due to the degradation of the reagent's hydroxyoximes. The degradation mechanism, acid-catalysed hydrolysis of the oxime, is accelerated by high temperature. This has a knock-on effect where the increase in degradation products increases the organic viscosity, leading to increased entrainment, crud formation, and ultimately to an increase in SX reagent consumption (Bender, Emmerich, and Nisbett, 2013).
SX reagents also show an increase in the co-extraction of iron as the ferric concentration in the PLS increases. Co-extracted iron is transferred to electrowinning (EW) during stripping of the loaded organic and can adversely affect EW current efficiency. Iron in the EW circuit is typically managed by bleeding the electrolyte; however, this can lead to excessive consumption of EW reagents such as acid, cobalt, and mist-eliminating surfactants. The electrolyte bleed can also affect upstream consumption of reagents, such as lime, due to the high acid concentration recycled to leach.
High PLS temperatures and iron concentrations are typically encountered when processing sulphide ores. Numerous technologies have been developed for the leaching of primary sulphide ores (Dreisinger, 2016). Table I is an adapted list from Dreisinger (2016). It is evident that while there are as many conditions as there are processes, the high temperature of the resulting PLS is ever-present.
BASF has developed a new generation of SX reagents that have a much greater stability than currently available reagents at higher temperatures. During extensive laboratory investigations and various customer pilotplant trials, the new high-temperature (HT) extractant exhibited higher copper-to-iron selectivity andwas nitration-proof. As the HT extractant is still in the research and development phase, a full-scale plant trial will be conducted in 2018. To evaluate the difference in operating costs when using a standard SX reagent compared with BASF's new HT extractant, a desktop study was conducted and is presented in this paper.
Technical evaluation: benefits and properties of BASF'S new HT extractant
BASF's new-generation HT extractant has various properties that exhibit significant advantages over the existing range of hydroxyoximes extractants (standard reagents/extractants) available to the market:
► Increased resistance to degradation
► Nitration-proof
► High copper-to-iron selectivity
► Comparable kinetics
► Lower entrainment, thereby reducing impurity transfer in copper SX circuits.
Increased resistance to degradation
Accelerated degradation testing was performed by batchwise contacting of the organic solution containing the extractant with a synthetic strip electrolyte. For comparison purposes, the stripping conditions used were more aggressive than these typically employed. Thus, they are not representative of the actual degradation in SX circuits.
Figure 1 shows the results of tests that were individually performed to illustrate the degradation rate of non-modified, modified, and HT extractants. As expected, the aldoximes were more susceptible to degradation, followed by the ketoxime. All the extractants, except for the HT extractants, exhibited similar degradation behaviour, with an initial high loss of extractant followed by a decrease in loss over time. The shape of these extractant curves suggests that as the concentration of degradation products increases with time, these products start to interact with the oximes and thus reduce the degradation rate.
It is evident from Figure 1 that the degradation rates of the HT extractants (RGT-1 and RGT-2) are substantially lower than those of the standard extractants. The RGT-2 extractant even stopped degrading once an initial loss of 7% was reached after 100 days. The RGT-1 extractant degradation also appeared to have stopped after a loss of between 20% and 25%.
High temperatures not only increase the rate of degradation of standard extractants but also increase the organic viscosity, due to the increase in concentration of the degradation products. This increase in viscosity would lead to an increase in entrainment, crud formation, and ultimately an increase in SX reagent consumption (Bender et al., 2013).
Nitration-proof
The degradation by nitration of the oxime molecule of standard SX extractants is given by Equations [1] and [2]. The nitronium molecule, a highly reactive species, will force electrophilic substitution (a), followed by loss of a proton (b), and results in the nitrated species (c). Catalytic formation of the nitronium ion:
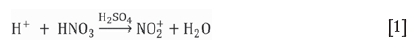
The nitration mechanism of oximes is expressed as follows:
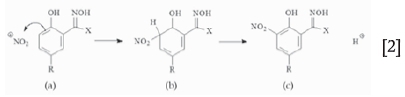
Nitration results in various degradation products:
► Nitrated oximes
► Nitrated nonylphenol
► Nitrated aldehydes and/or ketones.
