Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Journal of the Southern African Institute of Mining and Metallurgy
versión On-line ISSN 2411-9717
versión impresa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.118 no.10 Johannesburg oct. 2018
http://dx.doi.org/10.17159/2411-9717/2018/v118n10a7
FACTSAGE™ thermo-equilibrium simulations of mineral transformations in coal combustion ash
A.C. CollinsI; C.A. StrydomI; J.C. van DykI, II; J.R. BuntI, III
IChemical Resource Beneficiation, North-West University, South Africa
IIAfrican Carbon Energy, South Africa
IIISchool of Chemical and Mineras Engineering, North-West University, South Africa
SYNOPSIS
The aim of this investigation is to report on the influence of operating conditions, and of additives such as potassium carbonate, on the slagging behaviour of South African coal. This was done using a FACTSAGE™ model that was previously developed to simulate the chemistry and mineral transformations occurring during a fixed-bed countercurrent gasification process. The mineral transformations in K- and Al-containing inorganic compounds under certain thermal conditions were tracked to see whether these species remain in the minerals or are captured by the slag. The main contributors to slag formation and possible inorganic mineral transformations were identified. The addition of potassium carbonate to the coal before thermal processing decreases the melt formation temperature and the melt percentage. The mineral transformations and slagging behaviour depend on the percentage of potassium in the sample, as well as the basic components present in the coal.
Keywords: FACTSAGE™, coal combustion, potassium, aluminium, slagging behaviour.
Introduction
Coal is a heterogeneous material (sedimentary rock) composed of organic and inorganic components. The chemical and physical properties of coal vary depending on the source of the coal, i.e. the age and geological environment in which the coal was formed (Oboirien et al., 2011; Yu, Lucas, and Wall, 2007). During gasification of coal, the organic matter partially decomposes (Kong et al., 2011); while mineral transformations occur (Song et al., 2009). Coal ash is thus a collection of mineral and non-mineral inorganic components that has undergone transformation as a result of thermal processing. The ash composition will depend on the organic and inorganic compounds present in the coal and/or any materials added to the coal prior to thermal processing (Kong et al., 2014). This specific composition of the sample, the organic and inorganic components, determines the mineral matter transformation and thus the slag characteristics (van Dyk, 2006). Mineral behaviour during thermal processing depends on:
(i) The different types of minerals (modes of occurrence) and quantities present in the coal sample (Benson, Sondreal, and Hurley, 1995; Vassilev et al., 1995)
(ii) The operating temperature
(iii) The oxygen partial pressure in the atmosphere (Jak et al., 1998).
When the coal is subjected to high temperatures (> 1100°C), melting and reactions of the component mineral matter occur, forming slag (Song et al., 2009). It is assumed that the slag composition depends on the minerals present in the coal, coupled with the operating conditions (Guo et al., 2014). In addition, the slagging behaviour of coal ash depends on the ash composition, i.e. the inorganic species present in the ash, as these minerals determine the ash fusion temperature (AFT) (van Dyk and Waanders, 2008). Consequently, the ash fusibility is generally expressed as a function of the content of principal oxides: SiO2, Al2O3, TiO2, Fe2O3, CaO, MgO, Na2O, and K2O (Seggiani, 1999). The AFT is determined by the modes (vapour, solid mineral grains) in which these elements occur in the ash. Although AFT is still widely used as a parameter for determining ash fusibility and melting characteristics of minerals (Jak et al., 1998), accurate results are difficult to obtain due to the complex composition of coal ash. Because of the complex nature of coal and the associated minerals, prediction of the mineral behaviour/transformation during thermal processing is a difficult task (Jak et al., 1998) when applying tradition methods (Hanxu et al., 2006).
