Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Journal of the Southern African Institute of Mining and Metallurgy
versión On-line ISSN 2411-9717
versión impresa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.118 no.10 Johannesburg oct. 2018
http://dx.doi.org/10.17159/2411-9717/2018/v118n10a11
The effect of nC12-trithiocarbonate on pyrrhotite hydrophobicity and PGE flotation
C.F. VosI; J.C. DavidtzII; J.D. MillerIII
IImpala Platinum Limited, Concentrator Technical Department, South Africa
IIDepartment of Materials Science and Metallurgicäl Engineering, University of Pretoria, South Africa
IIIDepartment of Metallurgical Engineering, University of Utah, USA
SYNOPSIS
This work presents the potential for improving the flotation recovery of slow-floating sulphide minerals with the use of starvation dosages of a normal dodecyl (n-C12) trithiocarbonate (TTC) co-collector, together with a sodium isobutyl xanthate (SiBX) and dithiophosphate (DTP) collector mixture.
At potentials below -150 mV (SHE), addition of nC12-TTC with SiBX improves the hydrophobicity of pyrrhotite, yielding captive bubble contact angles greater than those measured for SiBX or nC12-TTC alone, suggesting a low potential synergistic effect. This synergistic effect is further studied using Fourier transform infrared (FTIR) spectroscopy, the results indicating an increase in the surface concentration of the collector species when in a mixture. Thus, nC12-TTC with SiBX may act as an immobile surface anchor to which SiBX/SiBX2 molecules bond, increasing the localized concentration of collector species.
Bench-scale flotation tests using mixtures of SiBX/DTP/nC12-TTC on a platinum group element (PGE)-bearing ore from the Bushveld Complex in South Africa confirm an improved metallurgical performance at very low substitutions (approx. 5 molar per cent) of SiBX. The improved recoveries for PGE, Cu, and Ni are correlated with improvements in the flotation kinetics of their slow-floating components.
Keywords: sulphide flotation, hydrophobicity, collector, nC12-trithiocarbonate, synergistic effect.
Introduction
Short-chain (less than C6) xanthates are very effective for the bulk flotation of sulphides (Fuerstenau, 1982), and early work (Leja, 1968; Gaudin, 1957; Plaksin and Bessonov, 1957; Taggart, Giudice, and Ziehl, 1934) indicated that the presence of oxygen in flotation slurries is necessary for the oxidation of the xanthate collector at the sulphide mineral surface to induce hydrophobicity. Fundamental research into the chemistry and adsorption mechanisms of xanthates over the years (Finkelstein and Poling, 1977; Woods, 1976; Winter and Woods, 1973) has revealed that for iron-bearing sulphide minerals, adsorption occurs predominantly through a charge transfer process. Oxidation of the adsorbed collector to the corresponding dimer takes place if the mixed potential of the system is greater than the reversible potential for dimer formation. In oxygenated slurries the
rate of adsorption and oxidation of the collector depends greatly on the substrate surfaces. For chalcopyrite (Guler et al., 2005; Leppinen, 1990; Roos, Celis, and Sudrassono, 1990) and pentlandite (Hodgson and Agar, 1989) a two-step interaction with xanthate is proposed in which the chemisorbed xanthate is further oxidized to the dimer. The initial interaction of xanthate with pyrrhotite is through physisorption where xanthate physisorbs onto positive surface sites followed by oxidation to dixanthogen (Khan and Kellebek, 2004; Bozkurt, Xu, and Finch., 1998). This process is, however, slow and increased reaction time is needed to improve flotation recovery (Buswell and Nicol, 2002).
Research into short-chain (less than C6) trithiocarbonate (TTC) molecules as sulphide collectors has been undertaken since the 1980s, and has shown that the short-chain molecules are superior compared to xanthates, dithiophosphates, or their mixtures (Steyn, 1996; Coetzer and Davidtz, 1989; Slabbert, 1985). This is brought about by the third sulphur atom replacing the oxygen atom in the xanthate molecule. It has been suggested that this reduces the interaction between the adsorbed TTC and the surrounding bulk water (Davidtz, 1999), improving sulphide flotation metallurgy (Davidtz, 1999; Steyn, 1996; Coetzer and Davidtz, 1989; Slabbert, 1985).
