Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.118 n.7 Johannesburg Jul. 2018
http://dx.doi.org/10.17159/2411-9717/2018/v118n7a5
PAPERS OF GENERAL INTEREST
Investigation of secondary zinc oxides as an alternative feed to the Skorpion Zinc process: Part 2 - Process considerations and economic analysis
C. LotteringI, II; C DorflingII
ISkorpion Zinc Mine, Rosh Pinah, Namibia
IIDepartment of Process Engineering, University of Stellenbosch, South Africa
SYNOPSIS
Skorpion Zinc is investigating the possibility of using secondary zinc oxides as an alternative feed to supplement the zinc oxide ore feed and to extend the life of mine. Part 1 of this communication provides the technical background on the leaching performance at the typical Skorpion Zinc operating conditions. This study reports on the process modelling and economic analysis that were performed to determine appropriate feed blending strategies for electric arc furnace (EAF) dust, zinc dross, and zinc fume dust based on process limitations and economic considerations.
The zinc fume dust had the highest zinc and lowest impurity content of the alternative oxide sources investigated; as a result, this alternative source resulted in the highest zinc production and profitability. At a blending ratio of 50% zinc fume in the solids feed, more than three times the current zinc production from ore could theoretically be achieved. Production from the zinc dross samples was limited by the amount of contained nickel; the maximum production was achieved at a blending ratio of 10% and was 20% higher than the current production from ore. Zinc production from EAF dust was very low at blending ratios exceeding 30%, due to Mg and Mn impurity limitations as well as the relatively low zinc content. Both zinc dross and EAF dust can also be processed economically to yield profit for Skorpion Zinc and the alternative oxide suppliers. Zinc dross was generally more profitable to process than EAF dust, despite its higher freight costs.
Keywords: leaching, process economics, secondary zinc oxide.
Introduction
Part 1 of this communication (Lottering and Dorfling, 2018 ) presented background on the Skorpion Zinc operation as well as a motivation for the work performed. In addition, results were presented on the leaching of alternative zinc oxide sources at conditions compatible with the current Skorpion Zinc operation. The dissolution of zinc and impurities such as Al and Fe was evaluated at temperatures between 40 and 70°C, and pH values between 1.2 and 2.1. It was concluded that electric arc furnace (EAF) dust, zinc dross, and zinc fume could all potentially be used to supplement the ore feed given the high extent of zinc dissolution and acceptably low acid consumption at the current Skorpion Zinc operating conditions.
In this part of the communication, the focus is therefore on determining the maximum amount of different alternative zinc oxides that could be fed to the plant considering operational limitations, and on determining the most economically feasible manner in which to supplement ore with alternative zinc oxide sources. Mass balances were performed taking into account the capacity of the respective unit operations in order to determine appropriate ore and alternative zinc oxide blending ratios. The mass balances were used as the basis to calculate production rates and operating costs, and for the associated economic analysis to compare the profitability of the different alternative zinc oxide sources and blending ratios. For the purpose of the mass balances and economic analysis, the leaching performance of the alternative zinc oxides at the current standard Skorpion Zinc operating conditions (pH 1.8 and 50°C) was used.
Skorpion Zinc process overview
Process description
The basic flow diagram of the Skorpion Zinc process is presented in Figure 1. Zinc ore from the open pit mine is crushed and milled to a d80 particle size of 180 μηι. Atmospheric sulphuric acid leaching of the ore takes place in a continuous operation consisting of five agitated leaching tanks arranged in series with a total residence time of approximately 2 hours. The slurry that leaves the ball mill contains 20% solids and flows into the first tank, where it is mixed with raffinate from the solvent extraction section. A small amount of pure acid is also added to adjust the pH to the required set-point for this tank. As the slurry flows into each subsequent leach tank, additional raffinate and pure acid are added to adjust the pH. The temperature in each tank is controlled to 50°C and the final pH at the end of the leaching section is maintained between 1.8 and 2 to maximize colloidal silica stability (Gnoinski, 2007). The main leaching reactions taking place have been presented in part 1 of this communication.