FACTSAGE™ simulation of the slagging process provides a means by which mineral behaviour/transformation towards equilibrium conditions can be predicted. It is an important tool that can be used to describe equilibrium ash properties, mineral transformation, behaviour of inorganic components, and the slagging tendency of coal ash at specific temperatures (van Dyk and Waanders, 2008), which can then be compared to experimental results. The modelling software was developed mainly for complex chemical equilibrium and process simulations, but can also be used to calculate and manipulate phase diagrams for minerals and mineral complexes (van Dyk et al., 2009). One of the advantages of using the FACTSAGE™ databases is that carbon reactions can be studied in conjunction with minerals, while still being able to change atmospheric conditions (van Dyk et al, 2006). The FACTSAGE™ software can also provide information on the phases that have reached equilibrium during thermal processing, the compositions of these phases and the proportions in which they are present (Hanxu et al., 2006). A wide range of thermochemical calculations can also be performed with the FACTSAGE™ software (Hanxu et al, 2006; Zhao et al, 2013).
A FACTSAGE™ model was developed by van Dyk et al. (2006) in order to understand the chemistry and mineral transformation during a fixed-bed countercurrent gasification process. This model consisted of a three-zone simulation:
1. Drying and devolatilization zone
2. Gasification zone
3. Combustion and ash zone.
Van Dyk and Waanders (2008) subsequently developed a modified model that consisted of a two-zone simulation:
1. Drying, devolatilization, and gasification zone (reduction zone)
2. Combustion and ash zone (oxidation zone).
Both the original and improved thermodynamic equilibrium models were validated with high-temperature X-ray diffraction (HT-XRD) (van Dyk and Waanders, 2008; van Dyk et al., 2008).
The recovery of inorganic compounds from coal ash produced during thermal processing may be economically viable. Potassium salts, for example, are used as gasification catalysts, i.e. they promote the production of methane during gasification (Nahas, 1983) and lower the operating temperatures of the gasification process (Green et al., 1988). Coal ash represents a good potential source of potassium for re-utilization in industrial processes (Ge, Jin, and Guo., 2014).
The main aim of this investigation is to not only evaluate the influence of operating temperatures on slagging behaviour of South African coal ash, but also to determine if the addition of a potassium compound to the coal influenced the slagging behaviour. Various percentages of potassium (as potassium carbonate) were added to the system (modelling simulation) for each of the coal samples and the mineral transformations, especially Al- and K- containing minerals, tracked.
Materials and methods
Coal samples
Three South African coal samples (SA1, SA2, and SA3) and one from the USA (US1) were used. The South African samples originated from mines in the Mpumalanga region,
and the USA coal from North Dakota. The samples were collected by the individual mines, and a representative sample of 50 kg was used during this study. The samples were selected so as to represent different ranks of coal, with ash fractions of various potassium contents and acidities. The coal rank was determined using the ASTM D388-12 standard. The acidity was determined from the XRF results (see Table III) using the following equation (van Dyk, Waanders, and van Heerden, 2008):
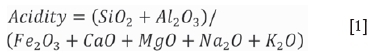
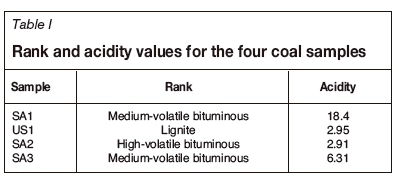
The rank and acidity for the four coal samples are presented in Table I.
Sample preparation
The coal was prepared by air drying the entire sample as received from the mine, to reduce the excess moisture not associated with the coal structure. After drying, the samples were crushed to < 1 mm using a crusher and ball mill. The SA2 blend sample was prepared by the addition of potassium carbonate (5 wt%) to the coal during the milling step in order to ensure a heterogeneous mixture of additive and coal.
Predicting the influence of potassium content on the mineral transformation and slagging behaviour was investigated using FACTSAGE™ modelling. The percentage potassium added was calculated according to the ash yield of the coal, i.e. a specific percentage of potassium was loaded to the sample according to coal ash percentage.