The flotation chemistry of short-chain TTC collectors was studied (du Plessis, Miller, and Davidtz, 2003, 2000) to understand the underlying flotation mechanisms brought about by the third sulphur atom and it was shown that, in the presence of oxygen, a hydrophobic sulphide mineral surface can be established well below the reversible potential for TTC dimer formation. This was the first evidence that a hydrophobic surface in the presence of TTC molecules is less sensitive to the formation of the corresponding dimer. It was postulated that one or more of the TTC decomposition or hydrolysis products, potentially the mercaptan, may be adsorbing under reduced conditions, rendering the mineral hydrophobic (du Plessis, 2003). Research on the interaction of the TTC with copper and pyrite electrodes (Venter and Vermaak, 2008a; Venter, 2007) confirmed the difference between the adsorption mechanisms of xanthate and the TTC and found that the TTC interacts with the sulphide surface independently of the surface potential, confirming du Plessis' unexpected findings. Two adsorption mechanisms were proposed, one under cathodic potentials during which the mineral acts as catalyst for TCC decomposition into its corresponding thiol or thiolate, and a second that takes place under anodic potentials during which TTC chemisorbs via a charge transfer process. Under anodic potentials, as with xanthate, the chemisorbed TTC is oxidized to the dimer (Venter and Vermaak, 2008a; du Plessis, 2003).
Although the mechanisms of short-chain TTCs are understood, the longer chain molecules (nC12) have also shown significant benefits when used in low-dosage applications (Breytenbach, Vermaak, and Davidtz, 2003, Vos Davidtz, and Miller, 2007) in combination with xanthate and dithiophosphate. The synergistic effects of this new mixed collector system have not been studied extensively to date. This paper reports on findings involving mixed SiBX/nC12-TTC contact angle measurements and adsorption studies on pyrrhotite, a major sulphide mineral component of the Merensky Reef ores, followed by bench-scale flotation tests on Merensky Reef ores to evaluate the nC12-TTC as a co-collector with a traditional SiBX/DTP mixture.
Experimental
The processing of the Merensky PGE-bearing ore used in this study takes place at the buffering pH of the ore, which is alkaline at approximately pH 9.0-9.5. In other studies (Vos, 2006) it was demonstrated that pyrrhotite hydrophobicity improved at more acid conditions. However, the overall
purpose here was to determine if its surface hydrophobicity could be improved at the natural or buffer pH of the Merensky ore used in the study, therefore for the batch tests and small-scale tests that follow, no pH modifications were done or reported for this paper.
Contact angle measurements and FTIR spectroscopy
Captive bubble contact angle measurements were performed on a polished pyrrhotite crystal (> 95 mass% purity) sourced from the Geology Curator at the University of Utah, USA. Prior to every measurement the mineral electrode was polished using a 1 m corundum suspension. The surface oxidation was minimized by transferring the polished mineral directly into the test solution. The electrode was then conditioned at the desired potential for one minute prior to collector addition (SiBX, nC12-TTC, or a mixture). The electrode was then conditioned in the collector solution for ten minutes at the desired potential. Whenever a mixture was used the electrode was conditioned for five minutes with nC12-TTC prior to SiBX addition, followed by a further five minutes of conditioning. An initial xanthate concentration of 10-3 mol/L was employed and the nC12-TTC additions were made according to Figure 2 and Figure 3. The solution pH was buffered at 9.2 using a 0.05 mol/L sodium borate solution.
The C-H stretching spectra were collected using a Biorad-Digilab FTS-6000 FTIR spectrometer with a liquid-nitrogen-cooled detector having a wide-band MCT. The FTIR chamber was flushed with dry air before any spectra were taken. All absorbance spectra are the result of 512 co-added scans ratioed against 512 co-added background scans, all at a resolution of 4 cm-1. Before placing the pyrrhotite crystal into the spectrometer the mineral was contacted with SiBX, nC12-TTC, or a mixture of the two. The same contact times given above were applied and care was taken not to contaminate the surface during handling.
Bench-scale flotation tests
A bulk sample (about 600 kg of 25 mm top size) of a PGE-bearing ore from a South African producer was used in this investigation. The bulk sample was crushed to -2.36 mm (moving from point A to B in Figure 1), homogenized, and split into smaller 3.3 kg sub-samples (moving from point B to C in Figure 1) which were used for the milling and flotation experiments. Because the bulk sample and sub-samples are located to the left of Gy's Safety Line their integrity has not been compromised during processing.
From a milling curve the required time to achieve a grind of 60% passing 75 u.m was established and used throughout the flotation experiments. The samples were milled at approximately 45% solids (w/w) in a stainless steel rod mill with rods of various sizes.
After milling, the slurry was transferred into an 8 L Denver float cell and topped up with potable water from the mine site in Rustenburg, South Africa to produce a slurry of approximately 32% solids (w/w). Reagent dosages and conditioning times are noted in Table I.