The pregnant leach solution (PLS) contains approximately 30 g/L zinc and high concentrations of impurities. The leaching step is followed by a neutralization step in which the pH of the leach solution is raised to 4 by calcium carbonate addition; this results in the agglomeration and precipitation of some of the major impurities, such as iron, aluminium, and silica.
The solids recovered after neutralization and thickening are re-acidified to recover any zinc that co-precipitated; this slurry is then filtered and the solids residue disposed of as tailings. The liquid filtrate is treated with milk of lime to precipitate the zinc in solution as a basic zinc sulphate, which is recycled to the neutralization section of the plant.
Approximately 25% of the PLS recovered from the thickener following neutralization is treated with zinc dust to precipitate Cu and Ni before being recycled to the process feed. The remaining 75% of the PLS is clarified before being sent to solvent extraction, where the zinc tenor is increased from approximately 30 g/L to 120 g/L and many of the impurities in the PLS are removed. Removal of the dissolved halides from the solution is of crucial interest in this section (Gnoinski, 2007). These impurities cause major corrosion of the lead anodes used in the electrowinning section of the process, which subsequently leads to unacceptable high lead levels in the electrolyte solution and in the plated zinc. The solvent extraction circuit is described in more detail by Cole and Sole (2003), Cole (2002), Cole, Sole, and Feather (2006), Musadaidzwa and Tshiningayamwe (2009,) and Sole, Feather, and Cole (2005). The loaded electrolyte from the solvent extraction section is passed to the electrowinning section, where zinc is electroplated onto aluminium cathodes.
The electrowinning process used by Skorpion Zinc to recover elemental zinc from the electrolyte is sensitive to the presence of impurities. It is therefore very important to ensure that the solvent extraction circuit can deal with the impurities introduced with the leach solution generated when supplementing the feed with alternative zinc oxides. Currently, Skorpion Zinc aims to obtain a PLS after neutralization with impurity concentrations as listed in Table I.
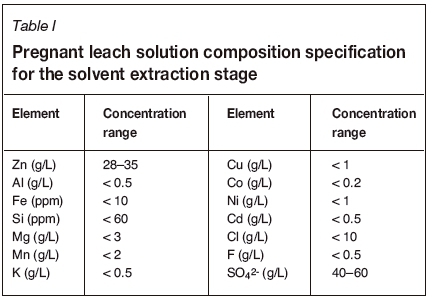
Since the electrowinning circuit regenerates the acid consumed for zinc leaching, the real net acid consumption is determined by the acid consumed as a result of gangue leaching as well as acid lost to the residue tailings. The main acid-consuming elements in the Skorpion Zinc circuit are calcium (as calcite), iron, and manganese. These elements are found in their oxide forms in the ore, and exist in similar forms in the secondary zinc oxides.
Effects ofimpurities on leaching and downstream processing
Certain elements associated with secondary zinc oxides are not present in the Skorpion Zinc ore. For this reason, there are currently no limits set on the concentrations of these impurities that the solvent extraction and electrowinning sections are capable of treating. The possible effects of the different impurities on solvent extraction and electrowinning are reviewed briefly to demonstrate the importance of effective impurity control in the process.
Aluminium in the electrolyte causes changes to the morphology of the zinc cathode, leading to larger grain sizes and therefore a coarser cathode deposit (Fukubayashi, 1972). In addition, aluminium can form complexes with fluorides, which would allow these ions to be transported to the electrowinning circuit where they damage the surface of the aluminium cathodes.