Analytical methods
The coal samples were subjected to ultimate, proximate, and XRF analysis. Sample preparation was done according to ISO 13909-4: 2001. The ISO standard characterization methods used on the coal samples are summarized in Table II. The composition of the coal ash samples was determined by XRF analysis, and is presented as elemental oxides.
FACTSAGE™ Modelling
FACTSAGE™ 7.2 modelling software was used to investigate the mineral transformations and speciation of the four coal samples. The two-zone gasification simulation model (van Dyk et al., 2008), described earlier, was used. In order to simulate a real gasification process, similar operating conditions (i.e. similar flows and conditions such as temperature, pressure, and mass flow) were used in the model, (van Dyk, Melzer, and Sobiecki, 2006). Although coal is a complex heterogeneous material that consists of different amounts of various organic and inorganic components, to accommodate the model used for the simulations it was assumed that coal consists of four basic components: moisture, fixed carbon, volatile matter, and minerals. The data was input to the FACTSAGE™ software in elemental form, i.e. carbon, hydrogen, nitrogen, sulphur, oxygen, and inorganic components. Input data can also be in mineral or compound form. For this investigation, the input data was derived from the results obtained from ultimate, proximate, and ash composition analyses. The mass flow data for the volatile matter and fixed carbon was normalized to an elemental composition, similar to that of ultimate analysis. Since the ash flow (melt) is composed of a variety of mineral species, it was normalized to a mass flow for the different mineral species (van Dyk, Melzer, and Sobiecki, 2006).
Simulation model
The model used in this investigation was developed on the principle that coal flows from the top into the gasifier as gas flows upwards into the zone that is being modelled. Thus, as the coal flows downwards into the drying, devolatilization, and gasification (reduction) zone, it comes into contact with and reacts with the gas that flows upwards from the combustion (oxidizing) zone. A similar approach was followed during the modelling of the combustion (oxidation) zone. As the organic and mineral components in coal enter the combustion (oxidation) zone, they react with the reagent gas that flows into the gasifier at 340°C (van Dyk et al., 2009). The two modelling zones, as described in van Dyk et al. (2208), differ in two main respects: the input data used for the simulations and the temperature range at which the simulations are run. The temperature range used for the drying, devolatilization, and gasification (reduction) zone started at 25°C when the coal enters the gasifier and reacts with the gas that flows up from the combustion (oxidation) zone at a maximum temperature of 1400°C (van Dyk and Keyser, 2014; van Dyk et al., 2009). The databases used for the FACTSAGE™ calculations were FactFS, FToxid, and FTmisc. The FactPS database was used for all pure and gaseous components during simulation, while the FTmisc database was used for the pure sulphur compound. The melt phase was imitated using the 'B-Slag-liq with SO4' phase, which forms part of the FToxid database. During the simulations, only pure compounds from these databases were considered.
Results and discussion
Characterization
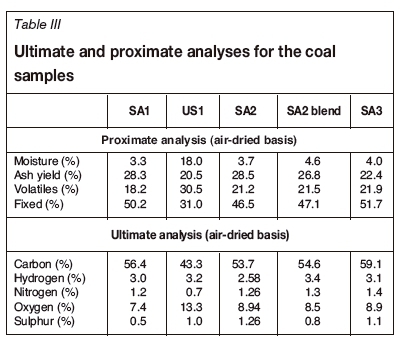
The ultimate and proximate analyses results are presented in Table III. The ash yield varied between 20% and 30% for the different samples. The South African coal samples had a high volatile content, similar to previous observations for South African coals (Hattingh et al., 2011).