Collector ratio (1) and the remainder of the chemicals (activator, collector spike, depressant, and frother dosages) were selected so as to represent the applied dosages on the Impala Platinum Merensky flotation circuit at that time. The dosages in collector ratio (2) were selected to align with those tested during the fundamental contact-angle measurements and FTIR spectroscopy studies.
After conditioning, the air flow to the cell was initiated and concentrates were collected at 1, 6, 16, and 30 minutes by scraping the froth every 15 seconds. A constant froth depth of approximately 2 cm was employed throughout each test. The concentrates and tailings samples were dried, weighed, and assayed for 4E-PGE (sum of Pt, Pd, Rh, and Au grades), copper, and nickel content.
Results and discussion
Contact angle measurements
The use of copper and lead activating ions had failed to improve the measured contact angle on the surface of this pyrrhotite crystal at pH 9.2 (Vos, 2006). Higher xanthate concentrations (10-3 M vs. 10-4 M) also failed to increase the measured angle beyond approximately 25-30° for open circuit potentials.
In alkaline systems the activation of pyrrhotite with copper ions is not fully understood, leading to many controversial conclusions. Some researchers (Nicol, 1984) have reported that at pH > 8 pyrrhotite activation is not possible due to the formation of insoluble copper hydroxide species. Others (Kelebek, Wells, and Fekete, 1996; Senior, Trahar, and Guy, 1995; Leppinen, 1990) reported improved pyrrhotite flotation after copper ion addition.
This contradiction was explained (Finkelstein, 1997) as being in part due to the presence of iron (from grinding media, mill liners) in contact with the sulphide minerals. This contact reduces the rest potential of the mineral and increases copper uptake significantly. As copper xanthate species are orders of magnitude less soluble than those of nickel or iron (Rao, 2004a; Chander, 1999) they form preferentially and subsequently stabilize the pyrrhotite surface (Buswell and Nicol, 2002).
The purpose of nC12-TTC addition with SiBX is to establish whether the surface hydrophobicity, as measured by captive bubble contact angles, can be improved in alkaline systems and to what extent this is affected by the electrode potential.
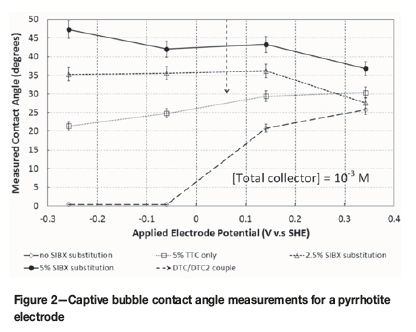
The results of the contact angle measurements, as a function of applied electrode potential and degree of xanthate substitution, are shown in Figure 2.
In Figure 2, TTC refers to solutions containing TTC only but at the indicated SiBX substitution concentrations. A 2.5% SiBX substitution refers to a solution containing SiBX at 97.5% of the initial molar concentration and the rest substituted with TTC. The 5% SiBX substitution tests are the same in that 5% of the initial SiBX is substituted with TTC.
The addition of pure nC12-TTC at 5 x 10-5 m (at 5 molar per cent of the initial xanthate concentration) produces a contact angle very similar to that with 10-3 M SiBX at potentials above 200 mV (vs. SHE). This result can be expected since the longer chain molecule is much more hydrophobic than the shorter chain SiBX. Furthermore, the size of the TTC molecule, compared to SiBX, results in it covering a larger substrate area upon adsorption, which results in a similar surface hydrophobicity but at much lower concentrations.
At lower potentials, a contact angle is measured with the nC12-TTC but none for SiBX only. This is because at lower potentials the TTC is more effective due to its much lower standard redox potential (du Plessis, 2003), implying that it forms the dithiolate much more readily at the mineral surface.
The formation of a hydrophobic pyrrhotite surface below the reversible potential for the DTC/DTC2 couple (indicated in Figure 2) is not possible with only SiBX, which is in agreement with the literature in that dixanthogen is a prerequisite for pyrrhotite hydrophobicity (Hodgson and Agar, 1989).
As the electrode potential is lowered even further (below -150 mV) for nC12-TTC-containing solutions, it is observed that a finite contact angle is maintained. This indicates that the formation of a hydrophobic pyrrhotite surface in the presence of nC12-TTC is much less sensitive to the substrate surface potential. This is in line with previous observations reported for a pyrite (Venter, 2007; du Plessis, 2003) and a pure copper (Venter, 2007) electrode respectively. These earlier works suggested that under reduced potentials the decomposition product (possibly a thiolate) is responsible for the observed hydrophobicity. As the thiolate is a poor collector on its own (Venter and Vermaak, 2008b; Venter, 2007) its adsorption is believed to be catalysed through the TTC ions as an intermediate (Venter and Vermaak, 2008a).