Nitrated organic species also cause higher organic viscosities, which can adversely affect organic behaviour, leading to poor phase separation and higher entrainment losses. Nitrated oximes also extract copper although the loaded copper cannot be stripped. To counter this destructive mechanism nonylphenol, which is preferentially nitrated, is added to the organic, thereby protecting the oxime. All current aromatic hydroxyoximes can undergo this form of attack, with the nitration rate depending on the substituent groups (Virnig et al., 2003).
The HT extractants do not have a chemically favourable site for nitration, therefore, the nitronium ion cannot nitrate the new-generation reagents, making them completely nitration-proof (Bender Emmerich, and Nisbett, 2014).
High copper-to-iron selectivity
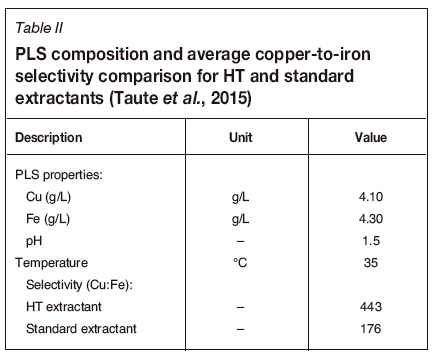
Extractant selectivity for copper over iron is very important in circuits with high PLS iron concentrations, for reasons already mentioned. Blended and unblended HT extractants have a high copper-to-iron selectivity compared with standard non-modified aldoxime/ketoxime blends, as shown in Figure 2 (pilot-plant trial).
Table II provides the PLS composition and average copper-to-iron selectivity achieved over the trial period shown in Figure 2. The copper-to-iron selectivity was two to three times higher for the HT extractant than it was for the standard, non-modified aldoxime-ketoxime blend.
Comparable kinetics
Extraction and stripping kinetics at 25°C were investigated for various blends of HT extractants (RGT-1 and RGT-2) and compared with the standard extractant LIX® 984N (see Figure 3). The BASF quality-control test for LIX® reagents (BASF, 2018) was used to generate the kinetic data. Figure 3 shows that the extraction kinetics for the HT blended extractants were comparable with LIX® 984N because they too have relatively high copper extractive strength (Bender et al., 2013).
Lower entrainment
Aqueous-in-organic entrainments for both the HT extractant and a standard modified aldoxime reagent were determined during a week-long pilot-plant campaign. Entrainment was determined by measuring the manganese concentration in the spent wash liquor and is given in Figure 4. Manganese is not extracted and can only transfer via aqueous entrainment of PLS to the loaded organic. In the wash stage, the loaded organic is contacted with a wash liquor and most of the entrained aqueous, which contains manganese, reports to the spent wash liquor. Entrainment of manganese in the HT extractant was approximately 20% to 30% less than in the standard modified aldoxime reagent (Bender et al., 2014).
Impurity transfer in copper SX circuits
The transfer of impurities such as iron, chloride, nitrate, and manganese from the PLS to the electrolyte occurs by means of two mechanisms: physical and chemical transfer. Physical transfer occurs in the following ways:
► Aqueous entrainment of the PLS in the loaded organic
► Micro-emulsions (tend to form when the organic phase contains surfactants and modifiers along with the copper extractant)
► Crud (which contains leach solution) moving with E1 loaded organic to the strip circuit
► Misdirected flow of the E1 aqueous into the E1 organic weir.
In many cases, the physical transfer of impurities into the EW circuit via the entrainment of E1 aqueous in loaded organic is far greater than the chemical transfer.