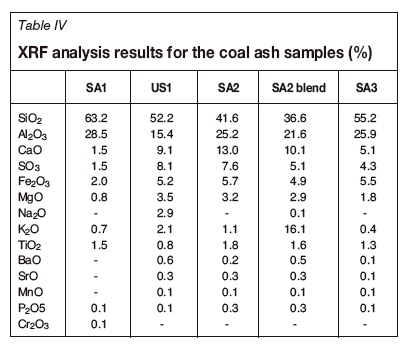
The ash compositions are presented in Table IV. These results indicate that the ash samples consist primarily of alumina (Al2O3) and silica (SiO2), with the K2O content between 0.43% and 2.06%. The SA2 blend sample with added potassium had a K2O content of 16.1%. CaO, Fe2O3, Na2O, and MgO, which were present in moderate percentages, are known for their fluxing potential during thermal processing of coal.
FACTSAGE™ modelling
Thermochemical calculations, using the EQUILIB uool which form part of the FACTSAGE™ modelling software, make it possible to predict the equilibrium behaviour of the inorganic compounds during thermal processing. Equilibrium mineral transformation and slag formation can therefore be predicted; under specific conditions. The influence of potassium additive was modelled using the following approach. Potassium addition was done according to the ash yield of the coal, i.e. a specific percentage (1, 5, or 10 mass%) of the ash yield is represented by the potassium oxide seen in Table IV. Figures 1-5 present the FACTSAGE™ simulation graphs for the feed coal and coal blend samples in the reduction zone. Figure 6 indicates the AFT versus the percentage of basic compounds, and Figures 7-9 present the FACTSAGE™ simulation graphs for the feed coal and coal blend samples in the oxidizing zone.
As SA1 and SA3, which containeds the lowest percentages of K2O and MgO, the highest percentages of SiO2, and had the highest acidity, behave similarly according to the FACTSAGE™ simulation results, only the results obtained for SA1 are discussed. US1 and SA2 had similar acidity values as calculated from the XRF data, and both contained high percentages of K2O and MgO; hence only the results from SA2 are discussed. The FACTSAGE™ results for both SA2 and the SA2 blend are discussed.
Drying, devolatilization, and gasification (reduction) zone
Mineral transformation
The mineral transformation simulation for SA1 is presented in Figure 1. From the graph it can be seen that the temperature at which melt stars to form was predicted to be 1175°C. The temperature at which the melt starts to form depends on the types of clays and fluxing minerals present in the coal, their concentrations in the sample, and their melting temperatures (Liu et al., 2013). The transformation of quartz (SiO2: S2) to quartz (SiO2: S4) took place as the temperature increased above 800°C. Even though quartz is inactive during thermal processing, transformation of the mineral to a more stable polymorph will take place as the temperature increases. SiO2 (Sx) refers to a stable phase for the mineral at a specific temperature (van Dyk, Waanders, and van Heerden, 2008). Stable phases of the different minerals present in the sample will influence the AFT. As the temperature increased above 1175°C, a decrease in the percentage quartz was observed during the simulation. This decrease may result from glass formation and partial melting of quartz (Zhou et al., 2012). According to the simulation results, sillimanite (Al2SiO5), anorthite (CaAl2Si2O8), cordierite (Mg2Al4Si5O18), and microcline (KAlSi3O8) contributed to the percentage melt as these minerals reached their melting temperatures. This same trend in mineral transformation was observed for SA3 during the simulation runs. However, the total percentage slag formed at 1400°C for SA3 (80%) was higher than that for SA1 (50%). This may be due to the higher anorthite and cordierite contents in SA3.
The mineral transformations for SA2 are presented in Figure 2. High percentages of anorthite are predicted. Other minerals such as microcline, quartz (S2), quartz (S4), and enstatite (MgSiO3) are also present but at levels lower than 10%. The percentage melt increased with temperature as the minerals reach their melting temperatures. US1 exhibited similar mineral transformation trends to SA2 during the simulations, although the melt starting temperature was lower (1025°C) than that of SA2 (1125°C). The total percentage of slag formed from US1 (86%) at 1400°C was higher than for SA2 (55%). This may be due to the high contents of anorthite, diopside, and K- and Na-feldspars (KAlSi3O8 and NaAlSi3O8) in US1.