When the same amount of nC12-TTC (5 molar per cent) is used with SiBX, a clear improvement in the surface hydrophobicity is measured for all electrode potentials tested. Although not that significant above 200 mV, the effect is clear at more reducing potentials. This is a significant observation as it provides early evidence of a low-potential synergistic effect between the nC12-TTC and SiBX. The result is an improved surface hydrophobicity for pyrrhotite. Even in the absence of the dimer at the mineral surface the nC12-TTC,
when attached to the pyrrhotite surface, seems to act as an immobile anchor for SiBX and the SiBX dimer at the surface. This can be seen as equivalent to the role of dithio-phosphates in collector mixtures (Bradshaw, 1997) but at reduced potentials.
When related to flotation, the contact angle measurements indicate that there is a potential to improve the recovery of slow-floating, possibly rapidly oxidizing minerals (which are associated with difficulty in the formation of hydrophobic surface states).
FTIR spectroscopy
To further study the synergistic mechanism between nC12-TTC and SiBX, FTIR spectroscopy was completed on a pyrrhotite crystal conditioned in solutions containing various concentrations of SiBX and nC12-TTC. This was done to evaluate the effect of nC12-TTC on the concentration of collector at the pyrrhotite surface, as can be inferred from the intensity of the absorbance peaks at 2925 cm-1 and 2850 cm-1. The effect of nC12-TCC substitutions is shown by the C-H absorbance peaks in Figure 3.
The absorbance peaks located at approximately 2925 cm-1 indicate that more collector is present at the pyrrhotite surface when contacted with 5% and 10% nC12-TTC solutions compared to SiBX only. This is mainly because the nC12-TTC molecule contains significantly more CH2-functional groups in its hydrophobic tails compared to SiBX. The SiBX peak is hardly visible.
When the two collectors are combined (spectra 4 and 5) an increase in the absorbance of the CH2- peaks is observed. The changes in the peak heights for spectra 4 and 5 are greater than the sum of the peak heights for spectra (1 + 2) and (1 + 3) respectively (see Table II). This observation further alludes to the presence of a synergistic effect, resulting in a localized increase in the collector concentration. A similar observation is made for the peaks at approximately 2850-2860 cm-1, which are the C-H symptotic stretching vibrations. For all the measurements, the total collector conditioning time was kept constant.
Bench-scale flotation tests
Based on the fundamental studies with pyrrhotite it seems that a collector mixture of SiBX with nCi2-TTC may offer an improved flotation recovery of sulphide minerals from the PGE ore, which in turn may translate to improved PGE recovery. The purpose of the bench-scale flotation tests was to establish the effect of mixed collector composition on the flotation performance of a PGE-bearing Merensky Reef ore, and to determine the best collector composition for optimum metallurgical performance.
For the bench-scale flotation tests, two levels of SiBX substitution with nC12-TTC were tested, namely 5% and 10% molar substitutions. The concentration of DTP remained constant.
Grade and recovery profiles
For the batch flotation tests, only the collector mixture was varied and as such all other dosages (refer to Table II) remained constant throughout. The standard collector suite consisted of a SiBX/DTP mixture (condition 1 in line 1 of Table I), with the SiBX replacement tests as potential alternatives.
For each condition, triplicate flotation experiments were conducted to determine reproducibility. Timed concentrates were collected after 1, 6, 16, and 30 minutes of flotation. These concentrate samples and final tailings samples were assayed and the results used in calculating the cumulative grade and recovery of the minerals/metals of interest at each time interval. This was done as follows.
► Cumulative elemental recovery after t = i minutes (Rm,t=i):
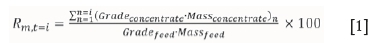
► Cumulative elemental grade after t = iminutes (Gm,t=i):

In the above equations the following are defined:
Gradeconcentrate : the elemental grade of the concentrate at time i
Gradefeed : the elemental grade of the feed
Massconcentrate : the incremental concentrate dry mass at time i
Massfeed : the initial dry mass of the feed
Rmtt=i : cumulative component recovery at time = i minutes
Gm,t=i : cumulative component grade at time = i minutes
Figure 4 shows the effect of SiBX substitutions on the PGE grade-recovery relationship. It is evident that with a very small substitution of SiBX (as low as 5 molar per cent) an improvement in the PGE grade and recovery profile is observed. At a very similar final concentrate grade of 40-41 g/t PGE a recovery improvement of approximately 4.4% is measured. With very small variability between triplicate tests for both conditions, this difference is statistically significant.