A high calcium content in the leach solution is an indication that a large amount of calcium has been leached, which causes excessive acid consumption in the leaching section. A large amount of calcium (> 4%) in the ore fed to the leaching section has previously caused the Skorpion Zinc leaching tanks to overflow due to gas generated by the reaction between calcium species and sulphuric acid. Most of the calcium in process solutions is removed in the neutralization step, so that the calcium can be removed from the circuit via the residue solids or tailings. Calcium concentrations exceeding 500 ppm in the PLS can lead to scaling of process equipment. This can be particularly detrimental in solvent extraction and electrowinning, where equipment must be taken offline to be cleaned. With the sensitivity of both these sections to process upsets, it is desirable to limit the calcium concentration to a maximum of 500 ppm.
The presence of nickel in the cellhouse electrolyte in quantities exceeding 200 ppb can cause dissolution of the plated zinc and decrease the current efficiency (Fukubayashi, 1972). Dissolution can cause large quantities of hydrogen to be emitted, which presents a serious fire and explosion risk in a zinc tankhouse. Copper, on the other hand, has a lower overpotential than zinc and would therefore plate preferentially to zinc. In addition, hydrogen will be released and the current efficiency reduced. Carryover of copper to the electrolyte can occur via entrainment of PLS in the loaded organic phase across solvent extraction. The copper cementation process treats a bleed stream of the PLS to prevent accumulation of copper in the leaching circuit.
Iron present in the leach solution entering the solvent extraction circuit will be preferentially extracted along with zinc. Unlike zinc, however, iron cannot be easily stripped off the organic phase by the spent electrolyte. This means that the iron accumulates in the organic, limiting the amount of extractant available for zinc to bind onto. For this reason, an organic stream is bled from the storage tank and contacted with 6 M HCl, which strips the iron from the organic (Alberts and Dorfling, 2013). During this process, the HCl becomes deactivated and must be regenerated in the Skorpion Zinc HCl plant. Iron from the HCl plant is bled from the process via a small bleed to the effluent treatment plant. In addition, any iron that enters the electrowinning circuit will reduce the current efficiency by continuously being oxidized to ferric iron at the anode, and then being reduced to ferrous iron at the cathode (Schlesinger etal., 2011).
Although the electrolyte entering the electrowinning circuit seldom contains large quantities of lead, lead-silver anodes are used for the electroplating process. Corrosion of these anodes by the highly acidic electrolyte solution introduces significant quantities of lead into the electrolyte in the form of PbSO4. This lead phase is very sparingly soluble, forming a fine precipitate that remains in suspension in the solution. During the plating process, the movement of zinc ions to the aluminium cathode traps the lead sulphate particles in the plated zinc matrix. The zinc that Skorpion Zinc aims to produce is classified as Special High Grade, with the specification that the zinc product should contain no more than 50 ppm total impurities, consisting of no more than 30 ppm lead, 10 ppm copper, and 20 ppm iron, as well as small quantities of other metal impurities. Thus, the entrapment of lead in the plated zinc matrix results in zinc that does not conform to the Special High Grade specifications. This will result in reduced profit for the company. Lead content in the feed solids to the leaching process should, however, not be a concern for electrowinning, as the lead should not be transferred across the solvent extraction circuit, and will therefore be removed from the process in the residue tailings and bleed streams.
Magnesium increases electrolyte viscosity and density, limiting the maximum attainable zinc concentration and raising the cell voltage. Manganese, although beneficial because it helps form a protective layer on the lead anode, also limits the maximum zinc content in the electrolyte (Sinclair, 2005). These elements are removed from the process via the tailings or residue stream, in both the solids and the liquids, as well as via the effluent treatment bleed stream.
When silica is dissolved as orthosilicic acid (H4SiO4) during leaching, the silica in solution may cause problems in the solvent extraction circuit. This form of silica is unstable in aqueous solutions and will decompose in acidic aqueous solutions (Moradi and Monhemius, 2011). The silica formed will precipitate as a polymer which can form hydrated gels, which cause problems with phase separation in the solvent extraction circuit (Sinclair, 2005).