The mineral transformation simulation for SA2 blend, which is the coal sample with added potassium prior to thermal processing, is presented in Figure 3. From these results it can be seen that the temperature at which the melt starts to form was below 1000°C.
The influence of potassium addition to the coal prior to thermal processing can be seen by comparing the results for the SA2 blend (Figure 3) with those for SA2 in Figure 2. A decrease in the melt formation temperature (1125°C to 975°C) and percentage melt formation (55% to 43%) is observed.
Influence ofadded potassium salt on the slagging behaviour of coal
The modelled influence of added potassium (described in the previous section) on the slagging behaviour is presented in Figures 4-6. For SA1, increasing the potassium loading led to an increase in the percentage melt (Figure 4). The temperature at which melt formation started remains the same for the feed coal and blended coal samples. This has also been observed in other studies (van Dyk, 2006). The same trend was observed for SA3. The melt starting temperature was within 25°C for all the samples. The influence of potassium loading on the coal was observed only after the melt formation temperature was reached.Sample SA2 exhibited the opposite trend - a decrease in the melt percentage was observed with increasing potassium loading (Figure 5). The melt starting temperature increased with additions of 5 and 10 mass% potassium. The same trends for melt percentage and melt starting temperature were observed for US1.
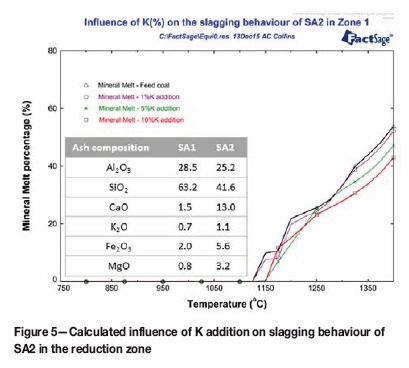
A comparison of the melt formation results for the SA2 blend (Figure 3) and the theoretical results calculated for SA2 (Figure 5) shows that the predicted percentages of melt formation were similar (within 5%). The melt formation temperature for the SA2 blend (Figure 3) was lower (975°C) than the theoretical prediction results (1150°C) (Figure 5).
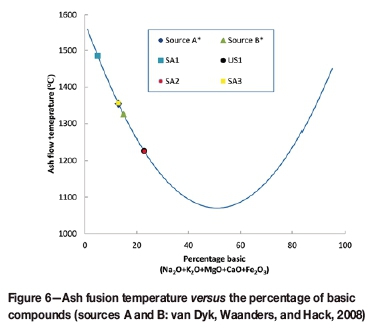
From Figure 4 and Figure 5, it can be seen that addition of potassium carbonate to coal at different loadings had various influences on the slagging behaviour. The slagging behaviour of coal depends on the mineral matter present in the coal. From the XRF results presented in Table IV, it can be seen that SA2 contained high percentages of basic (K-, Ca-, Fe-, Na-, and Mg- containing) compounds. The basic compounds influence the AFT (increasing or decreasing it) (Hanxu et al., 2006), and also act as fluxing agents when present in certain percentages in the coal (Jak et al., 1998; van Dyk, Waanders, Hack, 2008). The concentration of these compounds, especially Ca-containing species, will determine their combined influence on the AFT. When high percentages of basic mineral compounds (Ca2+, K+ Na+, and Fe2+) are present in a coal sample, an increase in the AFT will (possibly) be observed. This increase is due to the sub-liquidus transformation of the mineral phases (Song et al., 2009). This implies that high percentages of basic mineral compounds lead to maximum mineral formation/transformation and the stabilization of these mineral phases, which in turn increases the AFT (van Dyk, Waanders, and Hack, 2008). A high AFT will also be observed with low percentages of basic mineral compounds present in the coal (Jak et al., 1998). The influence of basic compounds, especially Ca, on the AFT is presented schematically in Figure 6. The results (source A and B) obtained by van Dyk, Waanders, and Hack (2008) are shown together with the coal samples used in this investigation (SA1, SA2, SA3, US1). The percentage of basic compounds in SA1 (4.9%) was lower than that of SA2 (22.9%). This indicates that SA2 would have a lower AFT than SA1. This influence of the basic compounds was also predicted by FACTSAGE™ modelling, and is presented in Figures 4 and 5.