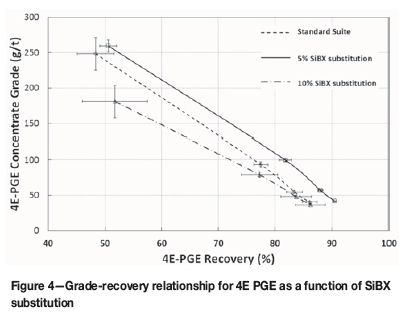
When 10% of the SiBX is replaced with the nC12-TTC no marked difference is observed in the final metallurgical performance. What is observed is a downwards shift in the grade-recovery relationship. The lower initial concentrate grade is attributed to an increase in the rate of recovery of gangue, and may imply overdosing conditions.
Figure 5 shows the grade-recovery relationship for copper (Cu) and nickel (Ni). An improvement in the recovery of Cu-and Ni-bearing sulphide minerals is also observed at 5% replacement. This improvement appears to be true for 10% replacement of SiBX with nC12-TTC as well, and is more evident for Cu than for Ni. Again, improved sulphide flotation with the preferred mix of collectors is evident.
Flotation kinetics
The grade and recovery data from the various flotation tests can be modelled using the two-parameter Kelsall equation.
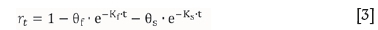
where
rt = cumulative recovery after t minutes
= fast and slow floating mass fractions respectively
Kf and Ks = fast and slow floating first-order flotation rate constants respectively
To evaluate the flotation kinetics under the different chemical conditions it is assumed that the ore in each test has the same mass fractions of fast-, slow-, and non-floating material, i.e. 6f and esare the same for all tests. Only the flotation rate constants are varied to fit the experimental data (grades and recoveries) by minimizing the sum of errors. This is a reasonable assumption since the various subsamples are taken from a homogenized bulk sample.
By only examining the rate constants it is not possible to rapidly assess the impact of the new collector mixture on the flotation kinetics. As the fast-floating fractions for PGE, Cu, and Ni achieve 100% recovery within the first two concentrates, it is reasonable to argue that an increase in their flotation rates will not influence the overall recoveries after half an hour. The only effect it may have is on the initial concentrate grade, if the fast-floating valuable minerals are recovered preferentially to the fast-floating gangue.
A summary of the recovery of the slow-floating fraction after 30 minutes of flotation for all valuable elements is presented in Table III. The predominant influence of the nCi2-TTC as a co-collector is evaluated in more detail by considering the response of the slow-floating fractions of each of the elements considered. Figure 6 presents the flotation-time profile for the slow-floating PGEs as modelled using the parameters determined from Equation [3].
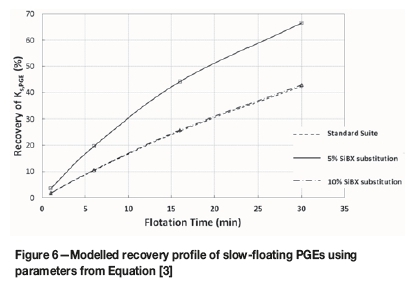
A very significant increase in the recovery of the slow-floating PGE fraction is observed at 5 molar per cent replacement of SiBX. As the feed consists of approximately 23.6% slow-floating PGEs (refer to Table III), an increase of this magnitude results in an overall PGE recovery improvement of approximately 4%.
As with PGEs, both Cu and Ni show a significant improvement in the recovery of the slow-floating component with the addition of nC12-TTC. As the dosage is increased to 10% of the initial SiBX dosage, the recovery of slow-floating Cu continues to increase and does not show the same maximum at a 5% SiBX replacement as found for the PGEs and Ni. This can possibly be explained in terms of the affinity of the collector molecule for the mineral of interest. Cu-xanthate complexes are known to be orders of magnitude less soluble than Ni and Fe complexes (Chander, 1999), and as the hydrocarbon chain length increases the solubility decreases further. In the same way it is expected that the nC12-TTC will have a much higher affinity for the Cu mineral and the recovery will improve as the dosage increases.
The Ni recoveries from the slow-floating fraction show maximum improvement at 5 molar per cent replacement of SiBX, in line with the observations for 4E-PGE. This is not surprising as pentlandite is known as a primary host for all of the PGEs present except for platinum (Godel, Barnes, and Maier, 2007).
Similar outcomes have been reported for Cu, S, Ni, and Fe elsewhere (Breytenbach, Vermaak, and Davidtz, 2003). In that work the optimum nC12-TTC dosage was found to be in the order of 7.5 molar per cent replacement of SiBX, which is in close agreement with our findings of 5 molar per cent. At complete substitution, however (Vos, 2006), selectivity is lost and the recovery of the valuable minerals decreases substantially.