Mass balance
Approach and assumptions
To determine the conditions under which maximum production could be achieved, the results from the experimental test work were used to perform mass balances for different blends of Skorpion Zinc ore and each of the alternative oxides. For each of the alternative oxides, blends of 10, 20, 30, 40, and 50% oxide with the ore were considered. It was assumed that the Skorpion Zinc ore grade remained constant at 9%. The compositions of the alternative zinc oxides were used as reported in part 1 of this communication. Based on historical plant data, the ore and limestone were specified to have a moisture content of 5%; the same moisture content was assumed for the alternative oxides.
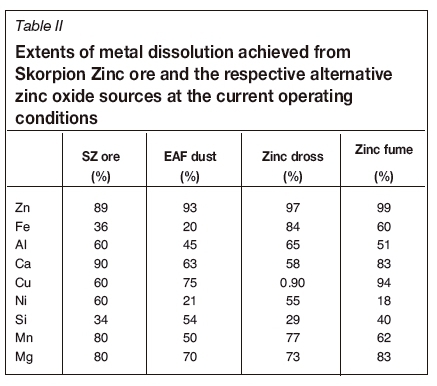
Dissolution of the different elements contained in the Skorpion Zinc ore was estimated based on historical plant data. Dissolution of the elements from the alternative oxides was determined from the experimental work presented in part 1 of this communication. A summary of the metal dissolutions achieved from the different sources at the typical Skorpion Zinc operating conditions is presented in Table II.
The key limiting factor for the different alternative zinc oxide sources was the impurity build-up in the Skorpion Zinc process. Calcium, aluminium, iron, silica, copper, nickel, manganese, and magnesium were the main impurities considered given their potential impact on the downstream unit operations. Calcium, silica, iron, and aluminium are normally completely removed from the circuit with the tailings, due to the combination of the neutralization section, effluent treatment, and the bleed stream from the solvent extraction circuit.
The system around which the mass balance was performed is shown in Figure 2. Sulphuric acid addition was calculated based on the Skorpion Zinc design target of 1.5 t per ton of zinc for ore, together with the experimentally determined acid consumptions for the oxides under investigation (0.11, 1.02, and 1.34 tons per ton for zinc fume, EAF dust, and zinc dross, respectively). Limestone addition was estimated based on actual plant data at approximately 16% by mass of the total dry solids feed to the refinery. It was assumed that a similar limestone addition would be used when blending alternative zinc oxides as supplementary feed material.
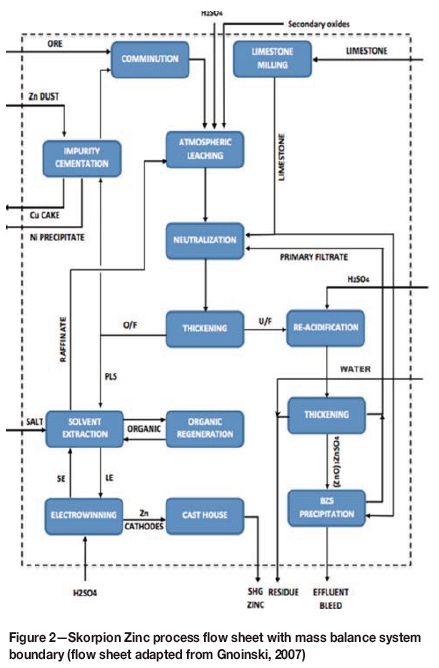
The mass balance was performed by specifying the desired feed ratio, whereafter the amount of oxide that could be fed under a certain set of conditions was determined by iteratively adjusting the amount of solids fed to the system until one of the impurities started to accumulate in the system. If impurity accumulation or removal was not found to be limiting, the total feed rate was set to the current maximum allowable feed rate of 230 t/h solids, and the plant throughput was then considered to be the limiting factor. A schematic overview of the mass balance approach is presented in Figure 3.