In terms of chemical transfer, there is no clear evidence that oxime-based copper extractants transfer chloride, nitrate, or manganese by chemical loading; however, this is the case for Fe(III). Unless the organic has been contaminated with a cationic functionality (such as an amine), transfer of chloride by chemical loading is very low. Although Fe(III) is co-extracted, it occurs to a lesser extent because the oxime-based extractants have a high selectivity for copper over iron. Kinetic experiments have shown rapid iron extraction and that freshly loaded iron strips faster and more efficiently than loaded iron that has been through the stripping circuit numerous times. This phenomenon is termed 'iron-staining'.
It is well known that copper will crowd iron from the typical oxime copper extractants; however, the rate of crowding is slower than the rate of iron loading. This necessitates a longer E1 mixer residence time for more efficient crowding. In general, the chemical loading of iron from copper leach solutions by oxime copper extractants is governed by the following:
► The pH of the leach liquor: higher pH favours iron extraction
► Copper loading on the organic: a high copper loading will crowd iron from the organic
► Addition of a modifier, particularly with an aldoxime: esters and BASF's low-viscosity modifiers reduce iron loading
► Impurities in the organic phase: certain species that are soluble in the organic phase (sulfonates or organic acids), may enhance iron loading. Clay treatment removes some of these species and, therefore, enhances copper-to-iron selectivity (Dudley et al., 2006).
In some copper SX plants there will be excessive transfer of impurities to the electrolyte, even if the plant is well designed and operated, and even when using an organic having good physical properties. Typical examples are leach solutions derived from:
► Leaching copper oxide ores with seawater or saline water
► Leaching ores with a high manganese content
► Leaching certain copper oxide ores in northern Chile where the soil has a high nitrate content
► Bioleaching of copper sulphide ores that have a high pyrite content
► Leaching of primary sulphides or roaster calcines containing high iron levels
► Plants where certain organic contaminants are present in the process water used in the plant.
In the abovementioned cases, the best solution is to install a scrub or wash stage for processing the loaded organic. The scrub or wash stage will:
► Remove entrained iron, nitrate, chloride, or manganese by a displacement wash using a dilute acid solution
► Reduce chemical iron transfer by approximately 50% to 60%. If the pH of the wash aqueous is lower than that of the E1 aqueous and the copper content is higher than the E1 aqueous, both Fe stripping by acid and crowding by copper will occur.
Economic evaluation - roast/leach SX/EW plant case study
The new HT extractant is currently not available commercially, therefore it was decided to conduct a desktop case study to compare its operating costs with those of a standard non-modified extractant.
Process description and flow sheet
The desktop case study used data and information gathered from a recent copper-cobalt project in the Democratic Republic of Congo. The project processes a sulphide ore, which is concentrated by flotation and then roasted in a sulphating roast before being leached. SX is employed to selectively extract the copper from the resulting PLS, which is then stripped and electrowon to produce London Metal Exchange (LME) Grade A copper, as shown in Figure 5.
Case study methodology
The comparison of the HT extractant with the standard, non-modified extractant required a process design basis to be compiled for the circuit described in Figure 5. It should be noted that this comparison considered only the differentials; areas such as comminution, flotation, and roasting were excluded. Capital cost (CAPEX) differentials were assumed to be negligible as the same unit operations were employed for both reagents, with a few unit operations differing only marginally in size. A more detailed flow sheet (see Figure 6) was used for this comparison, highlighting the operating cost (OPEX) differentials that could be expected, such as:
► Reducing PLS and electrolyte heat-transfer requirements
► Minimizing organic reagent top-up
► Reducing the electrolyte bleed requirement, thereby reducing the neutralization in leach as well as reducing valuable metal losses.
Table III provides a summary of the key design parameters used to compile the mass balance for both the HT and standard extractant. The following assumptions were used as the basis for the comparative evaluation.
► The excess acid in the leach circuit that is not neutralized by the oxide float concentrate is further neutralized with limestone. Calcined material has no neutralizing potential due to the upfront sulphating roast.
► The PLS has a ferric-to-ferrous ratio of 1.