Long-chain xanthates of seven or more carbon atoms in the hydrophobic tail are known to have surfactant properties similar to those of long-chain carboxylic acids. Beyond their critical micelle concentrations (CMC), the long-chain xanthates form micelles (Hamilton and Woods, 1986). At high dosages these molecules can absorb onto non-sulphide minerals such as oxides (silicates), in which case adsorption is through a physical mechanism (Rao, 2004b) and one deals with insoluble collector colloids or emulsions. A similar mechanism for adsorption of nC12-TTC molecules onto non-sulphide gangue at high dosages may be a possibility, and more work in this area is required to clarify this phenomenon.
Summary and conclusions
► Small replacements of SiBX with nC12-TTC improve the surface hydrophobicity of pyrrhotite in alkaline (pH 9.2) conditions, and the effect is more pronounced at reduced potentials (lower oxygen activity). This can have significant implications when viewed in conjunction with PGE and base metal grinding and flotation in a mild steel media environment, or where a valuable mineral is subjected to rapid oxidation.
► The synergistic effect at low concentration is believed to be in part due to a crowding of the collectors at the surface, which increases the localized surface concentration and improves hydrophobicity even at low substitutions of SiBX.
► At the bench scale, low substitutions of SiBX noticeably improve the recovery of PGE, Cu, and Ni. Overall recovery improvements are achieved at similar concentrate grades. Improved grades at the beginning of the flotation tests indicate that slow-floating, liberated minerals are being recovered.
Recommendations for future work
Further mineralogical data would add valuable information to this study. For this Merensky ore, copper is primarily associated with chalcopyrite and nickel with pentlandite, which are the two minerals expected to show recovery improvements in this regard. Overall, PGE recovery improvements as demonstrated may be due to the association of PGEs with these sulphide minerals, or to improvements in the recovery of PGMs. Detailed spare-phase mineralogical studies will be required to answer this question and it is important to include such studies in future work addressing this new chemical. Furthermore, since pyrrhotite flotation is pH-dependent, it will be useful for future experimenters to study the effect of pH using the methodologies in this paper and to determine if the correlations identified here are transferrable.
The application of nC12-TTC with mild steel grinding media to improve the flotation activity of xanthate and DTP is a subject that has not been explored in great detail. From preliminary captive bubble contact angle measurements under controlled potentials, there appears to be a benefit in using this new co-collector along with SiBX and potentially DTP. This is a novel application of the nC12-TTC due to its low sensitivity to oxygen activity and surface oxidation products (du Plessis, 2003).
It is well known that the choice of surfactants (especially collectors and frothers) has a significant effect on flotation metallurgy as the joint actions between them are widely acknowledged (Rao, 2004a, 2004b; Laskowski, 1993; Leja, 1989). The interaction of the nC12-TTC with various frother types, and how it affects bubble capture and flotation kinetics, also needs to be investigated.
It will be of value to further test this novel co-collector on problematic ore types as well, where rapid surface oxidation and poor collector adsorption are causes of substandard metallurgical performance.
Acknowledgements
The authors acknowledge and extend their appreciation to Impala Platinum Ltd, and in particular Johan Theron and Dave Marshall, for their generous involvement in the R&D as well as their academic support for this research. The NSF, Grant Int. - 0352807, for facilitating collaborative flotation research between South Africa and the USA is also acknowledged. Dr Ronel Kappes from Newmont is also acknowledged for very helpful discussions and a review of the initial manuscript.