Assuming that aluminium, iron, silica, and calcium were completely removed in the neutralization section, the residue solids composition was based on a balance of these elements over the system. In the case of Mn and Mg, the residual solids were specified to have a fixed Mn and Mg content, and the Mn and Mg contents of the residue moisture were calculated by mass balance. The Skorpion Zinc design limited the Mn and Mg contents in the residue moisture to 4 g/L; these impurities would thus accumulate in the system if the Mn and/or Mg content of the residue moisture exceeded 4 g/L. The moisture content in the tailings was assumed to be 40%, based on the average historical moisture content of the tailings material produced by Skorpion Zinc.
Copper and nickel were balanced by calculating the required capacities of the copper and nickel removal sections. If the required capacity exceeded the actual maximum capacity of either of these sectins, the feed rate of material was deemed to be limited by the copper or nickel removal capacity and the subsequent accumulation of these impurities that would occur at higher feed rates. Based on the plant design and actual operating data, the copper removal section was assumed to be able to handle a throughput of 250 m3/h at a copper concentration of 500 mg/L, resulting in a total copper removal of 125 kg/h. The assumptions for the nickel removal section were similarly based on plant experience, and resulted in an assumed capacity of 60 m3/h at 400 mg/L Ni, resulting in 24 kg/h nickel removal at maximum capacity.
Results
The base case mass balance for the Skorpion Zinc refinery assumed that a total of 230 t/h ore was fed to the circuit without any alternative zinc oxides supplementing the feed. This resulted in a total zinc production of 151 000 t/a in the mass balance calculation. Once the base case had been established, each of the alternative oxides was run through the mass balance in different blending ratios with the Skorpion Zinc ore, and the maximum achievable zinc production for each scenario was calculated. These results are summarized in Table III, along with the limiting factor for each scenario.
From Table III it is clear that the zinc fume dust provided the highest overall zinc throughput and production at all blending ratios. This is due to the combination of a high zinc content and low impurity levels in this material. Copper became the limiting impurity at the point where the solids feed contained 30% or more zinc fume oxide. Since this sample's production capacity was limited by the plant throughput in the two lowest proportion blends, and because it had a much higher zinc grade than the Skorpion ore, the zinc production decreased as the alternative oxide portion of the feed decreased.
Production from the zinc dross sample was limited by the amount of nickel contained in the sample at all the investigated blending ratios. This alternative oxide source contained the highest amount of nickel at 0.2%, which is an order of magnitude higher than the nickel content of the other two sources. This was most likely due to the type of steel used in the hot-dip galvanizing process. Zinc production from the zinc dross was fairly consistent for all blend scenarios, with an increase of only about 30 000 t per year in production across the range of blends tested.
EAF dust, despite containing the highest percentage of impurities and lowest zinc percentage, was not limited by its impurity content at ratios of 20% or less with the Skorpion Zinc ore. Instead, it was initially limited by the maximum plant throughput of 230 t/h solids feed. For solids blending ratios of 30% or more EAF dust, first copper, then magnesium, and finally manganese became limiting elements. Zinc production from EAF dust at these higher blending ratios was overall very low; this was partially due to high impurity contents, but also to the lower zinc content relative to the other alternative oxide sources investigated. Thus, the optimum zinc production was achieved at 20% blend before impurity accumulation started to limit the maximum total throughput to the refinery.
From the mass balance, it was evident that the zinc fume provided the highest overall zinc production potential. For each of the oxides, there is a scenario that would provide the maximum potential zinc production. For EAF dust, a blending ratio of 20% with ore is ideal, while the dross should be blended in a 10% ratio. Zinc fume provides the highest theoretical zinc production at a 50% blend with Skorpion Zinc ore.