► The copper-to-iron selectivity ratio for the HT extractant is 2.5 times higher than that of the standard non-modified reagent.
► Aqueous-in-organic entrainment for the HT extractant is 20% less than for the standard non-modified reagent.
► Organic-in-aqueous entrainment for both reagents is 50 mg/L with no reagent recovery from the raffinate ponds.
► A high wash-stage advance organic:aqueous (O:A) ratio was used to ensure a minimal effect on the overall water balance.
► The reagent degradation rate for the HT extractant is 2.7 times lower than for the standard non-modified reagent.
► A total of 3% of the copper and cobalt recycled to leach via the electrolyte bleed is lost during solid-liquid separation.
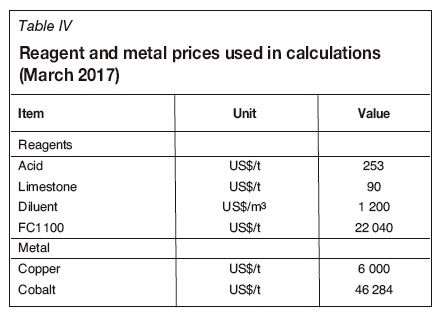
The provisional costs used for this case study assumed that the HT extractant is 40% more expensive than the standard non-modified reagent. The major reagents and metal prices used for the evaluation are provided in Table IV. Reagent prices include delivery to site. It should be noted that the copper and cobalt prices have increased significantly from those used for this evaluation.
Calculations and cost analysis
The SX circuit was modelled using BASF's Isocalc® software and the mass balance was calculated using SysCAD. The electrolyte bleed for each reagent was determined by maintaining the spent electrolyte's iron concentration at 1.5 g/L since the bleed is affected by both the chemical and physical transfer of iron from the washed organic into the electrolyte. Table V illustrates the calculated chemical and physical iron transfer to electrolyte based on the properties of the SX reagents used. The total iron transfer for the HT extractant was 35% less than for the standard extractant. This relates to a 35% lower electrolyte bleed rate, as shown in Table VI.
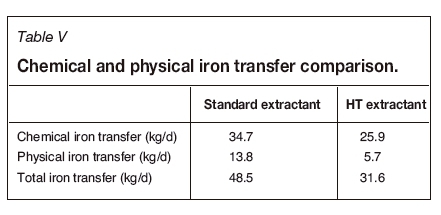
Table VI shows that the electrolyte bleed flow rate for the standard extractant is greater than that for the HT extractant, resulting in higher reagent consumptions and metal losses, and consequently greater annual bleed costs. The organic costs were calculated based on consumption resulting from degradation, entrainment to raffinate, and diluent make-up. The net annual cost resulting from bleeding electrolyte and topping up organic is significantly lower for the HT extractant; however, the once-off first-fill cost (extractant and diluent cost on start-up) for the HT extractant is higher than the standard non-modified extractant.
Discussion
To illustrate the long-term OPEX savings when using the HT extractant instead of the standard extractant, a five-year reagent cost calculation was done. Table VII shows the comparative cost and differential cost (saving) of the bleed, organic, and overall cost, including the first-fill cost over a five-year period. The total OPEX saving over five years is just over US$ 1.2 million.
Various sensitivity analyses have been conducted on the calculations to determine the impact of the various reagent costs on the total saving. The saving of US$329 082 (calculated in Table VI) is roughly 14% per annum. The following prices and factors will have the largest impact on the overall savings:
► HT extractant price
► Acid price
► Copper-to-iron selectivity
► Aqueous-in-organic entrainment (physical iron transfer)
► Cobalt price (except in the DRC).