References
Bozkurt, V., Xu, Z., and Finch, J.A. 1998. Pentlandite/pyrrhotite interaction and xanthate adsorption. International Journal of Mineral Processing,, vol. 52. pp. 203-214. [ Links ]
Bradshaw, D.J. 1997. Synergistic effects between thiol collectors used in the flotation of pyrite. PhD thesis, Faculty of Engineering and Built Environment, University of Cape Town. [ Links ]
Breytenbach, W., Vermaak, M.K.G., and Davidtz, J.C. 2003. Synergistic effects among dithiocarbonate (DTC), dithiophosphate (DTP) and trithiocarbonate (TTC) in the flotation of Merensky ores. Journal of the South African Institute of Mining and Metallurgy, vol. 103, no. 10. pp. 667-670. [ Links ]
Buswell, A.M. and Nicol, M.J. 2002. Some aspects of the electrochemistry of the flotation of pyrrhotite. Journal of Applied Electrochemistry, vol. 32. pp. 1321-1329. [ Links ]
Chander, S. 1999. Fundamentals of sulphide mineral flotation. Advances in Flotation Technology. Parekh, B.K. and Miller, J.D. (eds.). Society for Mining, Metallurgy & Exploration, Littleton, CO. [ Links ]
Coetzer, G. and Davidtz, J.C. 1989. Sulphydryl collectors in bulk and selective flotation. Part 1. Covalent trithiocarbonate derivatives. Journal of the South African Institute of MIning and Metallurgy, vol. 89, no. 10. pp. 307-311. [ Links ]
Davidtz, J.C. 1999. Quantification of the flotation activity by means of excess Gibbs free energies. Minerals Engineering, vol.12, no. 10. pp. 11471-11161. [ Links ]
Dippernaar, A. 1982. The destabilisation of froth by solids. I. The mechanism of film rupture. International Journal of Mineral Processing, vol.9. pp. 1-14. [ Links ]
Du Plessis, R. 2003. The thiocarbonate flotation chemistry of auriferous pyrite. PhD thesis. Department of Metallurgical Engineering, University of Utah, Salt Lake City, UT. [ Links ]
Du Plessis, R., Miller, J.D., and Davidtz, J.C. 2000. Preliminary examination of the electrochemical and spectroscopic features of trithiocarbonate collectors for sulfide mineral flotation. Transactions of Nonferrous Metals Society of China, vol. 10. pp. 12-18. [ Links ]
Du Plessis, R., MIller, J.D., and Davidtz, J.C. 2003. Thiocarbonate collectors in pyrite flotation - fundamentals and applications. Proceedings of the XXII International Mineral Processing Congress, Cape Town, South Africa, 29 September - 3 October 2003. Lorenzen, L. and Bradshaw, D.J. (eds.). Southern African Institute of Mining and Metallurgy, Johannesburg. pp. 892-901. [ Links ]
Finkelstein, N.P. 1997. The activation of sulphide mineral for flotation: a review. International Journal of Mineral Processing, vol. 52, no. 2-3. pp. 81-120. [ Links ]
Finkelstein, N.P. and Poling, G.W. 1977. The role of dithiolates in the flotation of sulphide minerals. Mineral Science and Engineering, vol.9, no. 4. pp. 177-197. [ Links ]
Fuerstenau, M.C. 1982. Sulfide mineral flotation. Principles of Flotation. King, R.P. (ed.). Southern African Institute of Mining and Metallurgy, Johannesburg. pp. 159-182. [ Links ]
Gaudin, A.M. 1957. Flotation. McGraw-Hill, New York. Chapter 9. [ Links ]
Godel, B., Barnes, S.J., and Maier, W.D. 2007. Platinum-group elements in sulfide minerals, platinum-group minerals, and whole-rock of the Merenksy Reef (Bushveld Complex, South Africa): Implications for the formation of the reef. Journal of Petrology, vol.48, no. 8. pp. 1569-1604. [ Links ]
Guler, T., Hicyilmaz, C., Gokagac, G., and Z.E. 2005. Electrochemical behaviour of chalcopyrite in the absence and presence of dithiophosphate. International Journal of Mineral Processing, vol. 75. pp. 217-228. [ Links ]
Hamilton, I.C. and Woods, R. 1986. Surfactant properties of alkyl xanthates. International Journal of Mineral Processing, vol. 17. pp. 113-120. [ Links ]
Hodgson, M. and Agar, G.E. 1989. Electrochemical investigations into the flotation chemistry of pentlandite and pyrrhotite. Canadian Metallurgy Quarterly, vol. 28. pp. 189-198. [ Links ]
Kelebek, S., Wells, P.F., and Fekete, S.O. 1996. Differential flotation of chalcopyrite, pentlandite and pyrrhotite in Ni-Cu sulphide ores. Canadian Metallurgy Quarterly, vol. 35, no. 4. pp. 329-336. [ Links ]
Khan, A. and Kellebek, S. 2004. Electrochemical aspects of pyrrhotite and pentlandite in relation to their flotation with xanthate. Part I: Cyclic voltammetry and rest potential measurements. Journal of Applied Electrochemistry, vol. 34. pp. 849-856. [ Links ]
Laskowski, J.S. 1993. Frothers and flotation froth. Mineral Processing and Extractive Metallurgy Review, vol. 12. pp. 61-89. [ Links ]