Combustion and ash (oxidation) zone
Figures 7-9 presents the results obtained for the mineral transformation simulations for the different coal samples in the combustion and ash (oxidation) zone. The graphs should be read from right to left to better understand the flow of the material as it moves from the top to the bottom of the gasifier (cooling process). As the graph is read from right to left, formation of mineral phases is observed, which may indicate crystallization of mineral phases from the slag. Also seen in the figures is the decrease in melt percentage as the temperature of the operating process decreases.
Mineral transformation of coal samples
The mineral transformation simulation for SA1 is presented in Figure 7. As the cooling process starts, sillimanite (Al2SiO5) and SiO2 (S4 and S2) minerals are formed. Small percentages of other minerals are also predicted to form during cooling. The same trend of mineral formation was again observed for SA3. Simulation of mineral transformation for SA2 is presented in Figure 8. Crystallization of minerals, such as calcium feldspar (CaAlSi3O8), cordierite (Mg2Al4Si5O18), calcium sulphate (CaSO4), SiO2 (S4 and S2), and potassium feldspar (KAlSi3O8) was predicted to occur as the temperature decreases. A similar trend was observed for US1. The simulation of mineral transformation for the SA2 blend is presented in Figure 9. During the cooling process, crystallization of minerals, such as leucite (KAlSi2O6) and kalisilite (KAlSiO4) was predicted as the temperature decreased. The influence of the potassium loading on behaviour in the oxidizing zone will remain constant, since slag formation is determined at the highest temperature in the reducing zone.
Conclusions
The mineral transformation and slagging tendencies of different coal samples were investigated using the FACTSAGE™ modelling database. The extent of melt formation increased with increasing operating temperature as more of the minerals present in the coal undergo melting. Lower melting temperatures may be due to the fluxing influence of basic components such as Ca-, Mg-, K , and Fe-containing minerals. The slagging tendencies of the coal samples were dependent on the specific mineral composition of the coal and the transformation of these minerals during thermal processing. Increased potassium loadings according to the mineral content resulted in an increase in melt formation for samples SA1 and SA3, while for samples US1 and SA2 it decreased the amount of melt formation. The latter observation can be explained by the ratios of specific species and elemental composition, and may be attributed to the already high content of basic components in US1 and SA2.
The FACTSAGE™ simulations were modelled according to an equilibrium model for the gasifier, which predicts mineral transformations on the assumption that all minerals in the sample have reached equilibrium. Prediction studies were done on this basis, even though mineral transformations (reactions) do not reach equilibrium during thermal processing. Although melt (slag) predictions have been modelled and verified with the use of this software, not all mineral interactions between phases can be predicted due the limitations of the software database.
The following conclusions can be drawn.
► The temperature at which thermal processing occurs plays an important role in the extent of melt formation, mineral transformation, and also the recoverability of compounds from the ash.
► The addition of potassium to the coal prior to thermal processing influences not only the extent of melt formation, but also the AFT, depending on the coal mineral composition.
► Simulation predictions of the melt percentage from the theoretical (assumed) addition of potassium to the coal compared well to the simulation run on the blended sample (coal sample with potassium). The simulation runs indicated a ±200°C difference in the melt formation temperatures. This may due to complex reactions taking place between the potassium and mineral phases in the coal, which could not be predicted by the equilibrium model conditions.
It needs to be remembered that FACTSAGE™ simulations are only a prediction of what might occur during thermal processing, and that these predictions are based on thermodynamic equilibrium conditions. These simulations provide only an indication on what might actually occur during thermal processing. Despite this limitation, the FACTSAGE™ modelling software can be a powerful tool for predicting coal behaviour, which can be used to optimize conditions for the different coal burning technologies available.