Other process considerations
The high iron and aluminium contents in the leach residue liquid generated from the EAF dust and zinc dross samples should be further investigated if the decision is made to use these samples as supplementary feed. Currently, the plant limits the allowable Fe and Al concentrations in the leach solution to 240 and 1450 mg/L, respectively. Leaching of only EAF dust would exceed the iron limit, while leaching of zinc dross would exceed both the Fe and Al limits. Neutralization tests should therefore be performed to determine whether the Skorpion Zinc circuit would be capable of handling these impurities, to obtain a more accurate indication of the expected reagent consumption in this section of the plant, and to determine the residue composition and its potential environmental impact. This might place an upper limit on the allowable percentage of alternative oxides in the blended feed stream to prevent extreme impurity levels in the circuit. In addition, specialized waste management procedures and additional environmental permits may be required, which may have an impact on the economic feasibility of using the alternative oxides.
The potentially hazardous nature of the EAF dust and zinc dross is an important factor to consider when it comes to disposal of the tailings generated by leaching these solids. Harmful lead, chromium, and cobalt are generally leached together with the zinc (Oustadakis et al., 2010). In the case of the Skorpion Zinc process, most of these impurities will be removed together with the copper or nickel in their respective removal sections.
The zinc content in the PLS for each of the alternative zinc oxide samples exceeded the design maximum zinc tenor in the leach solution for the Skorpion Zinc circuit. Depending on which alternative oxide is chosen as supplementary feed and what blending ratios are used, some adjustments to the operating philosophy in the solvent extraction circuit may be required. Careful consideration will also have to be given to the metal accounting if the feed consists of a mixture of ore and alternative oxides. Attention will need to be given to determining how to account for the feed to the circuit, and how to account for the recoveries obtained from the respective materials, if these need to be accounted for separately.
Some additional studies can be done on the possibility of blending the alternative zinc oxides investigated in this study with the stockpiled marginal zinc ore from the Skorpion Zinc pit. This material contains less than 4% zinc and high (> 7%) calcium with large amounts of copper. Given the high zinc and low impurity content of the zinc fume, for example, it may be possible to economically utilize the marginal zinc ore with appropriate blending strategies to reduce the acid costs and impurity levels.
Economic analysis
The potential profit that Skorpion Zinc could make from processing each of the respective oxides was calculated for the different blending ratios using an assumed London Metal Exchange (LME) zinc price with a fixed premium for Special High Grade zinc. The assumed zinc price (including the premium) per unit mass of zinc was scaled to a value of 100, and all other calculations were performed and standardized relative to this scaled zinc price. Since there is no standard price for the purchase of alternative zinc oxides, it was assumed that a certain percentage of the LME zinc price would be paid for the zinc contained in the alternative oxides. Two different scenarios were investigated to determine the profitability of processing the different zinc oxides: purchase prices of 20% and 30% of LME zinc price were used.
In each scenario, the potential revenue that Skorpion Zinc could achieve from each oxide was calculated using the total theoretical zinc production as determined by the mass balance. Next, the total operating cost for zinc production was calculated using the actual costs from the Skorpion Zinc refinery and adjusting the costs for the volumes of oxide that would be fed. Adjustments were also made to the mining and comminution costs to account for the fact that no mining or comminution would be required for these alternative oxide feed sources. The last cost element on the Skorpion Zinc side, the cost of purchasing the oxide, was calculated based on the assumed LME percentages. Finally, the earnings before interest, tax deductions, and amortization (EBITDA) were calculated for each scenario.
It was important to ensure that the prices of the alternative zinc oxide sources used in the calculations were reasonable. To determine this, the supplier's profit was also calculated based on the assumption that the supplier would be responsible for paying the freight costs to ship the alternative zinc oxide to the Skorpion Zinc refinery. Situations that resulted in a profit for both the supplier and Skorpion Zinc were considered plausible. A summary of the total profit for the supplier and Skorpion Zinc is shown in Table IV.
It is evident that if 20% of the LME zinc price is paid for the alternative zinc oxide sources, the suppliers of EAF dust and zinc dross will not make a profit even though Skorpion Zinc will. The zinc fume oxide source was the only plausible alternative oxide if suppliers are paid 20% of the LME zinc value, since this is the only source for which both the supplier and Skorpion Zinc would make a profit. If the prices of the alternative oxide sources are set at 30% of the LME zinc price, all parties will make a profit; however, Skorpion Zinc's profit per ton is reduced by between 27 and 38%, depending on the source and blending ratio.