Should the price difference between the HT extractant and the standard extractant be lower than the estimated 40%, then the annual saving would increase proportionally (3% annual saving for every 10% decrease in HT extractant price). Similarly, if the acid price increases, the saving on using the HT extractant would be higher: roughly 1% more for every US$50 increase in acid price. A change in the copper-to-iron selectivity could have a positive or negative impact on the annual saving. An increased copper-to-iron selectivity ratio would mean less iron is chemically transferred, thus reducing the electrolyte bleed rate and increasing the annual saving. The opposite would be true if the copper-to-iron selectivity ratio is lower. Should the average aqueous-in-organic entrainment increase, physical transfer of iron will increase, resulting in a higher electrolyte bleed rate. This will reduce the annual saving by roughly 3% for every 100 mg/L of additional aqueous entrained.
Conclusions
The HT extractant offers a viable alternative to the standard oxime-based extractants, for the following reasons.
► The HT extractant degradation rate is significantly lower than that of the standard oxime extractant. A lower degradation rate means a lower extractant consumption rate.
► The HT extractant cannot be nitrated, which would be a substantial benefit for certain plants that operate with high nitrates in the PLS. This would also result in a significant drop in extractant consumption.
► The HT extractant offers a step-change improvement in copper-to-iron selectivity, which results in less chemical transfer of iron to electrowinning.
► Pilot-plant trials showed that aqueous-in-organic entrainment is significantly lower when using the HT extractant, which results in less physical transfer of iron to electrowinning.
► The case study showed that the electrolyte bleed rate can be reduced by 35% when using the HT extractant, which results in an OPEX saving of approximately 14% (> US$300 000 per annum). This saving would result in first-fill extractant cost payback of less than 18 months on the higher priced HT extractant. The case study also showed that a loaded organic wash stage is necessary to minimize physical and chemical iron transfer, even when using the HT extractant. This would require additional CAPEX, but the payback period is less than 12 months.
It should be noted that the HT extractant is still in the research and development phase. A commercial plant trial is scheduled for Q3 2018.
Acknowledgements
The authors would like to acknowledge and thank Jaco Scheepers for the SysCAD mass balances done as part of this work.
References
BASF. 2018. Quality control test of LIX® reagents (TI-D/EVH 017 e). Technical Information Global Mining Solutions. http://www.basf.com/miningsolutions [ Links ]
Bender, J., Emmerich, N., and Nisbett, A. 2013. Development of a new generation of copper solvent extraction reagents. Proceedings of Copper 2013: Vol. IV. Hydrometallurgy. Ugarte, G. (ed.). Institute de Ingenieros de Minas de Chile, Santiago. pp. 381-391. [ Links ]
Bender, J., Emmerich, N., and Nisbett, A. 2014. Evaluation of the next generation of copper solvent extraction reagents. Proceedings of ALTA 2104 Nickel-Copper-Cobalt Conference. ALTA Metallurgical Services, Melbourne. pp. 292-304. [ Links ]
Dreisinger, D. 2016. Hydrometallurgical treatment of high grade copper ores and concentrates. Proceedings of Copper 2016: Vol. 5 (3) Hydrometallurgy. Mining and Materials Processing Institute of Japan (MMIJ) and Japan Mining Industry Association (JMIA), Kobe. pp. 1655-1666. [ Links ]
Dudley, K., Virnig, M., Crane, P., and Hein, H. 2006. Clay treatment for copper solvent extraction circuits. Proceedings of ALTA 2006 Copper Conference. ALTA Metallurgical Services, Melbourne. [ Links ]
Taute, J.J., Bwando, P., Chisakuta, G., Mitshabu, G., and Nisbett, A. 2015. Improved copper/iron selectivity in solvent extraction. Proceedings of Copper Cobalt Africa, incorporating the 8th Southern African Base Metals Conference. Southern African Institute of Mining and Metallurgy, Johannesburg. pp. 281-290. [ Links ]
Virnig, M.J., Eyzaguirre, D., Jo, M., and Calderon, J. 2003. Effects of nitrate on copper SX circuits: a case study. Proceedings of Copper2003: Vol VI (2) Hydrometallurgy of Copper. Canadian Institute of Mining, Metallurgy and Petroleum, Montreal. pp. 795-810. [ Links ] ♦