Leja, J. 1968. On the mechanisms of surfactant adsorption. Proceedings of the VIII International Mineral Processing Congress, Leningrad. vol. S-1. pp. 1-7. [ Links ]
Leja, J. 1989. Interactions among surfactants. Minerals Processing and Extractive MetallurgyReview, vol. 5. pp. 1-24. [ Links ]
Leppinen, J.O. 1990. FTIR and flotation investigation of the adsorption of ethyl xanthate on activated and non-activated minerals. International Journal of Mineral Processing, vol. 30. pp. 245-263. [ Links ]
Lotter, N.O., Whiteman, E., and Bradshaw, D.J. 2014. Modern practice of laboratory flotation testing for flowsheet development - A review. Minerals Engineering, vol. 66-68. pp. 2-12 [ Links ]
Nicol, M.J. 1984. An electrochemical study of the interaction of copper (II) ions with sulfide minerals. Proceedings of the International Symposium on Electrochemistry in Mineral and Metal Processing. Richardson, P.E., Srinivasan, S., and Woods, R. (eds.). The Electrochemical Society, Pennington, NJ. pp. 152-168. [ Links ]
Plaksin, I.N. and Bessonov, S.V. 1957. The role of gases in flotation reactions. Proceedings of the 2nd International Congress on Surface Activity, vol. 3. Butterworths, London. pp. 361-367. [ Links ]
Rao, S.R. 2004a. Flotation surfactants. Surface Chemistry of Froth Flotation. 2nd edn. Kluwer Academic/Plenum Publishers, New York. pp. 385-478. [ Links ]
Rao, S.R. 2004b. Physical chemistry of interfaces. Surface Chemistry of Froth Flotation. 2nd edn. Kluwer Academic/Plenum Publishers, New York. pp. 143-207. [ Links ]
Robertson, C., Bradshaw, D., and Harris, P. 2003. Decoupling the effect of depression and dispersion in the batch flotation of a platinum bearing ore, Proceedings of the XXII International Mineral Processing Congress, Cape Town, South Africa, 29 September - 3 October 2003. Lorenzen, L. and Bradshaw, D.J. (eds). Southern African Institute of Mining and Metallurgy, Johannesburg. pp. 920-928. [ Links ]
Roos, J.R., Celis, J.P., and Sudrassono, A.S. 1990. Electrochemical control of metallic copper and chalcopyrite-xanthate flotation. International Journal of Mineral Processing, vol. 28. pp. 231-245. [ Links ]
Senior, G.D., Trahar, W.J., and Guy, P.J. 1995. The selective flotation of pentlandite from a nickel ore. International Journal of Mineral Processing, vol. 43, no. 3-4. pp. 209-234. [ Links ]
Slabbert, W. 1985. The role of trithiocarbonate and thiols on the flotation of some selected South African sulfide ores. MSc thesis. Department of Chemical Engineering, Potchefstroom university for Christian Higher Education, Potchefstroom. [ Links ]
Steyn, J.J. 1996. The role of collector functional groups in the flotation activity of Merenky Reef samples. MSc thesis. Department of Chemical Engineering, Potchefstroom university for Christian Higher Education, Potchefstroom. [ Links ]
Taggart, A.F., Giudice, G.R.M., and Ziehl, O.A. 1934. The case of chemical theory of flotation. Transactions of the American Instritute of Mining and Metallurgical Engineers, vol. 112. pp. 348-381. [ Links ]
Venter, J.A. 2007. Dithiocarbonate and trithiocarbonate interactions with pyrite and copper. MSc thesis. Faculty of Engineering, Built Environment and Information Technology, University of Pretoria, Pretoria. [ Links ]
Venter, J.A. and Vermaak, M.K.G. 2008a. EIS measurements of dithiocarbonate and trithiocarbonate interactions with pyrite and copper. Minerals Engineering, vol. 21, no. 5. pp. 440-448. [ Links ]
Venter, J.A. and Vermaak, M.K.G. 2008b. Mechanisms of trithiocarbonate adsorption: A flotation perspective. Minerals Engineering, vol. 21. pp. 1044-1049. [ Links ]
Vos, C.F. 2006. The role of long-chain trithiocarbonates in the optimisation of Impala Platinum's flotation circuit. MSc thesis, Department Material Science and Metallurgical Engineering, university of Pretoria, Pretoria. [ Links ]
Vos, C.F., Davidtz, J.C., and Miller, J.D. 2007. Trithiocarbonates for PGM flotation. Journal of the Southern African Institute of Mining and Metallurgy, vol. 107. pp. 23-28. [ Links ]
Winter, G. and Woods, R. 1973. The relation of collector redox potential to flotation efficiency: monothiocarbonates. Separation Science, vol. 8, no. 2. pp. 261-267. [ Links ]
Woods, R. 1976. Electrochemistry of sulfide flotation. Flotation. Fuerstenau, M.C. (ed.). AIME, New York. pp. 298-333. [ Links ] ♦
Paper received Mar. 2018
Revised paper received Jun. 2018