In general, for zinc fume and dross, the total income and profit increased as the percentage of alternative oxides in the feed increased. In the case of EAF dust, increasing the blending ratio to the point where the feed contained more than 30% alternative oxides significantly limited the total feed and the zinc production rate due to the accumulation of magnesium and manganese, which resulted in reduced EBITDA.
It is clear from these results that the zinc fume oxide is the most profitable alternative oxide source for Skorpion Zinc. This is because of the low impurity levels in this source, which allows large zinc production volumes to be generated. At a 50% blending ratio, Skorpion Zinc could possibly afford to pay up to 57.3% of the LME zinc value for the zinc fume oxide before treatment of this alternative source would become unprofitable.
EAF dust and zinc dross can be used to supplement ore in a profitable manner. EAF dust provides the second highest supplier profit at a price based on 30% of the LME zinc value. This is due to the relatively high transport cost for zinc dross. EAF dust also results in the second highest overall income for Skorpion Zinc at blends of 30% oxide with ore or less, due to the greater amounts of zinc that can be recovered at this blending ratio relative to zinc production from zinc dross, which is limited by impurities. The EBITDA was, however, lower for EAF dust than for any of the other alternative zinc oxide sources at all blending ratios investigated. At a blending ratio of 30% EAF dust, a price of at least 23.3% of the LME zinc value needs to be paid to cover the supplier's freight costs, while processing at Skorpion Zinc would become unprofitable if the EAF dust price is more than 46.6% of the LME zinc value. The corresponding feasible alternative oxide price range for treatment of zinc dross at a 50% blending ratio is 26.952.6% of the LME zinc value.
Conclusions
This paper forms the second part of a two-part communication reporting on the evaluation of the technical and economic feasibility of using secondary zinc oxides as a supplementary feed to the Skorpion Zinc process. The leaching performance at the typical operating conditions currently used at Skorpion Zinc, as reported in part 1 of this communication, was used to perform mass balances and economic evaluations for the respective alternative zinc oxide sources and ore/alternative oxide blending ratios.
From the mass balance it was determined that the zinc fume sample would consistently provide the highest zinc production of the three samples, regardless of the blending ratio used. This material can theoretically lead to an annual zinc production of 495 kt when blended in a 50% ratio with the Skorpion Zinc ore. This is significantly more than the maximum production achievable when using EAF dust (220 kt/a at a 20% blending ratio) or zinc dross (183 kt/a at a ratio of 10%) as alternative oxide sources. This was due, in part, to the high zinc content of the zinc fume and its low impurity content, which resulted in low acid consumption and hence reduced operating costs. At a blending ratio of 30% or less, zinc production from EAF dust exceeded that from zinc dross, despite its lower zinc content. Production from zinc dross was limited to a large extent by its impurity levels and, more specifically, the nickel content, which was higher than that in either of the other two sources.
Impurity levels in the PLS produced from EAF dust and zinc dross may be a cause for concern. Leaching of EAF dust and zinc dross resulted in aluminium and iron concentrations in the PLS exceeding the original Skorpion Zinc circuit design specification limits of 1450 and 240 ppm, respectively. Appropriate blending ratios would thus be important to avoid overloading of the neutralization circuit, and further tests would be required to confirm the reagent requirements and precipitate characteristics. The operating philosophy for downstream units such as solvent extraction and electrowinning will also have to be evaluated for higher zinc tenors and production rates.
The financial feasibility study performed using the mass balance results showed that the zinc fume oxide would be the most profitable option of the three alternative oxides. This was the only source that was profitable for both the supplier and Skorpion Zinc at a 20% LME zinc purchase price for the zinc contained in the oxide. Increasing the proposed purchase price to 30% of the LME zinc price to make it more attractive to the supplier resulted in a decrease in Skorpion Zinc's profit of 27-38 %, while the supplier's profit increased by 90%.