Future work
► Prediction of the percentage potassium not captured in the melt or lost through gas formation. This may yield valuable insights when experimental work is conducted on the leachability of K-containing compounds.
► Simulation work on the influence of specific compounds, their percentages, and composition on the slagging behaviour of coal during thermal processing.
► Further investigations into the reactions of mineral phases with the potassium compound added to the coal.
Acknowledgements
The information presented in this paper is based on the research financially supported by the South African Research Chairs Initiative (SARChI) of the Department of Science and Technology and National Research Foundation of South Africa (Coal Research Chair Grant No. 86880).
Any opinion, finding, or conclusion or recommendation expressed in this material is that of the author(s) and the NRF does not accept any liability in this regard.
References
Benson, S.A., Sondreal, E.A., and Hurley, J.P. 1995. Status of coal ash behavior research. Fuel Processing Technology, vol. 44, no. 1-3. pp. 1-12. [ Links ]
Ge, Z., Jin, H., and Guo, L. 2014. Hydrogen production by catalytic gasification of coal in supercritical water with alkaline catalysts: Explore the way to complete gasification of coal. Internation Journal of Hydrogen Energy, vol. 39. pp. 19583-19592. [ Links ]
Green, P.D., Edwards, I.A.S., Marsh, H., Thomas, K.M., and Watson, R.F. 1988. Coal thermoplasticity and coke structure as related to gasification. Fuel, vol. 67. pp. 389-395. [ Links ]
Guo, Q., Zhou, Z., Wang, F., and Yu, G. 2014. Slag properties of blending coal in an industrial OMB coal water slurry entrained-flow gasifier. Energy Conversion and Management, vol. 86. pp. 683-688. [ Links ]
Hanxu, L.I., Ninomiya, Y., Zhongbing, D., and Mingxu, Z. 2006. Application of the FactSage to predict the ash melting behaviour in reducing conditions. Chinese Journal of Chemical Engineering, vol. 14. pp. 784-789. [ Links ]
Hattingh, B.B., Everson, R.C., Neomagus, H.W.J.P., and Bunt, J.R. 2011. Assessing the catalytic effect of coal ash constituents on the CO2 gasification rate of high ash, South Afican coal. Fuel Processing Technology, vol. 92. pp. 2048-2054. [ Links ]
Jak, E., Degterov, S., Hayes, P.C., and Pelton, A.D. 1998. Thermodynamic modelling of the system Al2o3-Sio2-Cao-Feo-Fe2o3 to predict the flux requirements for coal ash slags. Fuel, vol. 77. pp. 77-84. [ Links ]
Kong, L., Bai, J., Bai, Z., Guo, Q., and Li, W. 2014. Improvement of ash flow properties of low-rank coal for entrained glow gasifier. Fuel, vol. 120. pp. 122-129. [ Links ]
Kong, L., Bai, J., Li, W., Bai, Z., and Guo, Z. 2011. Effect of lime addition on slag fluidity of coal ash. Journal of Fuel Chemistry and Technology, vol. 39. pp. 407-411. [ Links ]
Liu, В., He, Q., Jiang, Z., Xu, R., and Hu, B. 2013. Relationship between coal ash composition and ash fusion tempretures. Fuel, vol. 105. pp. 293-300. [ Links ]
Nahas, N.C. 1983. Exxon catalytic coal gasification process. Fuel, vol. 62. pp. 239-241. [ Links ]
Oboirien, B.O., Engelbrecht, A.D., North, B.C., du Cann, V.M., Verryn, S., and Falcon, R. 2011. Study on the structure and gasification characteristics of selected South African bituminous coals in fluidised bed gasification. Fuel Processing Technology, vol. 92, no. 4. pp. 735-742. [ Links ]