Acknowledgements
The authors gratefully acknowledge Skorpion Zinc for the supply of materials and financial support.
References
Alberts, E. and Dorfling, C. 2013. Stripping conditions to prevent the accumulation of rare earth elements and iron on the organic phase in the solvent extraction circuit at Skorpion Zinc. Minerals Engineering, vol. 40. pp. 48-55. [ Links ]
Cole, P.M. 2002. The introduction of solvent-extraction steps during upgrading of a cobalt refinery. Hydrometallurgy, vol. 64, no.1. pp. 69-77. https://doi.org/10.1016/S0304-386X(02)00013-0 [ Links ]
Cole, P.M. and Sole, K C. 2003. Zinc solvent extraction in the process industries. Mineral Processing and Extractive Metallurgy Review, vol. 24, no. 2. pp. 91-137. https://doi.org/10.1080/08827500306897 [ Links ]
Cole, P.M., Sole, K.C., and Feather, A.M. 2006. Solvent extraction developments in Southern Africa. Tsinghua Science and Technology, vol. 11, no. 2. pp. 153-159. https://doi.org/10.1016/S1007-0214(06)70169-9 [ Links ]
Fukubayashi, H. 1972. The effect of impurities and additives on the electrowinning of zinc. Doctoral dissertation, University of Missouri. http://scholarsmine.mst.edu/cgi/viewcontent.cgi?article=308 7&context=doctoral_dissertations [ Links ]
Gnoinski, J. 2007. Skorpion Zinc: optimisation and innovation. Journal of the Southern African Institute of Mining and Metallurgy, vol. 107. pp. 657-662. [ Links ]
Lottering, C. and Dorfling, C. 2018. Investigation of secondary zinc oxides as an alternative feed to the Skorpion Zinc process: Part 1 - leaching alternative zinc oxides. Journal of the Southern Afican Institute of Mining and Metallurgy, vol. 118, no. 7. pp. 695-704. [ Links ]
Moradi, S. and Monhemius, A.J. 2011. Mixed sulphide-oxide lead and zinc ores: Problems and solutions. Minerals Engineering, vol. 24, no.10. pp. 1062-1076. https://doi.org/10.1016/j.mineng.2011.05.014 [ Links ]
Musadaidzwa, J.M., and Tshiningayamwe, E.I. 2009. Skorpion zinc solvent extraction: The upset conditions. Journal of the Southern African Institute of Mining and Metallurgy, vol. 109, no.11. pp. 691-695. [ Links ]
Oustadakis, P., Tsakiridis, P.E., Katsiapi, A., and Agatzini-Leonardou, S. 2010. Hydrometallurgical process for zinc recovery from electric arc furnace dust (EAFD). Part I: Characterization and leaching by diluted sulphuric acid. Journal of Hazardous Materials, vol. 179, no.1-3. pp. 1-7. https://doi.org/10.1016/j.jhazmat.2010.01.059 [ Links ]
Schlesinger, M.E., King, M.J., Sole, K.C., and Davenport, W.G. 2011. Electrowinning. Extractive Metallurgy of Copper (5th edn). Elsevier, Amsterdam. pp. 349-372. https://doi.org/10.1016/B978-0-08-096789-9.10017-4 [ Links ]
Sinclair, R.J. 2005. The Extractive Metallurgy of Zinc. Australasian Institute of Mining and Metallurgy, Melbourne. [ Links ]
Sole, K.C., Feather, A.M., and Cole, P.M. 2005. Solvent extraction in Southern Africa: An update of some recent hydrometallurgical developments. Hydrometallurgy, vol. 78. pp. 52-78. https://doi.org/10.1016/j.hydromet.2004.11.012 ♦ [ Links ]
Paper received May 2017
revised paper received Nov. 2017