Seggiani, M. 1999. Empirical correlations of the ash fusion temperatures and temperature of critical viscosity for coal and biomass ashes. Fuel, vol. 78, no. 9. pp. 1121-1125. [ Links ]
Song, W.J., Tang, L.H., Zhu, X.D., Wu, Y.Q., Rong, Y.Q., Zhu, Z.B., and Koyama, S. 2009. Fusibility and flow properties of coal ash and slag. Fuel, vol. 88, no. 2. pp. 297-304. [ Links ]
Van Dyk, J.C. 2006. Understanding the influence of acidic components (Si, Al, and Ti) on ash flow temperature of South African coal sources. Minerals Engineering, vol. 19. pp. 280-286. [ Links ]
Van Dyk, J.C. anD Keyser, M.J. 2014. Influence of discard mineral matter on slag-liquid formation and ash melting properties of coal - A FACTSAGE simulation study. Fuel, vol. 116. pp. 834-840. [ Links ]
Van Dyk, J.C., Melzer, S., and Sobiecki, A. 2006. Mineral matter transformation during Sasol-Lurgi fixed bed dry bottom gasification - utilization of HT-XRD and FactSage modelling. Minerals Engineering, vol. 19. pp. 1126-1135. [ Links ]
Van Dyk, J.C. and Waanders, F.B. 2008. An improved thermodynamic FACTSAGE simulation to simulate mineral matter transformation during a fixed bed counter-current gasification process, validated with HT-XRD. Proceedings of the XXIV International Mineral Processing Congress. Beijing, China, 24-28 September 2008, vol. 2. Wang. D.Z. (ed.). Science Press, Beijing. pp. 2314-2321. [ Links ]
Van Dyk, J.C., Waanders, F.B., Benson, S.A., Laumb, M.L., and Hack, K. 2009. Viscosity predictions of the slag composition of gasified coal, utilizing FacSage equilibrium modelling. Fuel, vol. 88. pp. 67-74. [ Links ]
Van Dyk, J.C., Waanders, F.B., and Hack, K. 2008. Behaviour of calcium- containing minerals in the mechanism towards in situ Co2 capture during gasification. Fuel, vol. 87. pp. 2388-2393. [ Links ]
Van Dyk, J.C., Waanders, F.B., Melzer, S., Baran, A., Hack, K., and Bunt, J.R. 2008. Validation of a thermodynamic equilibrium model developed on South-African coal sources in order to simulate mineral transformations of various coals during gasification. Proceedings of the 25th International Annual Pittsburgh Coal Conference Pittsburgh, PA. [ Links ]
Van Dyk, J.C., Waanders, F.B., anDVAN Heerden, J.H.P. 2008. Quantification of oxygen capture in mineral matter during gasification. Fuel, vol. 87. pp. 2735-2744. [ Links ]
Vassilev, S.V., Kitano, K., Takeda, S., and Tsurue, T. 1995. Influence of mineral and chemical composition of coal ashes on their fusibility. Fuel Processing Technology, vol. 45. pp. 27-51. [ Links ]
Yu, J.L., Lucas, J.A., and Wall, T.F. 2007. Formation of the structure of chars during devolatilization of pulverized coal and its thermoproperties: A review. Progress in Energy and Combustion Science, vol. 33, no. 2. pp. 135-170. [ Links ]
Zhao, Y.L., Zhang, Y.M., Bao, S.X., Chen, T.J., and Han, J. 2013. Calculation of mineral phase and liquid phase formation temperature during roasting of vanadium-bearing stone coal using FactSage software. International Journal of Mineral Processing, vol. 124. pp. 150-153. [ Links ]
Zhou, C., Liu, G., Yan, H., Fang, T., and Wang, R. 2012. Transformation behaviour of mineral composition and trace elements during coal gangue combustion. Fuel, vol. 97. pp. 644-650. [ Links ] ♦
Paper received Mar. 2018;
revised paper received Aug. 2018














